Abstract
Purpose
Total knee arthroplasty (TKA) implanted in patients with secondary osteonecrosis (ON) related to corticosteroids have relatively poor outcome (20% revision rate) at a mean follow-up of only eight years. With the hypothesis that subchondral bone marrow injection might improve knees in these patients, we evaluated 30 patients who had bilateral knee osteoarthritis with severe joint space narrowing and received TKA in one knee and subchondral bone marrow concentrate injection in the contralateral knee.
Material and methods
A prospective randomized controlled clinical trial was carried out in 60 knees of 30 patients (mean age 28 years, 18–41) who presented bilateral osteoarthritis secondary to knee ON related to corticosteroids in relation with different severe medical conditions. During the same anesthesia, one knee received TKA; for the other knee, a bone marrow graft containing an average of 6500 MSCs/mL (counted as CFU-F, range 3420 to 9830) was delivered to the subchondral bone of the femur and tibia. The length of anesthesia related to each procedure (bone marrow aspiration and subchondral injection of concentrated bone marrow versus total knee arthroplasty) was measured. Peri-operative outcomes, morbidity, complications, and safety of the two procedures were compared. Subsequent admissions for revision surgery were identified. At the most recent follow-up (average of 12 years, range 8 to 16 years), clinical outcomes of the patient (Knee Society score) were obtained along with radiological imaging outcomes (MRIs for knees with subchondral bone marrow injection).
Results
Anesthesia related to the TKA side was longer than for the cell therapy group. Medical and surgical complications were more frequent after TKA. A higher number of thrombophlebitis was observed on the side with TKA (15%) versus none on the side with cell therapy (0%). At the most recent follow-up (average of 12 years, range 8 to 16 years), six (out of 30) TKA knees needed subsequent surgery versus only one with cell therapy. The Knee Score had improved and remained similar in the TKA and cell therapy groups (respectively 80.3 points ± 11 versus 78.3 ± 23); 21 patients preferred the knee with cell therapy and 9 preferred the knee with TKA. Knees with cell therapy had improvement on cartilage and bone marrow lesions observed at the site of bone marrow subchondral injection.
Conclusions
Subchondral autologous bone marrow concentrate was an effective procedure for treating young patients with knee osteoarthritis following secondary ON of the knee related to corticosteroids with a lower complication rate and a quicker recovery as compared with TKA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Secondary osteonecrosis of the knee related to corticosteroids usually affects patients younger than 45 years of age, involves both femoral condyles, as well as the tibial epiphysis, and metaphyses of the femur and tibia are frequently affected (Fig. 1). In patients who have late stage IV disease, with lesions on both sides of the joint and collapsed articular cartilage with osteoarthritis (OA), total knee arthroplasty (TKA) is one of the solution. The therapeutic benefits after TKA performed for secondary atraumatic osteonecrosis of the knee should theoretically be comparable to those of TKA performed for OA. However, published results [1,2,3] of TKA for secondary osteonecrosis (ON) revealed only 74% good outcomes, with a 20% revision rate at a relatively short follow-up of eight years (range 4.2–10 years), and there is no data about the revision scores of the knees in this young population with associated severe comorbidities [4,5,6] as transplantation, lupus, or other causes of high doses of corticosteroids.
Due to these reasons, there is a growing need to postpone TKA in these patients and to propose less-invasive therapies in these young patients with comorbidities. One such therapy could be the use of autologous, bone marrow concentrate injected into the subchondral bone marrow of the affected joint(s). Our rationale for subchondral bone injection of bone marrow concentrate (BMC) was that intraosseous delivery of stem cells has been proven to be efficacious in bone fracture healing and ON [7, 8], and physiopathology has revealed that functional fibrocartilage tissue is synthesized only when the subchondral bone is involved through bone marrow-stimulating procedures such as Pridie drilling and microfractures [9, 10]. Pain associated with OA is likely multifactorial, but since cartilage has no innervation, the highly innervated subchondral bone may be the source of pain.
The aim of this study designed in 1999 was therefore to assess a novel approach to treating OA secondary to ON, by adding to the classical percutaneous drilling for the treatment of secondary osteonecrosis of the knee [11] subchondral bone intraosseous injection of BMC as proposed in hip ON [8], not only at early stages but also at the stage of OA. Since secondary ON is frequently bilateral [12,13,14], patients who had bilateral pain and were planned to undergo bilateral TKA were proposed to receive a subchondral injection of BMC on one of the knees during the same anaesthetic. Our hypothesis was that the subchondral injection of BMC should give at midterm a clinical improvement and could postpone TKA for one of the knee. This study was performed to assess how both techniques perform in the same patients and determine the following: (1) the number of cells obtained from the iliac crest as compared to the number of cells in the knee of these patients; (2) what were the general, local complications, and special considerations related to the two techniques; (3) whether they have the same durable effect on pain during the average follow-up of 12 years (range 8 to 16 years) and obtained the same functional scores at the most recent follow-up.
Methods
Patient selection and study design
After institutional review board approval, we conducted a study on 30 patients (18 women, 12 men) who had secondary bilateral ON of the knees related to corticosteroids. Age at time of surgery averaged 28 years ranging 18 to 41 years. All these young patients had taken high doses of corticosteroids (> 2000 mg) for different medical conditions including leukaemia (15 patients, 30 knees), variable organ (cardiac, renal transplantation (6 patients, 12 knees)), lupus erythematosus (4 patients, 8 knees), cerebral tumour (3 patients, 6 knees), and Crohn’s disease (2 patients, 4 knees). The delay between knee surgery and onset of severe medical condition with corticosteroid treatment was average eight years (range 5 to 15 years). At the time of surgery, 24 of these patients had received hip arthroplasties for associated hip ON [15], and 12 of the 30 patients were taking oral corticosteroids.
Analysis of the knees with pre-operative radiographs and MRI determined that there was collapse and degradation of the cartilage surface with secondary OA. According to the usual classifications [12], they were stage IV with collapse, degenerative changes present on both sides of the joint, joint space narrowing, osteophytes, and osteosclerosis on affected condyle and ipsilateral tibial plateau and bone marrow edema in the subchondral bone. For sizing [16], ON was present in all the quadrants of the knees.
The two knees of the same patient were randomized into either the TKA group or the MSC group. Simple randomization method conducted by a staff member blinded to the patients’ data was used; each patient was asked to choose either of two identical envelopes with either the TKA or MSC group indicated inside. Subchondral bone marrow concentrate injection and TKA were performed on the same day during the same anesthesia from 2000 to 2008 and this study was completed in 2016. At the most recent follow-up (average of 12 years, range 8 to 16 years), no knee was lost to follow-up.
Surgical technique
Surgery was performed under general anaesthesia three times. Each patient underwent knee-preserving surgery at the same time TKA surgery was performed. Patients were placed on supine position. The length of anaesthesia related to each procedure (bone marrow aspiration and subchondral injection of concentrated bone marrow versus total knee arthroplasty) was measured.
Mesenchymal stem cell aspiration from bone marrow was first performed
After installation, the patient for knee arthroplasty and before TKA incision, bone marrow was harvested on the anterior iliac crest as previously reported [17,18,19]. The quantity of bone marrow aspirate extracted from the patient’s anterior iliac crests averaged 105 mL (range 90 to 110), which was then concentrated by centrifugation. The concentration of MSCs in the bone marrow aspirate was on average 1370 MSCs/mL (range 637 to 2155).
Knee arthroplasty was performed secondly
Before anterior incision, the number of MSCs present in the subchondral bone of the knee with TKA was determined from samples (approximately 4 mL of bone marrow) aspirated percutaneously under general anaesthesia using the same technique from the tibia plateau and the femoral condyle. For TKA, cemented implants were utilized in all patients (Ceraver Total Knee System) and all were posterior-stabilized prostheses including patellar components. Stems (Fig. 2) were used when extension of the ON was present either on the femur or on the tibia. Protected weight bearing with crutches was instituted during the two post-operative weeks for these patients who had surgery on both knees at the same time.
Knee of Fig. 1 treated with arthroplasty and stems
MSCs were supercharged in the subchondral bone of the contralateral knee
Before reinjection of the bone marrow aspirated from the iliac crest, the number of MSCs present in the subchondral bone of the knee treated with MScs was also determined from samples aspirated percutaneously under general anaesthesia from the tibia plateau and the femoral condyle. Following this aspiration, each knee received a BMC graft containing an average of 6500 MSCs/mL (counted as CFU-F, range 3420 to 9830). Treatment was performed by percutaneous injection of 40 mL BMC in the subchondral medial and lateral femorotibial compartments of each knee, i.e., 10 mL in the medial tibial plateau and 10 mL in the medial condyle, 10 mL in the lateral tibial plateau and 10 mL in the lateral condyle. The bone marrow concentrate was injected slowly through the trocar and the wound closed using a single nylon suture. Patients were discharged with instructions for partial weight bearing using crutches for the first post-operative week, and then total weight bearing without crutches. Physical therapy was not necessary.
Comparative analysis of bone marrow of the iliac crest, and knee subchondral bone
The number of MSCs present in the subchondral bone before injection were determined from samples (approximately 4 mL of bone marrow) aspirated percutaneously under general anaesthesia from each patient recruited using the same technique from the anterior iliac spine, the tibia plateau, and the femoral condyle. Cell counts were carried out with a hemocytometer. Presence of connective tissue progenitor (MSC) in the subchondral bone before intraosseous injection as well as in the concentrated cell suspension was evaluated using colony-forming unit fibroblast (CFU-F) assays as previously reported [19]. Additional analysis included the number of nucleated cells, erythrocytes, and platelets, as well as the differential count of the nucleated cells. Estimates of MSC prevalence and biological performance based on CFU-F assays were performed for populations of freshly isolated cells. The number of CFU-F colonies was not the only parameter studied for these cells. Other parameters were used as previously reported [20] to explore the functional capacity of cell populations coming from the iliac crest and knee.
Peri-operative morbidity, complications, and safety of the two procedures
Patients were evaluated clinically at four to six weeks, three months, one year, and then yearly intervals thereafter. Acute medical complications were evaluated during the first 90 days after surgery. Additional complications of the procedures also included transfusions, deep-vein thrombosis, and pulmonary embolism.
Outcomes
Subsequent re-operations, causes of failures evaluation
Subsequent admissions for revision surgery were identified. All patients were encouraged to return for routine clinical and radiographic follow-up. Orthopaedic complications of arthroplasties included revision knee arthroplasty, arthroscopy, arthrotomy, debridement, synovectomy, or other surgery.
Clinical outcome of the patient
The clinical results were graded according to the Knee Society score [21]. Evaluation was scheduled at six months, one year, and yearly thereafter. At the most recent follow-up, three patients were unable or unwilling to return for full clinical evaluation, and in these patients, local radiographic evaluation was arranged and obtained, and clinical examination performed locally by their medical doctor. Thus, the status of the knee was known for all patients at final follow-up.
Radiological and imaging outcome of the patients
Radiographs and knee MRI acquisitions
During follow-up, in radiographic evaluation, standing antero-posterior and lateral views of the knee as well as skyline, and long-standing AP views were obtained for both knees. Knee joint space changes and subchondral changes were assessed at final follow-up on radiographs (narrowing, stabilization, improvement) and MRI. MRI was performed at baseline, at 24 months, five years, and at most recent follow-up on a 2.5-T MRI (Siemens) using a standard knee coil. The sequence acquisitions were for the cartilage [22,23,24] and subchondral bone marrow lesion (BML) volume measurement (Fig. 3). The cartilage volume was measured by using a computer program. The change in knee cartilage volume was obtained by subtracting the follow-up volume from the initial (baseline) volume. Bone marrow lesion (BML) volume were measured with a semi-automated segmentation method that detects, extracts, and quantifies the structure of BMLs based on the coronal and sagittal MRI sequences, using a graphical user interface (Photoshop) to manually identify the crude boundaries of the tibia and femur in each slice of the MR imaging data set by marking multiple points along the articular surface. We focused on BMLs adjacent to the chondral surface. BML volumes were calculated for four regions: medial femur, lateral femur, medial tibia, and lateral tibia.
Statistical analysis
Demographic and medical variables were determined by the mean, standard deviation, range, and percent. For this study, a pair protocol analysis was used. Comparisons were performed by Student’s 푡 test for paired-samples parametric data or the Wilcoxon signed-rank test for paired-samples nonparametric data.
Results
Progenitor counts in the cell therapy group
Differences were observed in the number of progenitor’s concentration at the three sites
CFU-F counts were performed on aspirations performed at different sites (femur, tibia) of both knees and iliac crests; the mean volumes of aspirate obtained from the pelvis, femur, and tibia were respectively 4.2 ml ± 2.68, 3.60 ml ± 2.05, and 4.5 ml ± 1.50. CFU-F counts showed a significant difference (p < 0.001) between the three sites in terms of both the CFU-F per million nucleated cells (respectively 29.4, 9.7, and 9.0) and the CFU-F per milliliter of bone marrow aspirate: 1370 MSCs/mL (range 637 to 2155) for the iliac crest, 125 MSCs/mL (range 78 to 195) for the femur, and 81 MSCs/mL (range 24 to 125) for the tibia. Nevertheless, the assessment of proliferative ability and cell differentiation did not reveal any obvious differences between the different locations: MSC growth was evaluated by comparing the cell number and the doubling time of MSCs during successive passages for bone marrow isolated from different sites for each patient (iliac crest versus femur and tibia).
The number of progenitor cells in the bone marrow concentrate graft implanted in the subchondral bone
The average BMC graft contained 6500 MSCs/mL (counted as CFU-F, range 3420 to 9830)/mL, which represents an average 30-fold increase compared to the concentration of MSCs present in the bone marrow aspirate of the subchondral bone of the OA knees of patients before implantation. Bone graft volume was 40 cm3 (20 cm3 in the tibia and 20 cm3 in the femur).
Peri-operative outcomes, morbidity, complications, and safety of the two procedures
When the duration of anesthesia in the operating room were calculated for each side, the duration was on average 1.5 times (range 1.2 to 1.9) longer for sides receiving TKA compared with sides receiving cell therapy. As compared with the usual length of hospital stay of patients receiving only cell therapy procedure for both sides (1 day for cell therapy procedure) in our usual protocol [8], the length of hospital stay of patients receiving simultaneous TKA and cell therapy was increased to average eight days in the hospital, the difference being related to the TKA side. With the same mode of comparison, the length of anticoagulation was lower for a bilateral cell therapy simultaneous procedure in our usual protocol (1 week maximum) as for simultaneous TKA and cell therapy procedures (4 weeks). The period of time for use of crutches was significantly different for the two procedures with average three days (range none to 7 days) for two crutches related to the bilateral procedure, but three weeks more (range 2 to 6 weeks) with the persistent crutch related to the TKA side.
During the post-operative period (6 months), the number of adverse events were higher (p = 0.04) in the TKA group. The TKA group had a higher rate of blood transfusion (30 versus 0%), and a higher number of thrombophlebitis was observed on the side with TKA (15%) versus none on the side with cell therapy (0%). One patient with TKA developed wound necrosis two weeks post-operatively. This was treated with plastic surgery and patient subsequently healed with healthy scar but loss of range of motion.
Outcomes of the two procedures as primary surgery
Subsequent re-operations and causes of failure in each group
At the most recent follow-up (average of 12 years, range 8 to 16 years), six of the 30 knees operated with TKA required a subsequent anaesthesia and surgery. One had haematoma at one month with surgical evacuation, two had a revision after their initial TKA (one for periprosthetic fracture and one for patella pain), and two had loosening and revision at seven and eight years follow-up). Among knees with cell therapy, three knees had a subsequent surgery for TKA at six, eight, and 12 years follow-up.
Functional outcome for knees without subsequent surgery
Clinical pre-operative knee scores of cell therapy knees before treatment (47 points ± 12) were similar (p = 0.08) to knee’s scores of the TKA group before treatment (knee 44 points ± 8; function 45 points ± 13). The mean overall changes in knee scores at three months follow-up were similar (p = 0.24) for the cell therapy group (81.3 points ± 12) when compared with the TKA group (79 points ± 21). At the time of the most recent follow-up, the Knee Score remained similar in the group with cell therapy as compared with the group with TKA (respectively 80.3 points ± 11 versus 78.3 ± 23). If the mean scores were similar, the distribution was different in the two groups: a greater proportion of patients had higher knee scores and described the results of their operation as excellent after TKA (8 of 30 respondents) when compared with cell therapy (4 of 30 respondents), which may reflect better joint involvement associated with TKA; but the proportion of patients with poor results not reporting significant improvements was also greater for the TKA group (four patients for TKA versus one patient with cell therapy), which may reflect a higher number of dissatisfied patients with knee arthroplasties than with cell therapy. Among the 30 patients, 21 preferred the knee with cell therapy and nine the knee with TKA (P < 0.05).
Radiological and imaging outcome of the patients without subsequent surgery
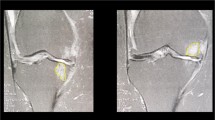
Figures 3 and 4 show the status of the knee before treatment and Figs. 5 and 6 the aspect of the same knee treated with subchondral injections at a ten years follow-up. Cartilage volume changes over time were measured on knees with cell therapy: In the group of knees treated with BMC injection, the percentage cartilage volume measured with MRI (without osteophytes) increased compared to baseline (2.3% ± 1.1% at two years, 3.8% ± 2.1% at five years, and 4.2% ± 2.5% at the most recent follow-up).
The same knee as Fig. 3; pre-operative radiograph
MRI of the same knee (as Fig. 3) at 10 years follow-up after cell therapy treatment. The subchondral lesions have decreased
Radiographs of the same knee after (as Fig. 4) at 10 years follow-up after cell therapy treatment
Subchondral bone marrow lesion changes
Increased subchondral bone marrow (BML) volume of the femorotibial compartment representing the size of the ON was associated with joint space narrowing in the same tibiofemoral compartment (odds ratio (OR) = 1.80; 95% confidence interval (CI) = 1.23 to 2.32; p = 0.01) at baseline before bone marrow injection. The average baseline BML volume of the femorotibial compartments (i.e., size of the necrotic bone) ranged from 6.4 to 12.5 cm3 (mean 8.4 cm3). Larger total baseline BML volumes were associated with greater knee pain (p = 0.01%). After treatment with bone marrow injection, femorotibial compartment BML volume experienced regression over 24 months and five years (respectively mean 3.5 cm3, range 1.2 to 5.3 cm3; mean 4.1 cm3, range 3.2 to 6.2 cm3), representing a decrease in size ON of average 40%. The regression models indicated that total BML volume decrease and pain change were significantly related (odds ratio (OR) = 3.42; 95% confidence interval (CI) = 2.46 to 4.62; p = 0.02), as BML volume decreases and cartilage increases.
The correlations between cartilage healing status, BML changes and the number of progenitors were analyzed and demonstrated that a poor response was correlated with a low dose of progenitors in the graft (less than 1200 MSCs/mL). However, for higher dose of progenitors, there was no correlation between the response and the dose of cells. To determine whether there were other reasons for the observed cartilage healing status, other patient demographic factors were analyzed. No correlations were not found between the cartilage healing status and patient body mass index, age, or limb alignment.
Revision surgery was easier and had better outcome in the cell therapy group
Knees with cell therapy undergoing revision with TKA had implants without stems
During the TKA of the three knees that had first cell therapy, evaluation of the repair process was performed: (1) a bone marrow sample was collected for cell analysis of the MSC content of the femur and tibia, after which the cuts were fixed for subsequent histological evaluation. The mean concentration of MSCs observed in the BMMSC-treated osteonecrotic knees (both in the necrotic area of the tibia and talus) had increased (p = 0.001) from 96 (range 72 to 115) progenitors per cubic centimeter obtained at the aspiration before injection to 1124 progenitors per cubic centimeter (range 910 to 1500) at aspiration at the time of arthroplasty. (2) Cartilage repair was evaluated with histology in the zones where MRI demonstrated some improvement: according to the Kanamiya grading system, fibrocartilage coverage (grade 4), or partial fibrocartilage coverage (grade 3), were observed as regenerative changes in these knees. (3) Bone repair was also observed in osteonecrotic lesions treated with MSCs; this resulted in the formation of new bone, which was determined by histological evaluation of sections of the fixed femoral and tibial sections obtained during the three TKA. An assessment of new bone formation showed that osteonecrotic lesions treated with BMMSCs had produced new bone (mean of 45%; range 25 to 55%) which allowed implantation of cemented arthroplasties without stems.
Revision of knees with TKA as the primary surgery needed extended stems
Knees with TKA as the primary surgery undergoing revision with a subsequent TKAs needed extended stems since they had short stems at the primary surgery; they were more likely to have a re-revision (two among six knees) than knees with cell therapy undergoing revision with TKA (no re-revision among three knees) for the same follow-up.
Discussion
Knee ON in young patients treated with high doses of corticosteroids is usually multifocal with locations in several joints, especially the large joints of the lower extremity [15] as hips and knees. The size and location of the lesion typically influence the rate of disease progression with quicker progression on the hip as compared with the knees. In some cases, small lesions of the knees may stabilize spontaneously, but large lesions usually progress [12,13,14,15,16]. Ischemia and necrosis limit repair, causing bone resorption of the affected condyle or tibial plateau. This acellular region weakens the mechanical stability of the surrounding bone, leading to subchondral fracture. The collapsed regions increase stress on their adjacent articular surfaces, inducing further degenerative changes. When secondary OA arrives with extensive arthritis and incongruity and when the lesions are large, one of the solutions is TKA. However, the longevity of knee arthroplasty in younger patients is limited [1], and this can be devastating in young patients when complication occurs.
Historically, microfracture surgery has been applied to treat cartilage defects. During the procedure, the subchondral bone is penetrated, allowing bone marrow-derived mesenchymal stem cells to migrate towards the defect site and form new cartilage tissue or fibrocartilage. Patients treated with bone marrow stimulation by microfracture generally show clinical improvements up to three years [25, 26]. If this time of clinical improvement could be extended by better stimulation of the bone marrow through increasing the concentration of bone marrow progenitors in the subchondral bone of the knee, this would be highly beneficial in young patients with OA.
There was a low concentration of progenitor cells in the subchondral bone lesions of knees with OA secondary to ON as compared to the iliac crest. So, if one concern is the quantity and quality of osteoprogenitor cells harvested from the pelvis in patients who have received medications such as corticosteroids [27], the arthritic knee or areas of focal bone marrow lesion under cartilage degeneration is much more deficient in the stem cell or progenitor cell population as compared with the iliac crest number of progenitors. This deficiency may be corrected by the harvest and transplantation of cells from the iliac crest of patients which could provide sufficient population of progenitor cells to have an effect on pain relief [28], resolve bone marrow lesions and decrease wear of the cartilage (by multiplication of cells or cytokine effect).
The < 40 years patient population of this study has a high risk of revision arthroplasty due to the age but also to prevalence of comorbidities, including knee osteoporosis. There are many studies of treatment of secondary knee ON [29,30,31,32,33]; but to our knowledge, this is the first long-term study with comparison between cell therapy and TKA in knee OA. Starting treatment of knee OA in patients with subchondral injection of bone marrow instead of the standard TKA suggests that surgeons and patients expect improvement in patient’s quality of life that would offset the potential risk of revision surgery during at least one decade, and if possible an easier revision surgery when necessary; this was observed in this young population when TKA and subchondral injection were compared in the same patients. However, this population is a specific population; most of them had multiple joint involvements in relation to multifocal ON; therefore, despite the young age of patients of our series, there activity is lower than other young patients of the same age without co-morbidities.
Despite randomization of patients, this study has some limits. We did not perform biopsies of cartilage in this population, and most probably, the new formation of cartilage observed on MRI is rather formation of fibrocartilage as observed on knees that received TKA. Another factor is that these young patients had severe medical disease and for some of them had the presence of three arthroplasties (two hips and one knee); therefore, we can question whether they would be willing to be volunteers for a second knee arthroplasty even if the cell therapy treatment proved ineffective. However, most of them preferred the knee with cell therapy, had less medical complications and equivalent results with cell therapy as compared with TKA during the decade of follow-up.
In conclusion, this study showed that subchondral bone marrow concentrate was an effective procedure as compared with TKA for knee OA in young patients with secondary ON related to corticosteroids.
References
Myers TG, Cui Q, Kuskowski M, Mihalko WM, Saleh KJ (2006) Outcomes of total and unicompartmental knee arthroplasty for secondary and spontaneous osteonecrosis of the knee. J Bone Joint Surg Am 88(Suppl 3):76–82
Mont MA, Myers TH, Krackow KA et al (1997) Total knee arthroplasty for corticosteroid associated avascular necrosis of the knee. Clin Orthop Relat Res 338:124
Seldes RM, Tan V, Duffy G, Rand JA, Lotke PA (1999) Total knee arthroplasty for steroid-induced osteonecrosis. J Arthroplast 14:533–537
Mont MA, Baumgarten KM, Rifai A et al (2000) Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am 82(9):1279
Vora A (2011) Management of osteonecrosis in children and young adults with acute lymphoblastic leukaemia. Br J Haematol 155(5):549
Mertelsmann-Voss C, Lyman S, Pan TJ et al (2014) Arthroplasty rates are increased among US patients with systemic lupus erythematosus: 1991-2005. J Rheumatol 41(5):867
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–1437
Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 405:14
Dray A, Read SJ (2007) Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Res Ther 9(3):212
Tat SK, Lajeunesse D, Pelletier J-P, Martel-Pelletier J (2010) Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol 24(1):51–70
Marulanda G, Seyler TM, Sheikh NH et al (2006) Percutaneous drilling for the treatment of secondary osteonecrosis of the knee. J Bone Joint Surg (Br) 88(6):740
Mont MA, Marker DR, Zywiel MG et al (2011) Osteonecrosis of the knee and related conditions. J Am Acad Orthop Surg 19(8):482
Karimova EJ, Wozniak A, Wu J et al (2010) How does osteonecrosis about the knee progress in young patients with leukemia?: a 2- to 7-year study. Clin Orthop Relat Res 468(9):2454
Shigemura T, Nakamura J, Kishida S et al (2011) Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology 50(11):202312
Flouzat-Lachaniette CH, Roubineau F, Heyberger C, Bouthors C, Hernigou P (2016) Multifocal osteonecrosis related to corticosteroid: ten years later, risk of progression and observation of subsequent new osteonecroses. Int Orthop 40(4):669–672
al-Rowaih A, Bjorkengren A, Egund N, et al. (1993) Size of osteonecrosis of the knee. Clin Orthop Relat Res (287):68
Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P (2014) Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop 38(11):2377–2384
Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P (2014) Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop 38(12):2585–2590
Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, Rouard H (2013) Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 37(11):2279–2287
Lebouvier A, Poignard A, Coquelin-Salsac L, Léotot J, Homma Y, Jullien N, Bierling P, Galactéros F, Hernigou P, Chevallier N, Rouard H (2015) Autologous bone marrow stromal cells are promising candidates for cell therapy approaches to treat bone degeneration in sickle cell disease. Stem Cell Res 15(3):584–594
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J (2008) A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthr Cartil 16(Suppl 3):S8–S13
Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, Felson DT, Lewis CE, Torner J, Nevitt MC (2009) Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss—the MOST study a longitudinal multicenter study of knee osteoarthritis. Ann Rheum Dis 68:1461–1465
Wluka AE, Hanna FS, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, Adams J, Cicuttini FM (2009) Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis 68:850–855
Hoemann CD, Chen G, Marchand C, Tran-Khanh N, Thibault M, Chevrier A, Sun J, Shive MS, Fernandes MJ, Poubelle PE, Centola M, El-Gabalawy H (2010) Scaffold-guided subchondral bone repair: implication of neutrophils and alternatively activated arginase-1C macrophages. Am J Sports Med 38:1845–1856
Van der Linden MH, Saris D, Bulstra SK, Buma P (2013) Treatment of cartilaginous defects in the knee: recommendations from the Dutch Orthopaedic Association. Ned Tijdschr Geneeskd 157(3):A5719
Hernigou P, Beaujean F, Lambotte JC (1999) Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg (Br) 81(2):349
Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, Torner J, Lewis CE, Nevitt MC (2007) Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 56:2986–2992
Lieberman JR, Varthi AG, Polkowski GG II (2014) Osteonecrosis of the knee—which joint preservation procedures work? J Arthroplast 29(1):52
Rijnen WH, Luttjeboer JS, Schreurs BW et al (2006) Bone impaction grafting for corticosteroid-associated osteonecrosis of the knee. J Bone Joint Surg Am 88(Suppl 3):62
Gortz S, De Young AJ, Bugbee WD (2010) Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res 468(5):1269
Lee K, Goodman SB (2009) Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplast 24(1):43
Sultan AA, Khlopas A, Sodhi N, Denzine ML, Ramkumar PN, Harwin SF, Mont MA (2017) Cementless total knee arthroplasty in Knee osteonecrosis demonstrated excellent survivorship and outcomes at three-year minimum follow-up. J Arthroplasty. Nov 8
Acknowledgments
We thank Richard Suzuki and Meghana Malur of Celling Biosciences for the review of the final manuscript and their help in translation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hernigou, P., Auregan, J.C., Dubory, A. et al. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. International Orthopaedics (SICOT) 42, 2563–2571 (2018). https://doi.org/10.1007/s00264-018-3916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-3916-9