Abstract
Purpose
The purpose of this study was to investigate whether mechanical stretch can enhance the bone morphogenetic protein 9 (BMP9)-induced osteogenic differentiation in MSCs.
Methods
Recombinant adenoviruses were used to overexpress the BMP9 in C3H10T1/2 MSCs. Cells were seeded onto six-well BioFlex collagen I-coated plates and subjected to cyclic mechanical stretch [6% elongation at 60 cycles/minute (1 Hz)] in a Flexercell FX-4000 strain unit for up to 12 hours. Immunostaining and confocal microscope were used to detect cytoskeleton organization. Cell cycle progression was checked by flow cytometry. Alkaline phosphatase activity was measured with a Chemiluminescence Assay Kit and was quantified with a histochemical staining assay. Matrix mineralization was examined by Alizarin Red S Staining.
Results
Mechanical stretch induces cytoskeleton reorganization and inhibits cell proliferation by preventing cells entry into S phase of the cell cycle. Although mechanical stretch alone does not induce the osteogenic differentiation of C3H10T1/2 MSCs, co-stimulation with mechanical stretch and BMP9 enhances alkaline phosphatase activity. The expression of key lineage-specific regulators (e.g., osteocalcin (OCN), SRY-related HMG-box 9, and runt-related transcription factor 2) is also increased after the co-stimulation, compared to the mechanical stretch stimulation along. Furthermore, mechanical stretch augments the BMP9-mediated bone matrix mineralization of C3H10T1/2 MSCs.
Conclusions
Our results suggest that mechanical stretch enhances BMP9-induced osteoblastic lineage specification in C3H10T1/2 MSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an attractive cell source for tissue engineering and regenerative medicine, mesenchymal stem cells (MSCs) can be isolated from different tissues to give rise to several lineages (e.g., bone, adipose, cartilage, and muscle) [1]. Osteogenic differentiation is a complex but well-regulated process controlled via a series of chemical and physical factors [2]. Therefore, a better understanding of the mechanisms underlying osteogenic differentiation will be instrumental for the clinical use of stem cells.
Bone morphogenetic proteins (BMPs) play important roles in the regulation of stem cell proliferation and osteogenic differentiation [3]. BMPs belong to the transforming growth factor β superfamily; its members are crucial for cell proliferation, adhesion, and differentiation, skeletal development, and bone formation [4]. BMPs are highly conserved secretory multifunctional proteins, with at least 14 members in humans [5]. Among these members, BMP2, BMP4, BMP6, BMP7, and BMP9 can effectively induce the osteogenic differentiation of MSCs in vitro and in vivo [6]. BMP9 is one of the most osteogenic yet least studied BMPs.
Mechanical stimulation also has remarkable effects on the regulation of stem cell fate. Recent studies have shown that mechanical stimulation induces changes in stem cell behaviour such as adhesion, migration, proliferation, differentiation, and survival [7]. Previous studies of MSCs have focused on the cellular responses to chemical and biological factors. However, information about the synergistic effects of physical cues and biochemical factors on osteogenesis is limited. To determine whether mechanical stretch acts synergistically with the BMP9-induced osteogenic differentiation of MSCs, we first tested whether C3H10T1/2 MSCs are responsive to mechanical stretch. We then examined the effects of BMP9 and mechanical stretch co-stimulation on cell proliferation, the expression of osteogenic genes, and bone matrix mineralization in C3H10T1/2 MSCs.
Materials and methods
Cell culture
Human embryonic kidney 293 (HEK293) and C3H10T1/2 MSCs were obtained from the American Type Culture Collection (Manassas, VA, USA). C3H10T1/2 cells are commonly used and well characterized MSCs [5, 6]. Cells were maintained in complete Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit-Haemek, Israel), 100 U/mL of penicillin, and 100 g/mL of streptomycin (Beyotime, Shanghai, China) in a humidified 37 °C incubator with 5% CO2.
Recombinant adenoviruses
Recombinant adenoviruses are generated based on a replication-defective adenoviral vector that provides a versatile system for gene expression studies. Recombinant adenoviruses were produced following the previously described AdEasy protocols [8]. The coding regions of human BMP9, green fluorescence protein (GFP), and red fluorescence protein (RFP) were amplified by polymerase chain reaction (PCR) and cloned into an adenoviral shuttle vector that was subsequently used to generate recombinant adenoviruses in HEK293 cells. The adenoviruses were designated AdBMP9 or AdR-BMP9 (AdBMP9 expressed GFP, whereas AdR-BMP9 expressed RFP as a marker to monitor the infection efficiency). Adenoviruses expressing only monomeric RFP (AdRFP) or GFP (AdGFP) were used as controls [9].
Application of mechanical stretch
A Flexcell Strain Unit Fx-4000 (Flexcell Corp., Burlington, NC, USA) was used to apply mechanical stretch to C3H10T1/2 MSCs. Cells were seeded onto six-well BioFlex collagen I-coated plates (Flexcell Corp.) at 10% confluence for cytoskeletal analysis and at 30–40% confluence for other studies. Four hours after seeding, cells were infected with adenoviruses. Thirty hours after infection, cells were subjected to serum starvation overnight and cyclic mechanical stretch [6% elongation at 60 cycles/minute (1 Hz)] for up to 12 hours. Static control cells were subjected to the same conditions, except mechanical stretch was not applied.
Cytoskeletal analysis
Actin, a cellular skeleton protein, was visualized with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Enzo Life Sciences, Lausen, Switzerland), which was added immediately after cells were stretched with mechanical stimuli. The medium was removed from each sample, which was then washed twice with phosphate-buffered saline (PBS). Samples were fixed with 4% paraformaldehyde in PBS for 10 min and washed three times with PBS. Fixed cells were permeabilized with Triton X-100 (0.1%) in PBS and blocked with 5% bovine serum albumin (Sigma-Aldrich Co., St. Louis, MO, USA) at room temperature, followed by incubation in 10 μg/mL of fluorescent phalloidin-conjugate solution in PBS overnight at 4 °C. After washing, the cells were stained with 4′,6-diamidino-2-phenylindole (Beyotime) for ten minutes protected from light at room temperature. The cells were then analyzed on an inverted confocal microscope (Leica SP8; Leica, Wetzlar, Germany) with a long working distance lens. Cell F-actin responses to mechano-stimuli are semi-quantified by the aspect ratio and the number of branch points of the cells by using ImageJ software.
Flow cytometry
Samples were harvested after trypsinization, fixed with cold 70% ethanol overnight, and resuspended in PBS followed by incubation with RNase A in propidium iodide staining solution (Suzhou New Med Cytomics Biotechnology Co., Ltd., Suzhou, China) for 15 minutes at room temperature. The DNA content was measured on a flow cytometer (BD Influx™; BD Biosciences, San Jose, CA, USA). Data were analyzed using ModFit LT software (Verity Software House Inc., Topsham, ME, USA).
ALP assay
ALP activity was measured quantitatively with a Great Escape SEAP Chemiluminescence Assay Kit (BD Clontech, Palo Alto, CA, USA) and was quantified with a histochemical staining assay using a mixture of 0.1 mg/mL of naphthol AS-MX phosphate and 0.6 mg/mL of Fast Blue BB Salt (Sigma-Aldrich Co.) as described previously [10, 11]. ALP activity was normalized to the total cellular protein concentration. Calcium mineral deposits were examined under bright field microscopy.
RNA isolation and reverse transcription qRT-PCR
To determine whether C3H10T1/2 MSCs are sensitive to mechanical stretch, total RNA was isolated from mechanically stimulated and unstimulated cells at early time points (0, 0.5, 1, 2, or 4 h). RNA was isolated using TRIzol reagent (Takara, Dalian, China) according to the manufacturer’s instructions. RNA was reverse-transcribed using a PrimeScript™ II First Strand cDNA Synthesis Kit (Takara). Real-time quantification of mRNA was performed in a total volume of 25 μL using the Bio-Rad CFX Connect PCR system with SYBR Premix ExTaq™ II kits (Takara) according to the manufacturer’s instructions. Target gene expression was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). RT-PCR primers for mouse osteocalcin (OCN; 5′-CCT TCA TGT CCA AGC AGG A-3′ and 5′-GGC GGT CTT CAA GCC ATA C-3′), runt-related transcription factor 2 (Runx2; 5′-CCG GTC TCC TTC CAG GAT-3′ and 5′-GGG AAC TGC TGT GGC TTC-3′), SRY-related HMG-box 9 (Sox9; 5′-AGC TCA CCA GAC CCT GAG AA-3′ and 5′-TCC CAG CAA TCG TTA CCT TC-3′), and GAPDH (5′-GGC TGC CCA GAA CAT CAT-3′ and 5’-CGG ACA CAT TGG GGG TAG-3′) transcripts were designed using Primer3 Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/ primer3plus.cgi).
Alizarin Red S staining
AdBMP9- or AdGFP-infected cells were first exposed to cyclic mechanical stretch and then cultured in the presence of 50 mg/mL of ascorbic acid (Sigma-Aldrich Co.) and 10 mM β-glycerophosphate (Sigma-Aldrich Co.). After 14 days of culture, mineralized matrix nodules were stained for calcium precipitation by means of Alizarin Red S staining as described previously [10, 11]. Cells were fixed with 0.05% (v/v) glutaraldehyde at room temperature for ten minutes and washed with PBS. Fixed cells were then incubated with 0.4% Alizarin Red S (Sigma-Aldrich Co.) for 5 min, followed by washing with PBS. Calcium mineral deposits were examined under bright field microscopy.
Statistical analysis
All quantitative experiments were performed in triplicate and/or repeated three times. Data are expressed as the mean ± standard deviation. Comparisons between two groups were analyzed using the two-tailed unpaired Student’s t test. A value of p < 0.05 was considered to indicate statistical significance.
Results
Mechanical stretch-induced actin cytoskeleton reorganization in MSCs
Cells infected with Ad-RFP or AdR-BMP9 were subjected to cyclic mechanical stretch and then stained with phalloidin-FITC to observe the organization of actin filaments. Static cells were polygonal and displayed randomly oriented stress fibers, whereas stretched cells were fusiform, exhibiting realigned stress fibers orientated with the direction of the longitudinal axis (Fig. 1a). AdR-BMP9-infected cells exhibited thicker and more differentiated stress fibers than Ad-RFP control cells. Furthermore, the stretched cells transduced with AdBMP9 had higher aspect ratio and more branching than static cells (Fig. 1b, c). These data suggest that both mechanical stretch and BMP9 are involved in actin cytoskeleton reorganization in MSCs.
Mechanical stretch remodeled actin filaments in C3H10T1/2 MSCs. a Microfilaments in stretched cells were remodeled and well organized compared to static cells. Actin filaments (green), BMP9 (red), RFP (red), and nuclei (blue). Scale bars represent 50.0 μm. All images were obtained under the same magnification. b, c Cell morphology quantified for aspect ratio (b), and the number of primary, secondary, tertiary, and total branch points (c) using ImageJ software. Fifteen cells were analyzed for each treatment (n = 15). **p < 0.01
Effects of mechanical stretch on the cell cycle in MSCs
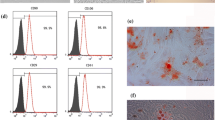
To assess the effects of mechanical stretch and BMP9 on the cell cycle, cell cycle profiles were examined by flow cytometry after DNA content staining (Fig. 2). After applying mechanical stretch for 12 hours, the accumulation of cells in G1 phase increased sharply (approximately 34% and 25% in GFP- and BMP9-infected groups, respectively). Meanwhile, the stretched cells in S phase decreased by almost 35% compared to static cells in both the GFP- and BMP9-infected groups. These data indicate that mechanical stretch inhibits cell proliferation by arresting the DNA content in G1 phase of the cell cycle.
Mechanical stretch prevented C3H10T1/2 MSCs from entering S phase of the cell cycle by arresting the cells in G1 phase. Cell cycle analysis of untreated cells or cells treated with tension. Flow cytometric data were analyzed using ModFit LT software. The percentage of cells in each cell cycle phase (G1, G2, and S) is reported in the boxed areas within each panel
Mechanical stretch enhances the BMP9-induced early osteogenic differentiation of MSCs
To determine the effect of mechanical stretch on BMP9-induced early osteogenesis, subconfluent C3H10T1/2 MSCs were infected with adenoviruses and mechanical stretch was applied. Successful infection of C3H10T1/2 MSCs by the adenoviruses was validated by examining green fluorescence (Fig. 3a). ALP activity was quantitatively determined and histochemical staining was conducted on days three, five and seven after applying mechanical stretch. Mechanical stretch alone did not induce ALP activity, whereas BMP9 alone significantly induced ALP activity in C3H10T1/2 MSCs. Interestingly, BMP9 and mechanical stretch co-stimulation further induced ALP activity in C3H10T1/2 MSCs relative to BMP9 alone (Fig. 3b). Similar results were obtained from ALP histochemical staining (Fig. 4a–d). These findings indicate that mechanical stretch enhances the BMP9-induced osteoblast lineage commitment of C3H10T1/2 MSCs.
a GFP shows efficient infection of C3H10T1/2 MSCs by adenovirus. Scale bars = 200 μm. b A quantitative ALP activity assay was performed at the indicated time points (n = 4). ALP activity was significantly higher in cells subjected to co-stimulation with mechanical stretch and BMP9 than in the BMP9-alone group. ALP activity was normalized to total cellular protein. *p < 0.05, ***p < 0.001
ALP staining of untreated cells or cells treated with mechanical stretch. a–c Overall ALP staining of the wells (top row) and microscopic images (bottom row) are shown in each panel. Scale bars = 100 μm. Positive staining is labeled in purple/blue. Arrows indicate the location of microscopic images. d Quantification of ALP staining area (pixels) is based on images from BMP9-infected groups (n = 3). **p < 0.01. Mechanical stretch enhanced BMP9-induced ALP activity in MSCs, consistent with the results of the quantitative ALP activity assay
Mechanical stretch enhances BMP9-induced late osteogenic differentiation and matrix mineralization in MSCs
To investigate the effect of mechanical stretch on late osteogenic and chondrogenic differentiation induced by BMP9, total RNA was collected at the indicated time points. Three chondro-osteogenic genes were analyzed by qRT-PCR: OCN, Runx2, and Sox9. When C3H10T1/2 MSCs were transduced with AdBMP9, all three genes were significantly upregulated compared to the AdGFP group (Fig. 5a–c). Furthermore, the expression of OCN and Runx2 was significantly increased in C3H10T1/2 MSCs after applying loading tension for two and four hours compared to the BMP9 alone group (Fig. 5a, b). Sox9, a key chondrogenic lineage-specific regulator, was also significantly upregulated in C3H10T1/2 cells after applying loading tension for four hours compared to the control group (Fig. 5c). These results confirm that mechanical stretch enhances BMP9-induced late osteogenic differentiation. Lastly, the effects of mechanical stretch and BMP9 on cell matrix mineralization were analyzed. Co-stimulation with mechanical stretch and BMP9 resulted in stronger Alizarin Red S staining indicating matrix mineralization in C3H10T1/2 MSCs compared to the static control group (Fig. 5d, e). Taken together, these results demonstrate that mechanical stretch enhances the BMP9-induced osteogenic differentiation of MSCs.
Mechanical stretch enhanced BMP9-induced late osteogenic differentiation and matrix mineralization in C3H10T1/2 MSCs. a–c qRT-PCR analysis of OCN, Runx2, and Sox9 expression (n = 3). Cultured cells were infected with adenoviruses and subjected to mechanical stretch. Samples were collected at 0 (static control), 0.5, 1, 2, or 4 hours for qRT-PCR analysis. Results were normalized to GAPDH and are expressed as the mean ± standard error of the mean (*p < 0.05, ***p < 0.001) relative to the GFP control group. d–e At 14 days post-infection, cells were fixed and stained with Alizarin Red S solution. Cells co-stimulated with mechanical stretch, and BMP9 exhibited more bone mineralization than the BMP9 infection group. Scale bar = 500 μm. **p < 0.01, ***p < 0.001
Discussion
We previously reported that BMP9 is a central BMP family member. BMP9 induces the osteogenic differentiation of MSCs by synergizing with a set of downstream targets, including growth hormone, retinoic acid, insulin-like growth factor 2, and hypoxia-inducible factor 1α [9,10,11,12]. Moreover, recent evidence revealed that the BMP signaling pathway is directly regulated by mechanotransduction [13]. However, it is unclear whether mechanical stretch affects BMP-induced cell fate. In this study, we applied cyclic mechanical stretch to C3H10T1/2 MSCs that were infected with BMP9-overexpressing adenoviruses. Mechanical stretch inhibited cell proliferation by arresting the cell cycle at G1 phase and acted synergistically with BMP9 to alter the cytoskeleton and promote osteogenesis in MSCs.
Putative mediators of mechanosensation include ion channels, integrins, surface receptors, cell adhesion, and the actin cytoskeleton [2, 14]. The actin cytoskeleton is a composite network that not only plays an important role in cell morphology, size, and movability, but it also acts as a mechanosensor in response to mechanical stimuli, reorganizes the cellular structure, and activates associated signaling pathways [15, 16]. Actin cytoskeleton functions as a coordinator in controlling cell proliferation, differentiation, and migration [17]. Recent reports have demonstrated that the cytoskeletal contractility and actin polymerization resulted in osteogenic differentiation on 2D substrates [18]. Our results indicate that, after mechanical stretch, the actin cytoskeleton in C3H10T1/2 MSCs was remodeled dramatically, being arranged regularly with the longitudinal axis. Moreover, the synergistic effects of mechanical stretch and BMP9 induced a thicker and polymerized actin network, higher aspect ratio, and more branch points.
Cell cycle regulation is required for stem cells to remain in a quiescent state or for entry into proliferation. Flow cytometric analysis showed that the percentage of cells in S phase was dramatically decreased and that cells in G1 phase were largely accumulated in the mechanical stress-treated group compared with the static control group. However, the present study shows that BMP9 has no significant effect on cell cycle transitions. These results indicate that mechanical stretch inhibits C3H10T1/2 MSC proliferation. Our results are in agreement with earlier findings that MSC proliferation can be inhibited by mechanical stretch [19]. Conversely, other researchers found that appropriate mechanical stretch treatment promoted the proliferation of MSCs [20]. These differing results may be due to differences in methods, time, frequency, and/or level of mechanical force loading [21].
The osteogenic potency of MSCs can be characterized by the expression of ALP as an early marker and key lineage-specific regulators, as well as matrix mineralization [6, 12]. Quantitative and qualitative measurements of ALP activity showed that mechanical stretch alone did not induce significant ALP activity, whereas co-stimulation with BMP9 and mechanical stretch induced ALP activity significantly in C3H10T1/2 MSCs. Our findings demonstrate that mechanical stretch is necessary but insufficient to induce MSCs to enter the early stage of osteogenesis without BMP9 stimulation. In accordance with these results, the qRT-PCR data also indicate that late osteogenic markers were significantly upregulated in C3H10T1/2 MSCs under mechanical stretch and BMP9 co-stimulation. The expression of OCN and Runx2 was significantly increased in C3H10T1/2 MSCs following co-treatment with mechanical stretch and BMP9 compared to the BMP9-induced group. OCN, also known as bone γ-carboxyglutamate protein, is the most abundant noncollagenous protein in bone and a key regulator of osteogenic maturation [22]. Runx2 is an essential regulator of osteoblast differentiation, chondrogenesis, and endochondral ossification. The expression of Runx2 can be induced by BMP2 or BMP7 in mouse calvarial mesenchymal progenitor cells and myoblast precursor cells [23, 24]. Interestingly, mechanical stretch and BMP9 also caused Sox9 mRNA upregulation four hours after exposure to force. Sox9 is mainly characterized as a key regulator in chondrogenesis. Previous study has demonstrated that mechanical load alone has no effect on stimulating the expression of cartilage-specific target genes (COL2A1 and ACAN). However, mechanical load enhances chondrogenesis in the presence of additional chondrogenic factors, such as dexamethasone, in a dose-dependent manner. This indicates that additional chondrogenic factors were required for chondrogenesis under in vitro conditions [25]. At present, the role of Sox9 in directing osteogenesis remains largely unknown. Since Sox9 is activated through BMP signaling pathway [26], we determined whether Sox9 can be induced by BMP9 and stretch treatments in our study. Our results indicate that Sox9 can be regulated by both chemical factors and physical cues. This is consistent with a report that Sox9 expression is increased in human inner meniscus cells which were treated by stretch for four hours. Sox9 expression is even more increased in these cells after stretch treatment for eight hours, indicating that stretch induces Sox9 expression in a time-dependent manner [27]. Although Sox9 expression can be regulated by both stretch and BMP9 at the early stage of stimulation, no further appreciable chondrogenesis is observed with the approaches used in our study. The reason is that stretch and BMP9 induce the differentiation of C3H10T1/2 MSCs into osteoblast lineage rather than other lineages. With respect to matrix mineralization, co-stimulation with BMP9 and mechanical stretch resulted in maximum Alizarin Red S staining in C3H10T1/2 MSCs, whereas mechanical stretch alone did not induce any detectable mineralization.
BMP ligands bind to type I and type II BMP receptors to activate BMP signaling pathways. After binding with BMP ligand, homomeric type II receptors form a tetrameric complex with homomeric type I receptors, which transphosphorylases homomeric type I receptor to trigger signal transduced through either Smads or MAPKs. The activation of Smads further activates the transcription of specific target genes involved in skeletal development and bone formation. In the BMP signaling pathway, Runx2 is a key transcriptional factor. The coordinated activity of Runx2 and activated Smads is critical for BMP9-mediated osteogenic differentiation and bone formation [26]. The way how mechanical stretch synergizes with BMP9 to induce osteogenic differentiation of MSCs remains further investigation. One possible mechanism is that the interaction between stretch and BMP9 increases the expression of Runx2, which has been found to regulate downstream targets of BMP signaling pathway.
In conclusion, the present study demonstrates that C3H10T1/2 MSCs are mechanosensitive. The tension-induced cytoskeleton reorganization in C3H10T1/2 MSCs inhibited cell proliferation by preventing DNA entry into S phase of the cell cycle. Mechanical stretch synergizes with BMP9 to induce the expression of key lineage-specific regulators (e.g., OCN, Sox9, and Runx2) and enhance matrix mineralization in MSCs. Stretch alone appears to be insufficient for inducing appreciable osteogenesis in MSCs without BMP9 stimulation, whereas BMP9 treatment is a more potent stimulus than stretch to initiate MSC differentiation. In addition, stretch can be effective at enhancing BMP9 induced MSC osteogenic differentiation. Our study expands the methods used to promote osteogenesis of MSCs and provides an opportunity for cell-based therapies in bone regenerative medicine.
References
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Lee JH, Park HK, Kim KS (2015) Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 473(3):752–757
Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Wei S, Zhang H, Kim SH (2013) BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng 6(8A):32–52
Varga AC, Wrana JL (2005) The disparate role of BMP in stem cell biology. Oncogene 24(37):5713–5721
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P (2003) Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg (Am Vol) 85-A(8):79–80
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18(4):545–559
Sun L, Qu L, Zhu R, Li H, Xue Y, Liu X, Fan J, Fan H (2016) Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cells Int 2016:1):1–1)10
Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2(5):1236–1247
Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, Gao JL, Kim SH, Liu X, Li M (2012) Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res 27(7):1566–1575
Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, Zhang BQ, Wagner ER, Rastegar F, Kim SH (2010) Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One 5(7):58–72
Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER (2010) Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res 25(11):2447–2459
Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, Kim SH, Chen X, Gao JL, Zhang H (2013) BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci 126(2):532–541
Iura A, Mcnerny EG, Zhang Y, Kamiya N, Tantillo M, Lynch M, Kohn DH, Mishina Y (2015) Mechanical loading synergistically increases trabecular bone volume and improves mechanical properties in the mouse when BMP signaling is specifically ablated in osteoblasts. PLoS One 10(10):e0141345
Xiao E, Yang HQ, Gan YH, Duan DH, He LH, Guo YB, Wang SQ, Zhang Y (2014) TRPM7 senses mechanical stimulation inducing Osteogenesis in human bone marrow Mesenchymal stem cells. Stem Cells 33(2):615–621
Xiao E, Chen C, Zhang Y (2016) The mechanosensor of mesenchymal stem cells: mechanosensitive channel or cytoskeleton? Stem Cell Res Ther 7(1):140
Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP (2011) Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin a. Nature 478(7368):260–263
Konstantinidis G, Moustakas A, Stournaras C (2011) Regulation of myosin light chain function by BMP signaling controls actin cytoskeleton remodeling. Cell Physiol Biochem 28(5):1031–1044
Das RK, Gocheva V, Hammink R, Zouani OF, Rowan A (2015) Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater 15(3):318–325
Wu Y, Zhang P, Dai Q, Yang X, Fu R, Jiang L, Fang B (2013) Effect of mechanical stretch on the proliferation and differentiation of BMSCs from ovariectomized rats. Mol Cell Biochem 382(1):273–282
Song G, Yang J, Shen X, Luo Q, Shi Y, Qin J (2007) Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces 58(2):271–277
Sun L, Ling Q, Rui Z, Li H, Xue Y, Liu X, Fan J, Fan H (2016) Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cells Int 2016:1):1–1)10
Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y (2009) Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods 15(2):169–180
Shenaq DS, Teven CM, Seitz IA, Rastegar F, Greives MR, He TC, Reid RR (2015) Characterization of reversibly immortalized calvarial mesenchymal progenitor cells. J Craniofac Surg 26(4):1207–1213
Gu K, Zhang L, Jin T, Rutherford RB (2004) Identification of potential modifiers of Runx2/Cbfa1 activity in C2C12 cells in response to bone morphogenetic protein-7. Cells Tissues Organs 176(1–3):28
Kupcsik L, Stoddart MJ, Li Z, Benneker LM, Alini M (2010) Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng A 16(6):1845–1855
Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH (2013) BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng 6(8A):32–52
Kanazawa T, Furumatsu T, Hachioji M, Oohashi T, Ninomiya Y, Ozaki T (2012) Mechanical stretch enhances COL2A1 expression on chromatin by inducing SOX9 nuclear translocalization in inner meniscus cells. J Orthop Res 30(3):468–474
Funding
This work was supported by the National Natural Science Foundation of China (81301551), the Chongqing Research Program of Basic Research and Frontier Technology (cstc2013jcyjA10022), the Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), the Ministry of Education (CQKLBST-2012-004), the Scientific and Technological Research Program of Chongqing Municipal Education Commission(KJ1702024), the Scientific and Technological Research Program of Chongqing Yubei district (2017 nongshe 42), and the Program for Innovation Team Building at Institutions of Higher Education in Chongqing in 2016 (CXTDG201602006). Mingxing Lei is supported by projects funded by China Postdoctoral Science Foundation (2016M590866) and Special Funding for Postdoctoral Research Projects in Chongqing (Xm2015093).
Author information
Authors and Affiliations
Contributions
Yang Song and Yinhong Tang contributed equally to this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Song, Y., Tang, Y., Song, J. et al. Cyclic mechanical stretch enhances BMP9-induced osteogenic differentiation of mesenchymal stem cells. International Orthopaedics (SICOT) 42, 947–955 (2018). https://doi.org/10.1007/s00264-018-3796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-3796-z