Abstract
Purpose
We present a minimally invasive tissue-sparing posterior superior (TSPS) approach that intends to protect the abductor muscles during total hip arthroplasty, prevents the release of the short rotator muscles, and provides the surgeon with the option to repair the posterior capsule. We hypothesized that the TSPS technique would produce a better clinical outcome, faster recovery, and lower complication rates, and that it would not jeopardize acetabular component position.
Methods
A retrospective, observational study was conducted in a consecutive series of patients. A cohort of 130 patients (130 hips) operated with a standard posterolateral approach were compared with a cohort of 132 patients (132 hips) operated with a TSPS approach. Patients were assessed with the Harris hip score (HHS) and Western Ontario and McMaster Universities index (WOMAC), which were carried out preoperatively, one month (HHS only), three months, one year, and at four years post-operatively.
Results
Compared with the standard group, patients in the TSPS group showed a faster return to ambulation as reflected in better post-operative HHS and WOMAC scores up until one year (p < 0.05). No significant differences in HHS (p = 0.564) and WOMAC (p = 0.796) scores were found at the four-year follow-up. No major adverse events were observed in either group.
Conclusion
The TSPS approach yielded better early clinical outcomes and appears to be a safe and reliable technique. However, these early differences do not appear to be sustained over time, as comparable mid-term clinical outcomes with similar complications rates were observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple minimally-invasive surgical (MIS) approaches have been described in an effort to improve short-term results of total hip arthroplasty (THA). Despite the popularity of such approaches, many MIS options present with specific associated shortcomings or complications [1].
The direct anterior approach (DAA) has been advocated as a muscle-sparing approach. However, due to sparse exposure of the proximal femur and abductor muscles, DAA has been associated with a high incidence of intra-operative complications, such as component malposition, femoral fractures, lateral femoral cutaneous nerve injury, and high rates of heterotopic ossification [1,2,3]. The learning curve to master the approach may require up to 100 cases [2], and a complication rate of 31% has been reported in the initial period [4].
A modification of the standard posterior approach, superior capsulotomy, has been suggested by Murphy et al. [5] as an alternative to DAA. Initial good results were reported by the surgeon who developed the approach [5]. However, the investigators used surgical navigation to ensure optimal component placement, which we consider a practical hurdle.
The first author of this paper sought to develop a technique that was able to overcome the shortcomings of the superior capsulotomy. Such a minimally invasive technique should be able to preserve the majority of soft tissues around the hip, allow for implant insertion without skin contact, protect the abductor muscles during the surgical procedure, prevent the release of the short external rotator muscles, and have the ability to repair the posterior capsule. Furthermore, the technique should allow a trial reduction and allow for a direct visualization during axial femoral broaching, and must provide a good visualization of the surgical field. Moreover, the approach should be easily extendable to a conventional posterolateral exposure if needed. The tissue-sparing posterior superior (TSPS) approach was developed in 2009 and successively introduced in our clinic. The technique can be interpreted as a proximal Moore approach without the detachment of the external rotator muscles. As detailed in the next section, the TSPS approach is initiated through an incision in the superior capsule. The femoral and acetabular components are inserted anterior to the posterior capsule and short rotators and just posterior to the gluteus medius.
We hypothesized that TSPS would produce better clinical outcome, faster recovery, and lower complication rates compared with the standard posterolateral approach.
Materials and methods
We conducted a retrospective, observational, single-center study in a consecutive series of patients based on prospectively collected data. In this study, 262 consecutive hips that underwent unilateral total hip arthroplasty by a single surgeon were retrospectively evaluated. The recruitment period was from March 2009 to December 2010. The first group of 130 consecutive hips (130 patients) were operated with a standard approach (“standard group”). The second group of 132 hips (132 patients) were operated on with a superior capsulotomy approach, i.e., the TSPS approach (“TSPS group”). Exclusion criteria for both groups were diagnosis of rheumatoid arthritis, body mass index greater than 35 m/kg2, and the need for revision THA.
Operative technique
The operative technique for the control group was a standard posterior approach without reconstruction of the capsule. The study group patients underwent minimally invasive surgery employing a TSPS approach, and the control group underwent a standard posterolateral approach. The first author of this paper performed all procedures in both groups.
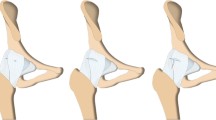
With the patient placed in a lateral decubitus position with the lower limb in maximum internal rotation and adduction (Fig. 1), a 6 cm-to −8 cm incision is made starting at the tip of the trochanter, parallel to the fibers of the gluteus maximus (Fig. 2). These fibers are then split, with care taken not to cut them. The gluteus medius muscle is located and divaricated. Next, the piriformis tendon is located and detached during retraction of the internal rotation to prevent sciatic nerve neuropraxia. Similarly, the gluteus minimus muscle is divaricated and detached from anterior superior capsule adhesions. A longitudinal capsulotomy with anterior flap of the superior capsule is completed, only exposing the cephalic end and carefully avoiding dislocation of the head, which can lead to possible pelvic trochanter muscle rupture.
Next, an intra-articular neck resection is performed. The osteotomy is completed in an oblique direction, beginning at the base of the greater trochanter (trochanteric fossa) and ending 1.5 cm above the apex of the lesser trochanter. A Schanz screw is used to excise the femoral head and is inserted in the most cranial direction possible while maintaining a cranial caudal direction toward the neck (Fig. 3). Next, with the leg in abduction to relax the gluteus muscles, the Schanz screw is turned in the cranial caudal direction to extract the femoral head (Fig. 4). The previously incised rear capsule is then fixed with stitches in order to protect the sciatic nerve, with subsequent suturing following the surgical intervention. A dual-offset reamer handle is used for acetabular preparation. We aim for 45 degrees of inclination for ceramic-on-highly crosslinked polyethylene bearings, and 40 degrees for ceramic-on-ceramic bearings. The final implant is then positioned employing a dual-offset impactor (Fig. 5). After positioning and impaction of the acetabular cup, the femur is prepared with the upper leg in maximum adduction and internal rotation (Fig. 6). Double-offset rasp handles are generally not required to rasp the femur (Fig. 7). A straight-handled impactor is used on the definitive femoral stem, and the femoral preparation is completed in the femoral axis to reduce the risk of intra-operative fractures. After implantation of the femoral component, the superior articular capsule is reconstructed and a tenorrhaphy of the piriformis tendon is performed.
A variety of cementless implants were used. In the standard group, Profemur Z and Profemur L femoral stems were combined with the Lineage acetabular cup (Wright Medical, Inc., Memphis, TN, USA). Ceramic-on-highly crosslinked polyethylene bearings were used in all cases. With the introduction of the minimally invasive techniques, we chose to use more bone-sparing implants. In the MIS group, Hydra (Adler Ortho S.R.L., Cormano, Italy), Taperloc Microplasty (Zimmer Biomet Inc., Warsaw, IN, USA), and Fitmore (Zimmer Biomet) femoral stems were used. These stems were combined with Fixa Ti-Por (Adler), Ringloc (Zimmer Biomet), and Trilogy (Zimmer Biomet) acetabular cups, respectively. The introduction of the TSPS approach coincided with the introduction of ceramic-on-ceramic bearings, which were used in all cases in the MIS group.
Post-operatively, both groups were allowed to progress to full weight-bearing and to full hip motion as tolerated without restriction.
All patients provided informed consent. In accordance with Italian law, ethics committee approval was not obtained, as the study was purely observational, with no changes to standard clinical care.
Study outcomes
Intra-operative blood loss was estimated by adding scavenged blood volume and changes in sponge weights (assuming a density of 1 g/mL). Transfusion criteria were a post-operative haemoglobin level of <8 g/dL or the patient presenting symptoms of tissue hypoperfusion with values higher than 8. For all patients, the haemoglobin (Hb) level was determined by blood draws pre-operatively and exactly 48 hours post-operatively. The decrease in Hb was calculated by subtracting the post-operative from the preoperative Hb level. Any instances of transfusions were noted with volumes recorded.
Both groups were clinically evaluated with the Harris Hip Score (HHS) [6] and Western Ontario and McMaster Universities Index (WOMAC) [7], which were carried out pre-operatively, and one month (HHS only), three months, one year, and at four years post-operatively. Complications were recorded until final follow-up. All patients provided informed consent. In accordance with Italian law, ethics committee approval was not obtained, as the study was purely observational, with no changes to standard clinical practice.
Statistical analysis
All outcome variables were analyzed by an independent statistician, using Stata/SE 12 (StataCorp, College Station, Texas). The mean ± standard deviation (SD) were calculated for continuous baseline variables, while numbers and percentages were calculated for group variables. Nominal variables were tested with the chi-squared test. Treatment comparisons for the continuous outcome variables were based on a mixed linear model with the pre-operative level of a variable used as a part of the outcome vector. The model included time as a linear spline. Separate intercepts and time terms were estimated for each technique. Random effects were included for each technique and the time term. Linear contrasts of fitted model estimates were constructed, and the Wald test was used to evaluate differences in clinical outcome for each single time point as well as for the overall difference in outcome patterns over time. The significance level was set at α = 0.05.
Results
As shown in Table 1, demographic data were comparable between the two groups with regard to sex, age, weight, and BMI. Mean (± SD) incision length was 10.7 ± 1.6 cm in the standard and 7.1 ± 0.9 cm in the MIS group (p < 0.001). Mean surgical time was 59.0 ± 11.1 min and 48.6 ± 10.6 min for the standard and TSPS approach, respectively (p < 0.001). Mean intra-operative blood loss was 380 ± 70 ml in the standard and 227 ± 34 ml in the MIS group (p < 0.001).
Significant differences in peri-operative Hb drop were found. Compared with the pre-operative level, a mean drop in Hb of 2.6 ± 1.2 g/dl for the standard group and 1.3 ± 0.7 g/dl for the MIS group was observed 48 hours post-operatively (p < 0.001). Post-operative transfusion was required in 39 patients in the standard group. None of the patients in the TSPS group required transfusion (p < 0.001). No patient was lost to follow-up, and none of the patients died during the course of the study. No serious adverse events directly related to the procedures were observed. Post-operatively, two cases of delayed wound healing were seen in the standard group. In the MIS group, one intra-operative calcar fracture was observed. The TSPS approach was extended to a standard approach, and cerclages were used to secure the fragmented bone. The patient then recovered uneventfully, and subsequently remained in the originally assigned treatment group.
Compared with the standard group, patients in the TSPS group showed faster return to normal activity. Average mean days of return to normal activities were 21 ± 2.7 (range, 18–25) days and 38 ± 4.9 (range, 30–45) days, respectively (p < 0.001).
A faster return to ambulation was also reflected in improved post-operative Harris hip (Fig. 8) and WOMAC scores at the short-term follow-up endpoints (up until 1 year post-operatively) for the TSPS group compared with the standard group. No significant differences in HHS and WOMAC scores were found at the 4-year follow-up (Table 2). Trajectory differences for HHS and WOMAC were statistically significant (p < 0.001).
Patients in both groups had accurate acetabular component positioning (Fig. 9). The mean cup adduction was 45 ± 3.3 degrees for the standard group and 40 ± 2.9 degrees in the MIS group, respectively(p < 0.001).
Discussion
Conventional total hip arthroplasty is a cost-effective procedure and generally yields a good long-term outcome. Still, the orthopaedic community has been searching for additional tissue-sparing approaches to minimize iatrogenic damage of muscle and soft tissue, enable faster rehabilitation, and reduce blood loss [8].
Although several less invasive approaches have been offered, variable outcomes and a high incidence of peri-operative and post-operative complications associated with minimally invasive approaches are not uncommon [1, 3].
To improve upon these techniques, the total hip arthroplasty through a superior capsulotomy without the use of surgical navigation is proposed. In this safety and effectiveness study of the TSPS approach in 132 hips, we presented the initial clinical results of this method and compared its functional outcome with the traditional technique. We believe that muscle preservation in the MIS TSPS translates into faster rehabilitation, which in turn may lead to a quicker return to normal activities of daily living. The present study found better clinical outcome scores and a shorter recovery time up until one year post-operatively. However, the differences in clinical outcomes seem to level over time, as no differences were found at the four year follow-up. The incidence of complications was low in both groups.
Another finding in this study was that the TSPS approach was associated with substantially less blood loss than the standard approach during THA. Blood loss can be a major cause of morbidity, leading to transfusion, pain, cardiac complications, slower rehabilitation due to haematoma formation, wound breakdown, and increased risk of infection [9]. Transfusions are associated with adverse events such as transmission of infectious diseases, immune sensitisation, and cardiovascular complications [10, 11].
TSPS appears to be a safe and effective approach for all primary elective hip replacement cases and can be used with a variety of implants, including those designed for tissue-sparing procedures or conventional canal-filling implants.
Acceptable acetabular component abduction angles with low standard deviations were seen in both TSPS and standard procedure study groups, which indicates that TSPS does not compromise the accuracy of component positioning.
While the placement of correctly positioned prosthetics without intra-operative femoral fracture in TSPS is similar to the supercapsular, percutaneously-assisted total hip (SuperPATH) technique [2, 12,13,14,15], TSPS has the advantages that fluoroscopic control during reaming of the femur is not necessary and the acetabulum can be reamed directly, rendering percutaneous assistance obsolete.
Furthermore, even though there were no post-operative mobility restrictions in the immediate post-operative period, there were no dislocations. Since reaming and broaching the femur occurs in situ without dislocation and before performing a femoral neck osteotomy, TSPS provides accurate measurement and produces accurate replication of the patient’s natural femoral offset and anteversion. In the first author’s experience, the learning curve of this approach involves ca. 50 cases; for those who come from the classical posterolateral approach, the learning curve drops to ca. 25–30 cases.
Strengths of the present study are that it is based on a consecutive series of patients and the absence of any attrition during the course of the study. A major limitation is that in our busy hospital setting a randomized controlled study was not feasible. We therefore employed a retrospective, observational study design with a multivariable data analysis to account for baseline differences in patient characteristics. Cohort studies are inherently vulnerable to selection bias. However, the investigators employed the same eligibility criteria for both study cohorts, which minimizes the risk of selection bias.
The different implant designs and bearing combinations used in the two study groups, and the differences in acetabular cup position (45° and 40° in the standard and MIS group, respectively) may have confounded our findings. The change in aimed inclination angle was employed based on the manufacturer’s recommendations. A final study limitation is that being reliant on clinical data from one single center implies that the conclusions may not be applicable to other institutions, where other surgical and rehabilitation protocols may be used. The relatively small sample size of the study also means that drawing robust conclusions about differences in clinical outcomes is difficult.
We conclude that the minimally invasive TSPS approach yielded similar low complication rates as the standard posterolateral approach, but exhibited significantly better early postoperative clinical outcomes and faster postoperative recovery. However, these early differences were not sustained over time, and comparable mid-term clinical outcomes were observed.
The TSPS approach appears to be a safe and reliable technique. Further studies will be required to substantiate our findings.
References
De Geest T, Fennema P, Lenaerts G, De Loore G (2015) Adverse effects associated with the direct anterior approach for total hip arthroplasty: a Bayesian meta-analysis. Arch Orthop Trauma Surg 135:1183–1192. https://doi.org/10.1007/s00402-015-2258-y
Penenberg BL, Campbell J, Woehnl A (2015) The mini anterior approach: optimizes total hip arthroplasty outcomes—opposes. Semin Arthoplasty 26:140–145
Macheras GA, Christofilopoulos P, Lepetsos P, Leonidou AO, Anastasopoulos PP, Galanakos SP (2016) Nerve injuries in total hip arthroplasty with a mini invasive anterior approach. Hip Int 26:338–343. https://doi.org/10.5301/hipint.5000352
Woolson ST, Pouliot MA, Huddleston JI (2009) Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplast 24:999–1005. https://doi.org/10.1016/j.arth.2009.04.001
Murphy SB, Ecker TM, Tannast M (2006) THA performed using conventional and navigated tissue-preserving techniques. Clin Orthop Relat Res 453:160–167
Harris WH (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg 51-A:737–755
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Gebel P, Oszwald M, Ishaque B, Ahmed G, Blessing R, Thorey F, Ottersbach A (2012) Process optimized minimally invasive total hip replacement. Orthop Rev (Pavia) 4:e3. https://doi.org/10.4081/or.2012.e3
Hochreiter J, Hejkrlik W, Emmanuel K, Hitzl W, Ortmaier R (2016) Blood loss and transfusion rate in short stem hip arthroplasty. A comparative study. Int Orthop. https://doi.org/10.1007/s00264-016-3365-2
Hourlier H, Reina N, Fennema P (2015) Single dose intravenous tranexamic acid as effective as continuous infusion in primary total knee arthroplasty: a randomised clinical trial. Arch Orthop Trauma Surg 135:465–471. https://doi.org/10.1007/s00402-015-2168-z
Gofton W, Chow J, Olsen KD, Fitch DA (2015) Thirty-day readmission rate and discharge status following total hip arthroplasty using the supercapsular percutaneously-assisted total hip surgical technique. Int Orthop 39:847–851. https://doi.org/10.1007/s00264-014-2587-4
Bodrogi AW, Sciortino R, Fitch DA, Gofton W (2016) Use of the supercapsular percutaneously assisted total hip approach for femoral neck fractures: surgical technique and case series. J Orthop Surg Res 11:113. https://doi.org/10.1186/s13018-016-0446-2
Cronin MD, Gofton W, Erwin L, Fitch DA, Chow J (2015) Early surgical and functional outcomes comparison of the supercapsular percutaneously-assisted total hip and traditional posterior surgical techniques for total hip arthroplasty: protocol for a randomized, controlled study. Ann Transl Med 3:335. https://doi.org/10.3978/j.issn.2305-5839.2015.12.15
Della Torre PK, Fitch DA, Chow JC (2015) Supercapsular percutaneously-assisted total hip arthroplasty: radiographic outcomes and surgical technique. Ann Transl Med 3:180. https://doi.org/10.3978/j.issn.2305-5839.2015.08.04
Gofton W, Fitch DA (2016) In-hospital cost comparison between the standard lateral and supercapsular percutaneously-assisted total hip surgical techniques for total hip replacement. Int Orthop 40:481–485. https://doi.org/10.1007/s00264-015-2878-4
Funding
This work was supported by Adler Ortho S.R.L., Cormano, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Capuano, N., Grillo, G., Carbone, F. et al. Total hip arthroplasty performed with a tissue-preserving technique using superior capsulotomy. International Orthopaedics (SICOT) 42, 281–287 (2018). https://doi.org/10.1007/s00264-017-3722-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3722-9