Abstract
Purpose
In order to test the validity of the selected surgical technique as a way to manage persistent synovitis of the knee joint, as well as to slow down the cartilage and bone destruction, we studied the dynamics of biomarkers of inflammatory conditions, and bone and cartilage destruction after total arthroscopic synovectomy (TAS) of the knee joint.
Methods
The sampling comprised 124 RA patients (158 knees) who had undergone the TAS procedure between 2003 and 2015. Before surgery the rheumatoid factor (IgM), C-reactive protein (CRP), erythrocyte sedimentation rate test was completed for all patients. Blood serum samples were collected (prior to surgery, and three, six and 12 months after surgery) and frozen at −70°С. The content of CRP, Matrix metalloproteinase-3 (MMP-3), Cartilage Oligomeric Matrix Protein, as well as cross-linked Ctelopeptides of types I and II were measured in blood serum by means of polarization fluoroimmunoassay with the use of a standard set of reagents.
Results
The average duration of the disease in the studied group was 8.7 ± 6.6 years. Concentration of the inflammation markers showed that only MMP-3 displayed statistical significance.
Conclusions
The obtained results can be used as basis in assessing the efficiency and effectiveness of this method of treatment of persistent knee joint synovitis associated with the RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical patterns and outcomes of the rheumatoid arthritis (RA) are in many ways determined by the inflammatory conditions in the synovium, which induce intraarticular lesion. Therefore, one of the main objectives of RA treatment is to prevent or slow down this destructive process. The modern rheumatology offers a wide range of capabilities; however, synovitis may take forms that are non-responsive even to the most advanced and aggressive methods of therapy. In such cases, the surgical removal of the synovial tissue (synovectomy) is the method of choice. Several parameters, including laboratory test values, need to be assessed for a surgical intervention to be effective.
In order to test the validity of the selected surgical technique as a way to manage persistent synovitis of the knee joint, reduce RA activity, and improve the quality-of-life (QOL) and joint function, as well as to slow down the cartilage and bone destruction, we studied the dynamics of biomarkers of inflammatory conditions, and bone and cartilage destruction after total arthroscopic synovectomy (TAS) of the knee joint.
Materials and methods
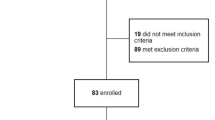
The sampling comprised 124 RA patients who had undergone TAS procedures on 158 knees; all the procedures had been performed in the period between 2003 and 2015. All the patients were referred for surgery based on recommendations of their rheumatologists and orthopaedic surgeons. Initially, the study covered 172 patients. Of them 48 (40 operated on in the period between 2003 and 2009, and 8 in the period between 2009 and 2011) were later excluded from the study due to unavailability of complete data.
The study inclusion criteria were as follows: definite RA diagnosis based on American College of Rheumatology (ACR) criteria [2]; unilateral or bilateral knee joint synovitis persisting for three months or longer during taking disease-modifying anti-rheumatic drugs (DMARD), biologic drugs, as well as oral and intraarticular glucocorticoid (GC) therapy; severe pain syndrome (50+ mm on the subjective visual analogue scale (VAS) for pain).
The study exclusion criteria were as follows: presence of other systemic inflammatory diseases, except RA, or erroneous RA diagnosis; unavailability of complete data; presence in the knee joint of gross destructive changes requiring total knee replacement; presence of systemic counter-indications.
The radiographic RA staging was based on the Steinbroker classification. The functional class was defined based on ACR criteria. To measure the RA activity prior to surgery (measured by examiner), the combined disease activity score (DAS28) was used. In prior therapy assessment, the average dose and duration of DMARD therapy, as well as oral GC therapy, were considered. The activity of disease (measured by patient) and functional status were also measured based on RAPID-3 criteria.
The clinical assessment of the severity of knee synovitis was performed with the use of a 4-point scale [11]: 0 points – no swelling; 1 point – moderate; 2 points – severe; 3 points – highly severe.
The signs of synovitis and number of erosions were assessed according to the OMERACT (the Outcome Measures in Rheumatology Clinical Trials) criteria based on MRI and ultrasonic data. The MRI scanning (GE Signa Ovation, Т1, Т2, STIR) and b-mode sonography (GE Voluson-i, 4–13-MHz linear probe) were performed over time (before surgery and 3, 6, and 12 months after surgery) to verify synovitis, measure the thickness of the synovium, and estimate the rate of the erosive process or formation of aseptic necrosis areas.
For morphological examination of the severity of synovitis, synovium biopsy samples were taken intra-operatively from the superior recess area, the inner (medial) compartment of the joint, and the area of contact between the synovia and the cartilage. The histologic specimens were hematoxylin-eosin stained, Van-Gieson-stained to assess the state of the connective tissue, and Mallory-stained to confirm the presence and maturity of fibrin. The severity of the inflammation was measured with the use of a 4-point scale, where: degree 0 – no inflammation; degree 1 – mild diffuse inflammation mostly in the undersurface of the synovial tissue (immediately under the synovial covering); degree 2 – moderate diffuse-local inflammation; degree 3 – severe superficial or deep diffuse or diffuse-local inflammation of the sub-synovial tissue [13, 14]. Should the severity of synovitis vary from one area within a joint to another, the higher-severity area would be used as reference.
The condition of the cartilage was assessed in the process of diagnostic arthroscopy. Depending on the nature of injury, it was scored from 0 to 4 based on the Outerbridge criteria [18].
Prior to the surgery and 12 months after it, biplane radiography (frontal and lateral view) of the knee joint was performed. All the radiograms were scored based on the Larsen method [16].
Special attention was paid to the duration and time periods of knee joint involvement in the process, time length and severity of persistent knee synovitis, as well as the number and effectiveness of intraarticular GC injections.
Before surgery, on top of standard blood tests, the rheumatoid factor (IgM), high sensitive C-reactive protein (hs-CRP), erythrocyte sedimentation rate, Matrix metalloproteinase-3 (MMP-3), Cartilage Oligomeric Matrix Protein (COMP), cross-linked C-telopeptides of type I (CTX-I) and type II (CTX-II) tests were completed for all patients. Moreover, blood serum samples were collected (prior to surgery, and 3, 6 and 12 months after surgery) and frozen at −70°С.
The concentration of hs-CRP in the blood serum was measured by means of highly sensitive latex-enhanced immunonephelometry with the use of a BN-ProSpec analyzer (Siemens, Germany) and CardioPhase hsCRP reagents (Siemens, Germany). The upper limit of normal was estimated at 5.0 mg/l, as per recommendations of the vendor.
The content of MMP-3 in the blood serum was measured by means of enzyme-linked immunoassay with the use of Bender MedSystems reagents (Austria) in accordance with the vendor’s recommendations. The sensitivity of MMP-3 measurement was 0.4 ng/ml. The upper limit of the norm was set at 9.3 ng/ml, i.e. the concentration level corresponding to the value of the 95th percentile in donors.
Similarly, the level of COMP in blood serum was measured by means of polarization fluoroimmunoassay with the use of a standard set of reagents (Human Cartilage Oligomeric Matrix Protein ELISA, BioVendor, Germany) according to the vendor’s guidelines.
The level of CTX-I and CTX-II was also measured in blood serum by means of polarization fluoroimmunoassay with the use of a standard set of reagents (CrossLapsTM test system from Nordic Bioscience Diagnostics A/S, Denmark).
The results were compared against reference values (for hs-CRP and MMP-3), as well as control group data (40 healthy controls).
For the statistical analysis of data, we used Statistica 8.0 for Windows (StatSoft Inc., USA). Study record data were entered manually, after which the completeness of data, acceptable ranges of values, and logical and clinical interrelationships were checked visually and with the use of software tools. The quantitative variables were described by means of standard variance analysis methods with calculation of the mean value (М), standard deviation (σ), 25th and 75th percentile, median value, and number of patients. The mean values were presented as М ± σ. The qualitative variables were described in absolute and relative frequency (percentage) terms. Differences were considered statistically significant at error levels p < 0.05. To calculate the probabilities that are subject to the normal distribution law, parametric statistics methods were employed (Student’s t-test). The predictive value of markers as a tool to predict recurrence of knee joint synovitis was tested with the use of receiver operating characteristic (ROC) analysis.
Results
The average duration of the disease in the studied group was 8.7 ± 6.6 years (from 0.5 to 35 years). First complaints of pain in the affected knee came 5.2 ± 4.5 years prior to surgery, while first signs of the synovitis of the knee joint were identified 4.5 ± 4.7 years prior to surgery. Persistent synovitis not responding to the DMARD or GC therapy was present in patients for two+ years prior to surgery. The DMARD therapy was received by 82.3% of patients (methotrexate – 32.3%, leflunimide −32.3%, sulfasalazine – 17.7%). In 6.5% of patients the methotrexate therapy was combined with biologic drugs (Infliximab, an anti-TNF-alpha-agent); 17.7% of patients did not undergo any DMARD therapy. Of the 158 knees in the study, synovectomy was a repeated surgery in 12 cases on the same knee previously operated on arthroscopically at another orthopaedic department for reasons not related to RA (meniscal tears, ligament tears, etc.).
Most of the patients covered by the study had a history of DMARD therapy, although, irregular. Moreover, more than one half of them had undergone oral GC therapy due to the aggressive nature of their disease. Intra-articular GC injections were received by all patients. In cases when synovitis did not respond to treatment, repeated, and at times multiple intraarticular GC injections, were required. The insufficiently sustainable effect of the DMARD therapy, which could be explained by insufficient doses or drug intolerance, along with recurrent arthritis called for adjustment of therapy, increased GC dosing, and in some cases biologic therapy to reduce disease activity and control the signs of synovitis. Should the signs of synovitis persist, synovectomy as a more curative treatment method would be called for to arrest the joint inflammation, and prevent further cartilage destruction, as well as malfunction and deformation of the knee joint (Table 1).
Dynamics of the biomarkers of inflammation, bone and cartilage destruction
Prior to surgery, all the markers exceeded the norm, which reflected both high inflammatory activity and progressing intraarticular and bone destruction. The fastest to respond to the surgery were the biomarkers of destruction. Their concentration decreased after three months, and by the end of the study it was statistically much lower than before surgery, except for CTX II—its concentration change was not statistically significant. The concentration of the inflammation markers only displayed a statistically significant decline six months after surgery; however, positive outcomes lasted up to 12 months after surgery (Table 2).
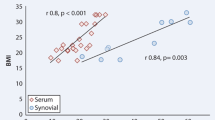
In addition to the dynamic data, we also assessed the markers of inflammation and destructions in terms of their correlation with each other, as well as disease activity indices, radiographic RA staging, degrees of chondromalacia and morphologic proliferation, and number of erosions on MRI (Table 3). The strongest interrelationship with the DAS28 and RAPID-3 indices of inflammatory activity was demonstrated by the CRP (r = 0.74 and 0.72, respectively). Besides, the CRP level correlated significantly with the radiographic RA staging according to Steinbroker and the level of CTX I (r = 0.54 and 0.43, respectively). The CRP and MMP-3 also correlated with each other, but this correlation was not statistically significant. The COMP and MMP-3 displayed a significant correlation with the degree of chondromalacia according to Outerbridge (r = 0.48 and 0.44, respectively). Similarly, a significant correlation was found between COMP and MMP-3, on the one hand, and the degree of chondromalacia according to Outerbridge and histological grade of proliferation of the synovium, on the other hand (r = 0.44 and 0.49, respectively). The number of erosions on MRI scans correlated with CTX II, MMP-3, and COMP (r = 0.58, 0.44, and 0.49, respectively). The morphologic traits of the synovium (severity of synovitis, signs of angiomatosis) demonstrated high ratios of correlation (from 0.45 to 0.75) with COMP, MMP-3, and СТХ II.
Apart from seeing the dynamics and correlations, it is necessary to understand how informative all these indicators can be from the perspective of the synovitis recurrence risk, as it is important to retain the positive outcome, rather than just prevent destruction in a short or longer run. One of the markers that appears to be useful is MMP-3. Of all the factors, this biomarker correlated with the baseline therapy. To assess the predictive value of MMP-3 as a predictor of recurrent synovitis, ROC curves were used. It was found that at values higher than 36 units with 73.3-% sensitivity and 69.2-% specificity, such a recurrence could be expected in the following year (Fig. 1).
Discussion
As the destructive changes in the cartilaginous and osseous tissue progress, the concentration of biomarkers in body fluids changes [4]. The most stable of them is the CRP, which reflects the degree of inflammation, cell-mediated response, and tissue damage [4, 11]. According to several authors, even an insignificant but steady increase in CRP concentration is associated with cartilage destruction [21]. Another marker that indicates the disease activity and intraarticular destruction is MMP-3. Houseman et al., Chu et al., and Galil et al. also showed these characteristics of MMP-3. The concentration of it was much lower in patients with no radiological signs of destruction, and higher in those patients who had intraarticular changes [6, 10, 12]. To evaluate cartilage destruction, COMP and СТХ-II are frequently used. The ability of these markers to show changes in the cartilaginous tissue has been confirmed by many researchers [8, 17, 23, 24]. Hunter et al., Chu et al., Benucci et al., Ahlen et al., and El Defrawy et al. consider COMP to be one of the markers of cartilage destruction [1, 3, 6, 8]. According to Fujikawa et al., the content of СОМР in blood plasma correlates with the degree of erosion on MRI scans [9]. Crnkic et al. [7] reported that the level of СОМР decreased in patients by the third month of Infliximab or Etanercept therapy, which confirms the protective effect of these drugs on the articular cartilage. Christqau et al. and Lohmander et al. reported that СТХ-II concentration was much lower in RA patients with no radiological signs of destruction, and higher in those patients who had intra-articular changes [5, 17]. Reijman et al. examined 1,235 patients and proved that the patients with exceedingly high СТХ-II concentration were prone to radiological progression of arthritis, while Li et al. believe that СТХ-II is one of the key markers that can indicate an articular cartilage damage at a stage when it is not possible to identify it with the use of instrumental examination data [19].
Thus, the products generated by inflamed synovium are responsible for intra-articular lesion [15, 20, 22]; therefore, its removal may not only result in a better joint function and higher QOL indicators, but also in a lower disease activity, as well as prevention or lowering down of further joint destruction.
In our study, the patients’ pre-surgery CRP, MMP-3, COMP, CTX-I, and CTX-II levels were high, which, according to literature, was an indication of intense inflammatory activity, as well as progressive intra-articular destruction. It is worth mentioning that not all the markers displayed a reduction in concentration three months after surgery. The fastest to decline was the concentration of COMP, CTX-I, and CTX-II. A significant improvement, from the perspective of concentration reduction, was only demonstrated by two of these markers—COMP and CTX-I, while CTX-II did not display further meaningful reduction. A meaningful decline in the concentration of CRP and MMP-3 only took place six months after surgery, with significant improvements being achieved 12 months post-operatively. Nevertheless, concentration levels of the markers above change much faster than instrumental examination values, and the established correlations allow using those changes as basis in making assumptions as to potential intra-articular processes.
With no sustained remission, synovitis recurrence may take place at any moment over the lifetime of an RA patient, whether he or she is treated with modern traditional antirheumatic drugs or genetically engineered biologic drugs, or even after a surgical removal of the synovium. By the time of surgery, most patients (76%) had Outerbridge Grade III-IV cartilage chondromalacia. Signs of angiomatosis and severe rheumatoid synovitis were found in bioptic samples of synovium of all our patients with disease recurrence. Although both COMP and MMP-3 correlate with these signs, only MMP-3 has a predictive value. This marker appears to have potential as a useful indicator of the risk of post-operative synovitis recurrence, however it requires further, and longer-term study.
Conclusions
The obtained results can be used as basis in assessing the efficiency and effectiveness of this method of treatment of persistent knee joint synovitis associated with the RA. Exceedingly high pre-operational values of biomarkers of inflammation, and bone and cartilage destruction reflect both high inflammatory activity and progressing intra-articular destruction. Statistically significant gradual reduction in the concentration of these markers indicating the presence of destructive and inflammatory changes confirms the suggested hypothesis. The identified correlations confirm this hypothesis even more making it possible to judge the informative value of biomarkers, which means that they can be useful in assessing the intra-articular changes, as well as early evaluation of the outcomes of surgery. Moreover, should these markers’ values grow in the post-operative period, they can give an impetus for the replacement of the current anti-rheumatic therapy for a more aggressive one.
References
Åhlén M, Roshani L, Lidén M, Struglics A, Rostgård-Christensen L, Kartus J (2015) Inflammatory cytokines and biomarkers of cartilage metabolism 8 years after anterior cruciate ligament reconstruction: results from operated and contralateral knees. Am J Sports Med 43(6):1460–1466
Arnett FC, Edworth SM, Bloch DA, et al (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315-24. https://doi.org/10.1002/art.1780310302
Benucci M, Meacci F, Manfredi M, Gobbi FL, Infantino M, Ricci C, Sarzi-Puttini P, Atzeni F (2014) Can Tocilizumab decrease cartilage Oligomeric matrix protein levels and disease activity in patients with long-standing rheumatoid arthritis? Curr Rheumatol Rev 10(2):131–135
Boers M, Brooks P, Simon LS et al (2005) OMERACT: an international initiative to improve outcome measurement in rheumatology. Clin Exp Rheumatol 23:10–13
Christgau S, Garnero P, Fledelius C, Moniz C et al (2001) Collagen type. II C-telopeptide fragments as an index of cartilage degradation Bone 29(3):209–215
Chu XQ, Wang JJ, Dou LD, Zhao G (2015) Cartilage oligomeric matrix protein and matrix metalloproteinase-3 expression in the serum and joint fluid of a reversible osteoarthritis rabbit model//. Genet Mol Res 14(4):14207–14215
Crnkic M, Månsson B, Larsson L et al (2003) Serum cartilage oligomeric matrix protein (COMP) decreases in rheumatoid arthritis patients treated with infliximab or etanercept. Arthritis Res Ther 5(4):181–185
El Defrawy AO, Gheita TA, Raslan HM, El Ansary MM, El Awar AH (2016) Serum and synovial cartilage oligomeric matrix protein levels in early and established rheumatoid arthritis. Z Rheumatol 75(9):917–923
Fujikawa K, Kawakami A, Tamai M et al (2009) High serum cartilage oligomeric matrix protein determines the subset of patients with early-stage rheumatoid arthritis with high serum C-reactive protein, matrix metalloproteinase-3, and MRI-proven bone erosion J. Rheumatol 36(6):1126–1129. https://doi.org/10.3899/jrheum.080926
Houseman M et al (2012) Baseline serum MMP-3 levels in patients with rheumatoid arthritis are still independently predictive of radiographic progression in a longitudinal observational cohort at 8 years follow up. Arthritis Res Ther 14(1):30. https://doi.org/10.1186/ar3734
Gabay C, Kushner I (1999) Acute phase proteins and other systemic responses to inflammation. Engl J Med 340:448–454
Galil SM, El-Shafey AM, Hagrass HA, Fawzy F, Sammak AE (2016) Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Int J Rheum Dis 19(4):377–384
Geiler G, Stiehl P (1974) Significance of the concepts basic activity and actual activity in the morphological evaluation of synovial membranes in rheumatoid arthritis. Z Rheumatol 33:73–86
Karateev DE, Radenska-Lopovok SG, Nasonova VA, Ivanova MM (2003) Synovial membrane in the early stage of rheumatoid arthritis: clinico-morphological comparisons. Ter Arkh 75(5):12–20
Karmakar S, Kay J, Gravallese EM (2010) Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin N Am 2:385–404
Larsen A, Dale K, Eek M (1977) Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diag 18(4):481–491
Lohmander LS, Atley LM, Pietka TA, Eyre DR (2003) The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum 11:3130–3139
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43:752–757
Reijman M, Hazes JM, Bierma-Zeinstra SM et al (2004) A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum 8:2471–2478
Smolen S, Aletaha D, Steiner G (2009) Does damage cause inflammation? Revisiting the link between joint damage and inflammation. Ann Rheum Dis 68:159–162
Spector TD, Hart DJ, Nandra D et al (1997) Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressivedisease. Arthr Rheum 40:723–727
Taylor PC (2002) VEGF and imaging of vessels in rheumatoid arthritis. Arthritis Res 4:99–107
Tseng S, Reddi AH, Di Cesare PE (2009) Cartilage Oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights 4:33–44
Turesson C, Bergstrom U, Jacobsson LTH et al (2011) Increased cartilage turnover and circulating autoantibodies in different subsets before the clinical onset of rheumatoid arthritis. Ann Rheum Dis 70:520–522
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors reported no conflicts of interest to declare in this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration ant its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Lipina, M., Makarov, M., Makarov, S. et al. The degree of cartilage degradation assessed by serum biomarker levels changes after arthroscopic knee synovectomy in rheumatoid arthritis patients. International Orthopaedics (SICOT) 41, 2259–2264 (2017). https://doi.org/10.1007/s00264-017-3634-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3634-8