Abstract
Purpose
Wear debris-induced osteolysis and aseptic loosening are the most frequent late complications of total joint arthroplasty leading to revision of the prosthesis. However, no effective measures for the prevention and treatment of particles-induced osteolysis currently exist. Here, we investigated the efficacy of local administration of osthole on tricalcium phosphate (TCP) particles-induced osteolysis in a murine calvarial model.
Methods
TCP particles were implanted over the calvaria of ICR mice, and established TCP particles-induced osteolysis model. On days one, four, seven, ten and thirteen post-surgery, osthole (10 mg/kg) or phosphate buffer saline (PBS) were subcutaneously injected into the calvaria of TCP particles-implanted or sham-operated mice. Two weeks later, blood, the periosteum and the calvaria were collected and processed for bone turnover markers, pro-inflammatory cytokine, histomorphometric and molecular analysis.
Results
Osthole (10 mg/kg) markedly prevented TCP particles-induced osteoclastogenesis and bone resorption in a mouse calvarial model. Osthole also inhibited the decrease of serum osteocalcin level and calvarial alkaline phosphatase (ALP) activity, and prevented the increase in the activity of tartrate resistant acid phosphatase (TRAP) and cathepsin K in the mouse calvaria. Furthermore, osthole obviously reduced the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) into the periosteum. Western blotting demonstrated TCP particles caused a remarkable endoplasmic reticulum (ER) stress response in the mouse calvaria, which was obviously blocked by osthole treatment.
Conclusion
These results suggest that local administration of osthole inhibits TCP particles-induced osteolysis in the mouse calvarial in vivo, which may be mediated by inhibition of the ER stress signaling pathway, and it will be developed as a new drug in the prevention and treatment of destructive diseases caused by prosthetic wear particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Joint arthroplasty is an effective treatment for severe trauma or arthritic joint diseases, as it restores joint function, diminishes pain and improves the overall quality of life for millions of people. Unfortunately, wear of these prostheses over time generates debris (such as metal, polyethylene and ceramic), which are released into the joint space and subsequently embedded into the surrounding synovial tissues [1]. These wear particles stimulate macrophages and other mesenchymal cells to produce a multitude of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, chemokines, metallo-proteinases and peptides [2–4]. These factors directly or indirectly induce osteoclasts differentiation and activation, ultimately leading to unexpected bone erosion around the prosthetic implant and possible implant failure. Thus, wear particles-induced periprosthetic osteolysis is the major cause of arthroplasty failure, requiring patients to receive an additional operation [5]. To date, the only established treatment for periprosthetic osteolysis is revision surgery, which is associated with greater morbidity and a poorer functional outcome. Therefore, it is urgent to identify drugs that can effectively inhibit wear particle-induced osteolysis and prosthetic loosening.
Osthole (7-methoxy-8-isopentenoxycoumarin) is a natural coumarin isolated from Cnidium monnieri (L.). It has received considerable attention because of its significant and diverse pharmacological activities such as antioxidation, antitumor, antidiabetes and neuroprotective effects, which make it a promising natural compound for new drug discovery and therapeutic application. Particularly, osthole has been reported to inhibit inflammation and regulate the expression of inflammatory mediators like TNF-α, IL-6 and IL-1β in HepG2 cells or in lipopolysaccharide (LPS)-stimulated 3T3-L1 adipocyte [6, 7]; and it also attenuates carrageenan-induced lung inflammation and renal ischemia-reperfusion injury by inhibiting inflammatory response in rats [8–10]. These results suggest that osthole with anti-inflammatory activity may affect wear particles induced with the release of pro-inflammatory cytokines, which enhances osteoclast differentiation and periprosthetic osteolysis. However, whether osthole can prevent TCP particles-induced inflammatory osteolysis remains unclear.
In this in vivo study, we investigated the potential inhibition of osthole on particles-induced calvarial osteolysis in mice, mainly focusing on osteoclasts number, osteolysis area, bone turnover markers and pro-inflammatory cytokines. To explore its possible molecular mechanism, western blotting was used to examine whether ER stress response is involved in the inhibition of osthole on the TCP particle-induced inflammatory osteolysis. Our results clearly suggest that osthole prevents TCP particles-induced osteoclastogenesis and osteolysis in vivo through inhibition of the ER stress signaling pathway, suggesting that osthole possesses therapeutic potential for the treatment of periprosthetic osteolysis and aseptic loosening.
Materials and methods
Chemical and reagents
The E-Toxate-Kit and the Acid Phosphatase Leukocyte TRAP kit were purchased from Sigma-Aldrich (St. Louis, USA). The mouse kits of osteocalcin, ALP, TRAP and cathepsin K were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). RIPA lysis buffer and BCA™ Protein Quantification Kit were supplied by Beyotime Institute of Biotechnology (Shanghai, China). The ELISA kits of TNF-α, IL-6 and IL-1β were from the Shanghai branch of biological science and Technology (Shanghai, China). Chemiluminescence detection system and the rabbit specific antibodies against glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP) and β-actin were purchased from Cell Signaling Technology (Waltham, USA). Osthole was provided by the National Institute for the Control of Pharmaceutical and Biological products (Beijing, China), the purity was 98 % determined by high performance liquid chromatography (HPLC). Other chemicals were AR (expect for special instructions).
Particles characterization
TCP particles were obtained from a commercial source (Ensail Beijing Corp., Beijing, China). The mean size of the particles was 1.997 μm (range 0.05–11.06 μm; Fig. 1), determined by scanning electron microscopy (SEM; Hitachi S-2400, Japan); these particles easily form aggregates of various sizes. The particles were washed in fresh 70 % ethanol solution at room temperature with shaking overnight to remove possible endotoxin. The particles’ endotoxin was tested with the E-Toxate-Kit, and endotoxin levels were below the detection limit of 0.05 U/mL.
Animal experiment
All procedures described below were performed in accordance with protocols approved by the Animal Care Research Committee of Shaoxing University. A total of 36 male ICR mice aged six to eight weeks (Zhejiang Academy of Medical Sciences, Hangzhou, China) were randomly divided into three groups: sham, TCP particles and osthole (10 mg/kg) with TCP particles.
Mouse calvarial osteolysis model
As previously described [11–13], animals were anaesthetized with pentobarbital sodium (65 mg/kg) by intra-peritoneal injection and then implanted with TCP particles in a warm environment. The hair overlying the skull was carefully shaved and sterilized by scrubbing three times topically. By means of an ophthalmological scalpel, an area of periosteum over the calvarium was exposed by making a 1-cm midline sagittal incision in front of the line connecting both external ears. Except for the sham group, TCP particles (30 mg) were implanted over the periosteum around the middle suture of calvaria in mice. On days one, four, seven, ten and thirteen post-surgery, osthole (10 mg/kg) or PBS was subcutaneously injected into the calvaria of TCP particles-implanted or sham-operated mice. The animals were allowed free access to a standard diet and water. Body weights were recorded weekly. After two weeks, blood, the periosteum and the calvaria were collected and processed for bone turnover markers, pro-inflammatory cytokine, histomorphometry and molecular analysis.
Histological assay
Three samples of calvaria per group were removed and fixed in 4 % paraformaldehyde for 48 h, followed by decalcifying in 10 % EDTA (pH 7.4) for two weeks and paraffin embedding. Sections (5 μm) were stained with H&E to visualize inflammatory response and multinucleated osteoclasts, which were counted under an IX70 microscope at the midsagittal suture with 0.25-mm intervals calculated with Image Pro-Plus 5.0 (Media Cybernetics, USA).
Another three samples per group were rinsed with PBS twice, and fixed in 2.5 % glutaraldehyde for seven minutes at room temperature, then dehydrated with a series of ethanol. After airing naturally, the bone slices were stained with the Acid Phosphatase Leukocyte TRAP kit following the manufacturer’s protocol after PBS washing processes. Bone resorption pits were observed under an IX70 light microscope (Olympus, Japan), and osteolysis area was analysis by NIH Image J.
Quantification of bone turnover markers and pro-inflammatory cytokines
Serum concentration of osteocalcin and pro-inflammatory cytokines including TNF-α, IL-6 and IL-1β in the periosteum were measured using specific ELISA kits, according to the manufacturer’s instructions. The activities of ALP, TRAP and cathepsin K in the calvaria were examined by their detection kits, respectively.
Western blotting
Three calvaria per group were rinsed three times in saline, and were cut into about 1 mm³ size. These bone fragments were grinded in liquid nitrogen, and then tardily homogenized in RIPA buffer (400 μL) containing protease and phosphatase inhibitors at 4 °C. Total protein was collected after centrifugation at 12,000 rpm/min for 15 minutes, and quantified by BCA™ Protein Quantification Kit. The protein extracts of calvaria were used for western blotting. Equal amounts of total protein were separated by 12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked in Tris-buffered saline containing 0.05 % (v/v) Tween-20 (TBST) with 5 % (w/v) bovine serum albumin (BSA) for one hour and probed overnight at 4 °C with appropriate rabbit GRP78 (1:1000 dilution), CHOP (1:1000 dilution) and β-actin (1:1000 dilution). After washing in TBST, the membrane was incubated with HRP conjugated goat anti rabbit-IgG secondary antibody (1:2500 dilution) at 37 °C for two hours. Washing in TBST again later, the protein bands were detected using a chemiluminescence detection system. Densitometric analysis was made using Image J 1.41 (National Institutes of Health, Bethesda, MD, USA).
Statistics and data presentation
All results were expressed as mean ± SD. Statistical comparisons were determined by the analysis of variance (ANOVA) among three or more groups or Bonferroni’s t-test between two groups. p-values < 0.05 were considered statistically significant.
Results
Effect of osthole on TCP particles-induced calvarial osteolysis in mice
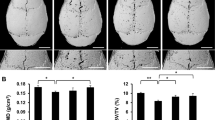
To investigate the effect of osthole on TCP particles-induced osteolysis in vivo, TCP particles were implanted, and a particles-induced calvarial osteolysis model in mice was established. During the treatment, we did not observe weight loss (Fig. 2a) and abnormal behavior in TCP particles-implanted or osthole-treated mice. TCP particles implantation caused calvarial bone loss, confirmed by an obvious decrease in calvaria weight (Fig. 2b); osthole treatment significantly increased calvaria weight, which is still lower than that of the sham-operated group (Fig. 2b).
Histological H&E assessment and histomorphometric analysis revealed TCP particles implantation resulted in a localized inflammatory response, as well as multiple osteoclasts lining the extensive erosion (Figs. 3a and 4a). By comparison, sham-operated mice show no obvious signs of osteolytic response. Importantly, local administration of osthole (10 mg/kg) significantly reduced osteoclaststogensis and bone loss caused by TCP particles (Figs. 3a and 4a); Osteoclasts number per 0.25 mm interval calvarial sections was decreased from 28.7 ± 4.7 to 13.6 ± 2.1, and osteolysis area was reduced from 0.3014 ± 0.052 mm² to 0.1732 ± 0.091 mm², respectively (Figs. 3b and 4b).
H&E staining for osteoclastogenesis in the mouse calvaria (n = 3). a All animals were sacrificed and the calvaria were collected (five per group), histological sections were processed and stained for H&E. b Average osteoclasts per 0.25 mm interval calvarial sections (three sections per calvaria) were counted, and data were expressed as mean ± SD. Red arrows show multinucleated osteoclasts. p < 0.05 compared with sham group (a); p < 0.05 compared with TCP particles group (b). Bar = 50 μm
TRAP staining for the calvarial osteolysis (n = 3). a TRAP staining of the calvaria from sham, TCP particles and osthole (10 mg/kg) groups. b Osteolysis area was calculated as described in the Materials and Methods section, and data are expressed as mean ± SD. Red arrows show bone resorption pits. p < 0.05 compared with sham group (a); p < 0.05 compared with TCP particles group (b). Bar = 50 μm
Effect of osthole on bone turnover markers
Next, we determine the effect of osthole on bone turnover markers such as osteocalcin, ALP, TRAP and cathepsin K [14, 15]. In the TCP particles-implanted mice, serum osteocalcin level and calvarial ALP activity were decreased to 8.39 % and to 73.25 % of sham-operated group, respectively. Whereas, the activity of TRAP and cathepsin K in the calvaria were increased to 3.79-fold, and to 1.85-fold compared with the sham group (Table 1). Osthole treatment significantly suppressed TCP particles-induced and the decrease of serum osteocalcin level and calvarial ALP activity, as well as the increased activity of TRAP and cathepsin K; their levels were nearly restored to that of the sham group after osthole treatment (Table 1).
Effect of osthole on the release of pro-inflammatory cytokines
TNF-α is one of the inflammatory mediators of osteolysis induced by wear particles, along with IL-1β and IL-6. These pro-inflammatory cytokines were significantly elevated in patients with total hip arthroplasty (THA) with periprosthetic osteolysis compared to control patients with THA with no radiographic signs of osteolysis. As shown in Table 2, TCP particles implantation triggered the release of TNF-α and IL-6 into the periosteum, and their levels were obviously increased to 106.23 ± 2.39 pg/mL (TNF-α) and to 303.42 ± 7.57 pg/mL (IL-6), which were much higher than that of the sham group (59.46 ± 2.18, 122.84 ± 4.34 pg/mL). Osthole treatment remarkably decreased the production of TNF-α and IL-6 in the periosteum, and the levels of TNF-α and IL-6 were decreased to 57.27 % and to 45.23 % of the TCP particles-implanted group, respectively (Table 2). However, the level of IL-1 β in the periosteum was not affected after TCP particles implantation or osthole treatment (Table 2). Taken together, osthole suppressed TCP particles-induced the production of TNF-α and IL-6, which enhances osteoclasts formation and improves osteolytic responses.
Effect of osthole on TCP particles induced an ER stress response
Recent studies showed that cytokines have been shown to impair ER homeostasis by inducing depletion of ER Ca2+ and altering chaperone function, proinsulin processing and ER-Golgi trafficking, triggering accumulation of misfolded proteins and ER stress [16, 17], that is closely associated with inflammatory osteolysis diseases [18, 19]. To explore the role of the ER stress signaling pathway in the osthole’s inhibition on TCP-induced inflammatory osteolysis in mice, western blotting was performed to examine the expression of ER stress markers glucose-regulated protein 78 (GRP78) and C/EBP homologous protein (CHOP). As shown in Fig. 5, TCP particles implantation significantly triggered an ER stress response in the mouse calvaria (Fig. 5a), and protein expression of GRP78 and CHOP were up-regulated by 9.47-fold and by 7.82-fold of the untreated sham group, respectively (Fig. 5b). Osthole administration blocked the ER stress response, which exhibited a marked decrease in GRP78 expression by 89.97 %, and CHOP expression by 45.91 % in the mouse calvaria, compared with the TCP particles-implanted group. Therefore, osthole prevents TCP particles-induced osteolysis via inhibition of ER stress response in vivo.
Western blotting analysis of the ER stress signaling pathway (n = 4). a The protein extracts of calvaria from sham, TCP particles and osthole (10 mg/kg) groups were separated by 12 % SDS-PAGE and transferred electrophoretically onto a PVDF membrane. Protein expression of GRP78, CHOP and β-actin were detected by western blotting. b Densitometric analysis used image analyzing software, and relative level of GRP78 or CHOP was expressed as percentage of the sham group (means ± SD). p < 0.05 compared with sham group (a); p < 0.05 compared with TCP particles group (b)
Discussion
Prosthetic wear particles play a pathological role in the initiation and development of periprosthetic osteolysis, leading to irreversible aseptic loosening of prostheses. Ceramics (such as TCP and PMMA), polymethylmethacrylate and titanium particles have been commonly associated with clinical aseptic joint loosening and over-activation of osteoclasts [1]. Many orthopaedic implants fail due to particles-stimulated aseptic loosening. Currently, therapy against particle-induced osteolysis is limited to surgical revision. Thus, identification of drugs that can prevent particles-induced osteolysis will improve treatment options for particle-stimulated aseptic loosening. Here, we demonstrated that osthole, as a classic drug commonly used in clinics, significantly prevents TCP particles-induced osteolysis. Hence, it has great potential for the treatment of particles-induced periprosthesic osteolysis and aseptic loosening.
In recent years, Chinese herbal medicine (CHM) has attracted the attention of many orthopaedics researchers because some of its herbs, formulations, or extracts have osteoprotective but few side effects, and are low in cost since thousands of years of history in osteoporosis clinical practice. Osthole, a natural coumarin extracted from the fruit of Cnidium monnieri (L.) Cusson, has been shown to display a therapeutic effect on solid tumour, diabetes, vasorelaxatio, and a variety of inflammatory disorders, which often result in increased osteoclasts formation [20]. Some preclinical in vivo and in vitro studies reported that osthole was able to enhance new bone formation [21–23], and prevent bone loss through an estrogen-like effect [24, 25]. Consistent with their results, we found that local administration of osthole at a dose of 10 mg/kg is effective to prevent TCP particles-induced bone loss (Fig. 2), confirmed by the significant decreases of osteoclasts number and osteolysis area in the mouse calvaria (Figs. 3 and 4). Next, we demonstrated that osthole obviously increased serum level of osteocalcin and calvarial ALP activity (Table 1), and significantly inhibited the activity of TRAP as well as cathepsin K (Table 1) in the TCP particles-implanted calvaria. These data strongly suggest that osthole effectively prevents TCP particles-induced mouse calvarial osteolysis in vivo via promotion of new bone formation and inhibition of osteoclastic bone resorption.
Besides these bone turnover markers, the pro-inflammatory cytokines induced by wear particles are also responsible for bone resorption around joint prostheses [1–4]. For example, previous in vitro or in vivo studies suggested that TNF-α and IL-6 are the two major pro-inflammatory cytokines provoking osteolysis, and could be largely produced in patients during implant loosening [1, 2, 4]. Our ELISA assay showed that TCP particles did trigger the release of TNF-α (Table 2), which is similar to the results from a recent study that compared the biological activity of ceramic and titanium particles [26]. Furthermore, TCP particles significantly increased IL-6 production both in TCP particles-implanted mice (Table 2) and in cultured RAW264.7 cells (data not shown), inconsistent with results from Ding et al. or Obando-Pereda GA et al. [26, 27]. They reported that zirconia ceramic particles had little effect on the release of IL-6 from cultured peritoneal macrophages. As the cells used in experiments were different, we speculated this discrepancy might be due to the different cell types. Fortunately, osthole treatment reduced the release of TNF-α and IL-6 induced by TCP particles, and the level of TNF-α and IL-6 were reduced to 43.07 %, and 44.77 % of the TCP particles-treated group (Table 2), which is more significant than the effect of it on bone turnover markers. Hence, we consider that suppressing the release of pro-inflammatory cytokines may be one of the mechanisms underlining ostholes’ prevention of TCP particles-induced osteolysis in mice.
As mentioned above, wear debris-induced local inflammation and consequent bone resorption are critical pathogenic factors of osteolysis. On the other hand, pro-inflammatory cytokines have shown to impair ER homeostasis by inducing depletion of ER Ca2+ and alter chaperone function, proinsulin processing and ER-Golgi trafficking, triggering unfolded protein response (UPR) and ER stress [16, 17, 28], which also contributes to inflammatory osteolysis caused by wear particles [18, 19]. Similarly, the present study demonstrated that TCP particles significantly trigger an ER stress response in the mouse calvaria (Fig. 5), which may be the consequence of the TCP particles-caused production of TNF-α and IL-6. Consistent results were reported by Wang and Liu’s research groups. They showed that inhibition of ER stress with 4-phenylbutyric acid (an ER stress inhibitor) dramatically decreased TiPs/CoPs particles-induced release of TNF-α and IL-1β, that led to less osteoclastogensis and resorption area than that of the TiPs/CoPs particles-treated group [18, 19]. Together, these facts suggest that the ER stress signaling pathway may be another novel pathway in potentiating cytokine production, osteoclast differentiation, and bone loss induced by orthopaedic wear particles; and thus targeting the ER stress pathway may lead to novel therapeutic approaches for the treatment of aseptic prosthesis loosening. Our current work indicated that osthole treatment did dramatically block TCP particles-triggered ER stress response (Fig. 5a), which exhibited a 89.97 % decrease in GRP78 expression, and 45.91 % in CHOP expression in mouse calvaria (Fig. 5b). Therefore, osthole prevents TCP particles-induced osteolysis in vivo via inhibition of the ER stress signaling pathway.
Conclusions
Local administration of osthole can prevent TCP particle-induced osteolysis in vivo via inhibition of the ER stress signaling pathway. These results strongly suggest osthole can be developed as a novel therapeutic approach for controlling bone destruction during the treatment of implants loosening. All our findings also provide a potential mechanism underling aseptic prosthesis loosening and identify a potential therapeutic target for this disease.
References
Harris WH (2001) Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res 393:66–70
Fu CF, Xie J, Hu N, Liang X, Chen RF, Wang CL, Chen C, Xu CM, Huang W, Paul Sung KL (2014) Titanium particles up-regulate the activity of matrix metalloproteinase-2 in human synovial cells. Int Orthop 38(5):1091–1098
Katsuyama E, Miyamoto H, Kobayashi T, Sato Y, Hao W, Kanagawa H, Fujie A, Tando T, Watanabe R, Morita M, Miyamoto K, Niki Y, Morioka H, Matsumoto M, Toyama Y, Miyamoto T (2015) Interleukin-1 receptor-associated kinase-4 (IRAK4) promotes inflammatory osteolysis by activating osteoclasts and inhibiting formation of foreign body giant cells. J Biol Chem 290(2):716–726
Vallés G, Pérez C, Boré A, Martín-Saavedra F, Saldaña L, Vilaboa N (2013) Simvastatin prevents the induction of interleukin-6 gene expression by titanium particles in human osteoblastic cells. Acta Biomater 9(1):4916–4925
Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C (2006) Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop 77(2):177–197
Wu SJ (2015) Osthole attenuates inflammatory responses and regulates the expression of inflammatory mediators in HepG2 cells grown in differentiated medium from 3T3-L1 preadipocytes. J Med Food 18(9):972–979
Wang XL, Shang X, Cui Y, Zhao X, Zhang Y, Xie ML (2015) Osthole inhibits inflammatory cytokine release through PPARα/γ-mediated mechanisms in LPS-stimulated 3T3-L1 adipocytes. Immunopharmacol Immunotoxicol 37(2):185–192
Li ZP, Ji HJ, Song XY, Hu JF, Han N, Chen NH (2014) Osthole attenuates the development of carrageenan-induced lung inflammation in rats. Int Immunopharmacol 20(1):33–36
Zheng Y, Lu M, Ma L, Zhang S, Qiu M, Ma X (2013) Osthole ameliorates renal ischemia-reperfusion injury by inhibiting inflammatory response. Urol Int 91(3):350–356
Liu J, Zhang W, Zhou L, Wang X, Lian Q (2005) Anti-inflammatory effect and mechanism of osthole in rats. Zhong Yao Cai 28(11):1002–1006
Tian B, Jiang T, Shao Z, Zhai Z, Li H, Fan Q, Liu X, Ouyang Z, Tang T, Jiang Q, Zheng M, Dai K, Qin A, Yu Y, Zhu Z (2014) The prevention of titanium-particle-induced osteolysis by OA-14 through the suppression of the p38 signaling pathway and inhibition of osteoclastogenesis. Biomaterials 35(32):8937–8950
Kim JA, Ihn HJ, Park JY, Lim J, Hong JM, Kim SH, Kim SY, Shin HI, Park EK (2015) Inhibitory effects of triptolide on titanium particle-induced osteolysis and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation. Int Orthop 39(1):173–182
Rao AJ, Zwingenberger S, Valladares R, Li C, Lane Smith R, Goodman SB, Nich C (2013) Direct subcutaneous injection of polyethylene particles over the murine calvaria results in dramatic osteolysis. Int Orthop 37(7):1393–1398
Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, Clarke SL, Cullen DM, Lucas EA (2014) A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone 58:151–159
Iitsuka N, Hie M, Tsukamoto I (2013) Zinc supplementation inhibits the increase in osteoclastogenesis and decrease in osteoblastogenesis in streptozotocin-induced diabetic rats. Eur J Pharmacol 714(1–3):41–47
Kandel-Kfir M, Almog T, Shaish A, Shlomai G, Anafi L, Avivi C, Barshack I, Grosskopf I, Harats D, Kamari Y (2015) Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice. J Hepatol 63(4):926–933
Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54(2):452–461
Liu G, Liu N, Xu Y, Ti Y, Chen J, Chen J, Zhang J, Zhao J (2015) Endoplasmic reticulum stress-mediated inflammatory signaling pathways within the osteolytic periosteum and interface membrane in particle-induced osteolysis. Cell Tissue Res. 2015 May 26. [Epub ahead of print]
Wang R, Wang ZH, Ma YT, Liu GY, Shi H, Chen JN, Dong L, Zhao JN, Zhang JF (2013) Particle-induced osteolysis mediated by endoplasmic reticulum stress in prosthesis loosening. Biomaterials 34(11):2611–2623
Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, Koers-Wunrau C, Stratis A, Korb-Pap A, Pap T (2015) Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 21(9):1085–1090
Ming LG, Zhou J, Cheng GZ, Ma HP, Chen KM (2011) Osthol, a coumarin isolated from common cnidium fruit, enhances the differentiation and maturation of osteoblasts in vitro. Pharmacology 88(1–2):33–43
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J, Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, Chen D, Wang YJ (2010) Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res 25(6):1234–1245
Meng F, Xiong Z, Sun Y, Li F (2004) Coumarins from Cnidium monnieri (L.) and their proliferation stimulating activity on osteoblast-like UMR106 cells. Pharmazie 59(8):643–645
Ming LG, Wang MG, Chen KM, Zhou J, Han GQ, Zhu RQ (2012) Effect of osthole on apoptosis and bone resorption of osteoclasts cultured in vitro. Yao Xue Xue Bao 47(2):174–179
Li XX, Hara I, Matsumiya T (2002) Effects of osthole on postmenopausal osteoporosis using ovariectomized rats; comparison to the effects of estradiol. Biol Pharm Bull 25(6):738–742
Ding Y, Qin CQ, Fu YR, Xu J, Huang DS (2012) In vitro comparison of the biological activity of alumina ceramic and titanium particles associated with aseptic loosening. Biomed Mater 7(4):045019–045019
Obando-Pereda GA, Fischer L, Stach-Machado DR (2014) Titanium and zirconia particle-induced pro-inflammatory gene expression in cultured macrophages and osteolysis, inflammatory hyperalgesia and edema in vivo. Life Sci 97(2):96–106
Kim S, Joe Y, Kim HJ, Kim YS, Jeong SO, Pae HO, Ryter SW, Surh YJ, Chung HT (2015) Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J Immunol 194(9):4498–4506
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY13H060003 and No. LY15H180012), Scientific Research Foundation of Traditional Chinese Medicine in Zhejiang Province (No.2012ZB161) and Science & Technology Innovation Project of College Students in Zhejiang Province (No. 2013R426025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lv, S., Zhang, Y., Yan, M. et al. Inhibition of osteolysis after local administration of osthole in a TCP particles-induced osteolysis model. International Orthopaedics (SICOT) 40, 1545–1552 (2016). https://doi.org/10.1007/s00264-015-3021-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3021-2