Abstract
Purpose
The purpose of this study was to evaluate the efficiency of biologic augmentation of rotator cuff repair with iliac crest bone marrow-derived mesenchymal stem cells (MSCs). The prevalence of healing and prevention of re-tears were correlated with the number of MSCs received at the tendon-to-bone interface.
Methods
Forty-five patients in the study group received concentrated bone marrow-derived MSCs as an adjunct to single-row rotator cuff repair at the time of arthroscopy. The average number of MSCs returned to the patient was 51,000 ± 25,000. Outcomes of patients receiving MSCs during their repair were compared to those of a matched control group of 45 patients who did not receive MSCs. All patients underwent imaging studies of the shoulder with iterative ultrasound performed every month from the first postoperative month to the 24th month. The rotator cuff healing or re-tear was confirmed with MRI postoperatively at three and six months, one and two years and at the most recent follow up MRI (minimum ten-year follow-up).
Results
Bone marrow-derived MSC injection as an adjunctive therapy during rotator cuff repair enhanced the healing rate and improved the quality of the repaired surface as determined by ultrasound and MRI. Forty-five (100 %) of the 45 repairs with MSC augmentation had healed by six months, versus 30 (67 %) of the 45 repairs without MSC treatment by six months. Bone marrow concentrate (BMC) injection also prevented further ruptures during the next ten years. At the most recent follow-up of ten years, intact rotator cuffs were found in 39 (87 %) of the 45 patients in the MSC-treated group, but just 20 (44 %) of the 45 patients in the control group. The number of transplanted MSCs was determined to be the most relevant to the outcome in the study group, since patients with a loss of tendon integrity at any time up to the ten-year follow-up milestone received fewer MSCs as compared with those who had maintained a successful repair during the same interval.
Conclusion
This study showed that significant improvement in healing outcomes could be achieved by the use of BMC containing MSC as an adjunct therapy in standard of care rotator cuff repair. Furthermore, our study showed a substantial improvement in the level of tendon integrity present at the ten-year milestone between the MSC-treated group and the control patients. These results support the use of bone marrow-derived MSC augmentation in rotator cuff repair, especially due to the enhanced rate of healing and the reduced number of re-tears observed over time in the MSC-treated patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of bone marrow-derived MSCs harvested from the iliac crest has been explored in humans since 1990 by our team, with the result that our studies have supported the use of MSCs in treating osteonecrosis and non-unions [18–20], as well as improving surgical procedures involving allografts. MSCs have the potential [5, 7, 25] to become a variety of adult tissue cells, including tenocytes, chondrocytes and osteoblasts, as well as being a source of multiple growth factors to establish an environment conducive to soft and hard tissue regeneration. Therefore, we also have used MSCs as an adjunct to rotator cuff repair in order to improve iterative rotator cuff repair of re-tears, to improve open surgery repair of large tears and to improve repairs performed during arthroscopy.
Rotator cuff repair is a frequent surgical procedure, performed mainly today with arthroscopy. In the literature, the reported frequency of re-tears [2–4, 13, 14, 22] after surgery exceeds 25 %, resulting in a persistent defect noted on postoperative MRI. Some patients have asymptomatic re-tears, but remain improved despite this re-tear. However, most such patients have persistent pain, dissatisfaction, and limited function after a re-tear and will require an iterative surgery. Management of symptomatic re-ruptures is a challenge to surgeons, because revision surgery is technically more demanding. Quality of the soft tissue is poorer, and results usually are less successful during subsequent repair procedures. The introduction of anchors [16] has facilitated tendon reintegration near the bone, while the use of synthetic non-absorbable sutures has significantly increased its resistance. With the advent of arthroscopy, these procedures became increasingly popular and widespread in the orthopaedic community, but none of these advances seems to have brought significant improvement to the quality of postoperative results in patients with recurrent rotator cuff injuries, e.g. the overall rate of re-rupture over the first postoperative year ranges from 15 % to 45 % depending on grade of lesion. As a consequence, even if re-rupture does not require surgical intervention, because the healing of recurrent tears is not always predictable, there is significant interest in improving the effectiveness of rotator cuff re-repair. Consequently, the goal of this study was to determine if the use of bone marrow-derived MSCs injected into the fixation site of rotator cuff ruptures during arthroscopy results in improved outcomes, including faster healing and higher quality tendon integrity over time.
We evaluated the following: (1) the biologic effect of bone marrow MSCs on healing (time and surface) of the rotator cuff as compared with the results of case-matched control patients who had the same technical surgical repair without MSCs augmentation; and (2) the prevention of re-tears in the same two groups. Our purpose was to test the hypothesis that a biologic augmentation of rotator cuff repair with MSCs during arthroscopy with a single-row repair would produce an improvement in healing at the tendon-to-bone interface as determined by diagnostic ultrasound and MRI.
Material and methods
Study design
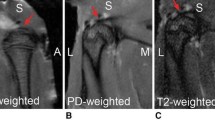
The study group was composed of 45 patients with symptomatic rupture (and tear size from 1.5 to 3.5 cm) of the rotator cuff that had undergone surgical repair with an arthroscopic protocol and adjunct therapy using autologous MSCs during a period of five years between 2000 and 2005. Patients were enrolled in this study when a rotator cuff tear was diagnosed on clinical examination by ultrasonography and magnetic resonance imaging (MRI). Inclusion criteria included the following: (1) a full thickness tear of the suprasupinatus tendon measuring 1.5 (±0.5) to 2.5 (±0.5) cm in diameter (Figs. 1 and 2) without extension to an adjacent tendon and (2) a tear pattern that was amenable to repair without open surgery; we included patients with arthroscopic subacromial decompression and acromioclavicular joint resection. The exclusion criteria included the following: (1) tear size more than 3 cm (or less than 4.5 cm²) or when the tear appeared irreparable at surgery by arthroscopy technique; (2) extension of the tear to either the subscapularis tendon and the infrasupinatus tendon; (3) degenerative arthritis of the glenohumeral joint; and (4) patients in whom we performed either a biceps tenotomy or tenodesis. In addition, patients presenting with prior operations on the shoulder were excluded.
The footprint of the greater tuberosity was abraded and sufficient bone removed to expose cancellous bone .The surface of healing was considered to be the surface of the footprint where the cancellous bone was removed; schematically it corresponds to the larger L of the tear multiplied by 10 mm (d) which is usually the distance where the cancellous bone can be removed, The surface of healing is in this example 3 cm*1 cm = 3 cm²
For the control group, patients were matched for the following: (1) tear size and tendon rupture location; (2) dominant shoulder; and (3) gender and age among patients who had the same technique of repair but without MSCs before 2000. In the control group, there were 45 patients (28 men and 25 women) and the mean age was 61 years (range, 49–71 years). The dominant shoulder was affected in 26 patients. Based on the patients selected for the two groups, there were no significant differences in age, gender, affected shoulder, size or location of tear, when the two groups were compared. The average follow-up between the two groups was different, but the comparison between the two groups was done at the same time from the repair out to a follow-up of ten years.
Surgical technique, procedures, and post-operative management
In the study group associated with MSCs biologic augmentation, bone marrow aspiration was performed after installation of the patient in a beach-chair position and before arthroscopy. A volume of 150 mL of marrow (that was concentrated later) was aspirated from the anterior iliac crest. Marrow was aspirated in small fractions (four mL) as previously described, to reduce the degree of dilution by peripheral blood. Several perforations (between three and five) were made through the same skin opening. Each perforation into the anterior iliac crest was spaced at approximately 2 cm apart to avoid dilution from aspiration in the previous channel. All aspirates (approximately 150 mL) were pooled in plastic bags containing an anticoagulant solution (citric acid, sodium citrate, dextrose). The bone marrow aspirate was concentrated in the Cellular and Molecular Therapy Laboratory. The buffy coat containing MSCs and other progenitor cells was returned to the operative room after 30 minutes.
Repair of the tear
All patients underwent a pre-operative interscalene block (ropivicaine 0.5 %) performed by an anesthesiologist to assist with postoperative pain control. After the induction of general anaesthesia, we placed the patient in the beach-chair position and prepared and draped the patient so that the shoulder was available as per protocol, including access to the patient’s ipsilateral anterior iliac crest. A standard arthroscopic pump was used, and fluid pressure was maintained at 60 mm Hg. Standard posterior and anterior portals were established to perform a thorough intra-articular diagnostic examination and locate any lesions of the biceps tendon and other associated lesions. After intra-articular examination, the arthroscope was placed in the subacromial space through the posterior portal, and a lateral working portal was established approximately 3 cm lateral to the anterolateral corner of the acromion. Subacromial bursal tissue was removed with the aid of an electrocautery ablation device to obtain a clear view of the rotator cuff and undersurface of the acromion. Acromioplasty and coracoacromial ligament release were routinely performed before the cuff repair in every case. An accessory posterolateral viewing portal was established approximately 1 to 2 cm anterior and lateral to the posterolateral corner of the acromion. The configuration and mobility of the torn cuff were assessed. The torn tendon was then grasped and mobilized to ensure adequate excursion to prevent undue tension on the repair and to determine the appropriate repair site. If the tendon was retracted and mobility of the tendon was insufficient, release of the coracohumeral ligament and detachment of the tendon from the bursal and articular sides were performed. The footprint of the greater tuberosity was abraded (Fig. 2) with a power shaver and sufficient bone removed to expose cancellous bone (1–2 mm depth of bone removal). No attempt was made to create a bone trough. Single-row repair was used in this period, with suture anchors placed on the lateral edge of the greater tuberosity. Sutures from these anchors were passed sequentially through the supraspinatus tendon in a simple fashion from anterior to posterior using a suture passing instrument. These sutures then were tied sequentially using arthroscopic sliding square knots.
Biological augmentation with MSCs and evaluation of the number of MSCs received by patients
The buffy coat containing MSCs and other progenitor cells was returned to the operating room from the Cellular and Molecular Therapy Laboratory after 30 minutes. Injection of MSCs was performed at the end of rotator cuff tendon fixation. MSCs were injected in the tendon at the junction between the bone and tendon (4 mL), and in the bone at the site of the footprint (8 mL). Each patient in the MSC-treated group received a total of 12 mL of bone marrow concentrate.
The concentration of MSCs (the number of connective-tissue progenitor cells per 1.0 cc of aspirate) and the prevalence of MSCs (the number of MSCs per million nucleated cells) were estimated by counting the number of colony forming unit fibroblasts (CFU-Fs). The CFU-F assay was performed by plating two million cells from the bone marrow aspirate into 25 cm² tissue culture flasks. Following ten days under standard growth conditions as previously described, the number of colonies containing at least 50 cells was evaluated. The concentration of connective-tissue progenitor cells was calculated for each sample as the product of the nucleated cell count and the prevalence of connective-tissue progenitor cells. The dilution of nucleated cells coming from the bone marrow by cells in the peripheral blood was calculated as previously described.
The average number of MSCs per mL (mean and standard error) of concentrated cells returned to the 45 patients was 4,300 + 1,800 per mL of BMC. The average total number of MSCs returned to the patient was 51,000 ± 25,000 cells in 12 mL of injected BMC.
Postoperative management
The two groups underwent the same program of rehabilitation. In the first week after surgery, an arm sling was used and gentle passive forward flexion started for all patients. Active range-of-motion exercise was initiated at six weeks. Active resistance muscle strengthening exercise started at the eighth week. Patients were allowed to perform light daily activities from two to three months postoperatively, whereas heavy manual work and sports activity were allowed only after six months.
Outcome measurement
All patients underwent imaging studies of the shoulder with anteroposterior and scapular lateral views pre- and postoperatively. A rotator cuff re-tear was confirmed by MRI or CT scan with arthrography. All patients underwent imaging studies of the shoulder with iterative ultrasound performed every month from the first postoperative month to the 24th month. Rotator cuff healing or re-tear was assessed with MRI postoperatively at three and six months, one and two years and at the most recent follow-up MRI (minimum ten-year follow-up).
Ultrasound criteria of rupture
The echographic surface of healing was checked every month for the following criteria: presence of intrasubstance, partial-thickness (bursal or articular surface), or full-thickness marginated hypoechoic defects; presence and location of focal thinning of the repair; presence of bursal or joint fluid; and location of the suture anchors. A compression test was performed by increasing pressure of the ultrasound probe over any apparent cuff defect. Ultrasound criteria for healed repair included visualization of a tendon with normal thickness, convexity, and length. A thinned cuff or one with a subtle concave contour was considered to be intact in the absence of a focal defect. A finding of a full-thickness rotator cuff tear was recorded when the rotator cuff could not be visualized because of complete avulsion and retraction under the acromion, when there was a focal defect in the rotator cuff or when the torn cuff had retracted a variable degree from the surgical repair site . A positive compression test with displacement of underlying fluid and loss of the normal convex contour of the peribursal fat also indicated a re-tear. Increasing hypoechoic lesion or gap, which is more clearly demarcated on sequential ultrasound exam, also indicated re-tear. When a tear on ultrasound was suspected, an MRI was performed to confirm the diagnosis.
Evaluation of healing surface on ultrasound
The surface of healing was considered to be the surface of the footprint where the cancellous bone was removed; schematically it corresponds (Fig. 2) to the larger (L) of the tear multiplied by 10 mm (d) which is usually the distance where the cancellous bone can be removed. The tear size was evaluated both on the preoperative MRI and during the arthroscopy. The larger was graded as 5, 10, 15, 20, 25 and 30 mm. So the surface of healing was graded from 0.5 cm² to 3 cm².
A subjective scoring system was employed to assess healing of the footprint area as follows: absent (grade 0), sparse (grade 1), moderate (grade 2), prominent (grade 3), or total (grade 4), so that a maximum healing score of 4 was possible on a scale from 0 to 4.
The MRI studies
MR imaging was performed with a 2.5-T closed-system magnetic resonance scanner and included the use of a dedicated shoulder coil. The pulse sequences included fast spin echo axial, coronal, and sagittal T1-weighted images with fat saturation, as well as coronal T2-weighted images with fat saturation. Cuff integrity was diagnosed as intact in the presence of complete footprint coverage by tendon. Oblique-coronal and oblique-sagittal fluid-sensitive sequences were evaluated for the presence and largest size in millimetres of full-thickness RCT. Measurement was made following the contour of the humeral head. In those tears involving subscapularis and supraspinatus tendons, measurement was made across the interval region. We chose to concentrate on previously published criteria of full thickness re-rupture, thus obviating intratendinous cleavages and partial re-ruptures.
Statistical analysis
Sample size estimation (power analysis) was conducted before data collection based on a review of the literature and a large series of prior single-row arthroscopic rotator cuff repairs conducted at our institution. We concluded that 45 patients should be enrolled into each group to detect a large (15–20 %) difference in supraspinatus healing rates between groups as determined by diagnostic ultrasound, with one-tailed type-I (alpha) error set at 0.05 and type-II (beta) error set at 0.2 (80 % power). A chi-square test was used to compare the final tendon healing rates between patients receiving MSCs or not. The paired t-test was used to compare the differences between control and MSCs groups. The independent t-test, 2 test, and one-way analysis-of-variance test were used to compare the differences between the two groups in terms of clinical outcomes and imaging findings. The significance was set at P < 0.05.
Results
Healing failures and re-tears
Demographic data
Ninety patients were enrolled in the study with 45 in the MSC-treatment study group and 45 in the control group. Among these 90 repairs, 31 (34 %) shoulders had non-healing or re-tear diagnosed on ultrasound, which subsequently was confirmed by MRI. We did not find a significant difference in pre-operative cuff tear size between the tendons that healed and those that showed deficient healing or a frank re-tear according to MRI criteria. The average cuff tear size (and standard deviation) as estimated by the surgeon at the time of screening or during surgery was 2.3 ± 0.5 cm (range, 1–4 cm) in the group with healing versus 2.2 ± 0.5 in those without healing (p = 0.24). The majority of patients had grade 0 or grade 1 fatty infiltration [17], and the two grades were equally distributed. There was no significant pre-operative difference in fatty infiltration between the healed and unhealed groups.
Bone marrow concentrate (BMC) injection as an adjunct therapy during the rotator cuff repair resulted in a statistically significant (p < 0.05) decrease in the number of ruptures diagnosed on ultrasound and confirmed by MRI. At the ten-year follow-up milestone, intact rotator cuffs were found in 39 (87 %) of the 45 patients in the MSC-treatment group, but just in 20 (44 %) of the 45 patients in the control group. Patients in the control group were approximately four times more likely to have experienced a poor outcome, including deficient or no healing or frank re-rupture when compared with the MSC-treatment group at the ten-year follow-up. Furthermore, failures in the MSC-treatment group had poor or no healing or re-tear in only one tendon (suprasupinatus), while in the control group, there were six cases of rotator cuff re-tear in which both the supraspinatus and the infraspinatus were involved, and four cases in which three tendons (the supraspinatus, the infraspinatus, and the subscapularis) were involved. Since even patients with a re-tear had a MRI at ten years, it was possible to perform a longitudinal assessment of fatty infiltration in all 90 patients. Patients with a re-tear had follow-up MRI at ten years, allowing a longitudinal assessment of fatty infiltration from pre-operative out to ten years. For those patients without a re-tear, 40 of the 59 patients had no change in grade (34 in the MSC-treatment group and six in the control group), while 19 had an increase of one grade. The remaining 31 patients with failed healing or a re-tear all had an increase of either one grade (19 patients) or two grades (12 patients).
Cell analysis in the MSC-treatment group demonstrated that the number of MSCs was relevant to the outcome. Since the volume of the graft was fixed (approximately 12 mL), the concentration and total number of transplanted cells were analysed together. The average number of MSCs returned to the 45 patients in the study group who received concentrated bone marrow-derived MSCs as an adjunct to single-row rotator cuff repair at the time of arthroscopy was 51,000 ± 25,000. For the 39 patients with healing at the most recent follow-up in the MSC-treatment group, the bone marrow-derived graft contained a mean of 4200 + 1900 progenitors per cubic centimeter and the mean total number of progenitors injected on the shoulder site was 54000 + 23000. For the six patients whose treatment failed (no healing or re-tear) there were significantly (p <0.01) fewer MSCs per cubic centimeter in the graft (mean of 1500 + 1200 versus 4200 + 1900) and a significant decrease (p < 0.01) in the mean total number of progenitor cells as compared with the other patients whose treatment was successful (14000+ 9000 versus 54000 + 23000).
Biologic effect of augmentation with MSCs on healing (time and surface) and prevention of repeated tears
With the numbers studied, the size of rupture at time of surgery, age at time of surgery, and time between diagnosis and repair were not predictive factors of deficient healing or re-tear in patients who had received MSCs. However, these variables were predictive in the control group patients who did not received MSCs. The best predictor of rotator cuff integrity in the control group was pre-operative tear size (correlation coefficient, r = 0.33, p < 0.01). Patients with small (≤2 cm²) rotator cuff tears were least likely to re-tear (re-tear rate, 10 %). As the tear-size increased, the re-tear rate increased in a linear fashion, i.e. ≤ 1.5 cm² (10 %), 3 cm² (16 %), 4.5 cm² (33 %).
The efficiency of MSC augmentation of the standard of care rotator cuff repair was analysed for the healing time, the quality of the healing surface on the footprint, and the absence of re-tear. Of all of the variables explored with multivariate analysis, the number of transplanted cells was determined to be the most relevant to the outcome. We considered that the healing process could require a significant passage of time, as previously reported, so that a failure of the initial repair that occurred in the first six months was considered as non-healing, but that a subsequent rupture of the repaired tendon or of other tendons after the sixth month was considered as a repeated tear, not a re-tear due to a deficient healing process.
Healing time during the first six months
Bone marrow-derived MSC injection improved the rate of healing. Failures in the MSC-treatment group did not occur during the first six months. During the same six-month period the control group experienced eight failures between two and three months and the remaining seven occurred between three and six months, resulting in a mean time to failure of 3.4 months. Thus, there was a dramatically higher risk of non-healing during the six-month period after surgery in the control group. Non-healing over the first three months in the control group accounted for 47 % (7/15) of all of the failed repairs that occurred during the first six months in this study. It demonstrates that natural rotator cuff healing may be abnormally prolonged in some patients, which suggests that there is an opportunity to enhance and improve healing in these patients by using MSC augmentation, since no failed repairs were observed in the MSC-treatment group during the first six months.
For patients who received MSCs of the footprint there was a negative correlation between the time to obtain total healing of a same surface and the concentration of MSCs in the graft (Rs = –0.4; p = 0.04). Size of the tear also had a significant relation (p = 0.03) with time to healing, i.e. patients in the MSC-treatment group with tears larger than 2 cm needed a longer time to reach total healing (average six months) compared to the patients with tears smaller than 2 cm (average three months). Regardless of the size of the tear, patients in the control group took two months longer to reach total healing when matched for tear size compared to the patients in the MSC-treatment group.
Surface of healing
For patients who received MSCs, there was a positive correlation between the surface of healing at three months and the number and concentration of MSCs in the graft (correlation coefficient 0.4 and 0.6, significant at 0.03 and 0.02 levels, respectively). From a schematic point of view, patients who received more than 30,000 MSCs had healed 2 cm² or more over the footprint at three months, while those who received fewer than 30,000 MSCs needed at least four months or more to achieve the same degree of surface healing. For control group patients, none had achieved healing of 2 cm² at three months, but required on average six months for surface healing to reach 2 cm².
Repeated tears between the sixth month and the tenth year
Ten failures in the control group were identified between the second and the ninth years involving the suprasupinatus and other tendons. Repeated tears in six cases involved both the supraspinatus and the infraspinatus, and in four cases three tendons (the supraspinatus, the infraspinatus, and the subscapularis). Six repeated tears of the suprasupinatus were also observed in the study group; they were identified in the second and fourth years among six patients who received a low number of cells in the MSC-treatment group. The subjective scoring system employed to assess healing surface of the footprint at the two-year follow-up visit demonstrated that healing was considered at one-year follow-up as sparse (six cases), or moderate (four cases) among the ten failures, although there was no evidence of fluid signal density within the rotator cuff tendon indicating an absence of full-thickness tendon gaps. Healing was at one-year followup more frequently graded prominent, or total in the MSC-treatment group as compared with the control group, which may explain the absence of subsequent tears in the MSC-treatment group.
Cell analysis in the study group demonstrated that the number of MSCs correlated to the grade of healing with a score of total healing (28 cases) more frequently obtained when the concentration of MSCs exceeded 2500 per mL and a risk of absence of healing (six cases) when the concentration of MSCs was lower than 1500 per mL.
Discussion
The frequency of re-tears in our control group is consistent with that reported for this type of tear and surgical technique [4, 14]. We believe that this is the first study with long-term follow-up (i.e., ten years) that evaluated whether patients’ shoulders with a rotator cuff repair augmented with autologous bone marrow-derived MSCs healed better than those without MSC augmentation (control group). We considered an abnormal MRI finding of a high-intensity signal consistent with fluid in an area that should be occupied by the tendon to represent a non-healing tendon when present before six months follow-up. On this basis, we observed a distinction between tendons that did not heal and those that healed but later had a repeated tear during the ten-year follow-up period. There was a significant risk of non-healing up to six months after surgery in the control group, with about 50 % of the non-healing patients being diagnosed between three and six months after surgery. These results demonstrate that rotator cuff healing is prolonged, suggesting that enhancing the rate of healing will aid in protecting the repair from excessive loading. Biologic augmentation of the repair with MSCs appears to be a successful strategy to improve the rate of tendon healing. In particular, all the tendons of patients in the MSC-treatment group had healed within the first six months, and when failure was observed it was later and associated with a significantly lower number of MSCs in the injectate used during the treatment.
The most important and novel finding in this study is that some re-tears or new tears occurred after one year. These re-tears were more frequently associated with the control group patients, who weren’t treated with MSCs. While the risk of a re-tear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (>three years) are relatively limited. Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent postoperative complications, especially re-tear. Observations in the MSC-treatment group support the potential that MSC treatment has both a short-term and a long-term benefit in reducing the rate of tendon re-tear.
Our results are similar to the data reported in animal models in the literature. During the last 15 years, a number of studies in animal models [1, 6, 8–10] have evaluated the effect of MSCs on the histologic appearance, microstructure, biomechanics, and strength of tendon healing after repair. Overall, the results have been promising with a trend toward improvements in histology and strength of repair. Taken together, these studies [12, 15, 21, 23–28] are supportive of the use of cell therapy in tendon repair, not only by improving regeneration of the enthesis (an application that may prove especially useful for rotator cuff repair), but by highlighting the therapeutic potential of autologous BMC (containing MSCs and other progenitor cells) to improve the strength and quality of tissue formed when used in tendon repairs [29–33]. The idea of using autologous BMC is especially attractive, since the bone marrow aspirate can be concentrated and available within 30 minutes, negating the need for prolonged culture-based expansion. Clearly, autologous BMC, as described in this study, is more practical in surgical applications than cultured MSCs.
In another human clinical study [11] that was begun ten years after our study, the authors were able to enroll 14 patients in a short time with complete RC tears repaired with transosseous stitches through miniopen incisions. Prior to cuff repairs, autologous BMMCs were harvested from the iliac crest and subsequently injected into the repaired tendon borders. The BMMC fractions were obtained by cell sorting and resuspended in saline enriched with 10 % autologous serum. These patients were monitored for a minimum of 12 months, and tendon integrity was demonstrated by magnetic resonance imaging in all 14 patients. No control group was included in this study, but historically for this procedure, overall rates of rerupture during the first postoperative year range from 25 % to 65 %, depending on lesion extent.
However, implantation of BM-MCs in rotator cuff tendon borders appears to be a safe and promising approach to enhance the healing of tendon repairs. Further research will be critical to better investigate the use of this biologic approach.
The purpose of the present study was to compare tendon healing between a rotator cuff repair with and without MSC augmentation. We chose tendon healing as an endpoint for this phase of the study rather than clinical outcomes. Our reasoning was that unless the MSCs augmented tendon repair demonstrated a statistically significant improved healing rate, clinical studies focusing on pain, range of motion, strength, etc. were moot. It would make no sense to perform a more costly and difficult operation with a lower tendon healing rate. In order to extract the most meaningful data for the investigation, attempts were made to keep the cohort as uniform as possible. To that end, we included only patients with a full-thickness supraspinatus tear <30 mm in the anterior to posterior dimension.
A potential limitation to this study is that the study group is relatively small. We acknowledge that additional longitudinal studies and evaluations of greater numbers of patients are necessary to fully understand the importance of rotator cuff integrity with regard to functional outcome at the time of long-term follow-up. We used both MRI and ultrasound (US) to check the healing process, since sutures and suture anchors used in most arthroscopic repairs can introduce artifacts when assessed by MRI, which can be misinterpreted as a re-tear. Moreover, metallic debris from the arthroscopic instrumentation may cause metallic artifacts compromising the quality of the MR images. Ultrasound is not affected by such artifacts, and the images are not distorted by suture anchors. Therefore, it has become a highly accurate diagnostic method in evaluating the integrity of the rotator cuff after surgical repair. The spectrum of normal US findings after arthroscopic rotator cuff repair were compared with pre-operative US or MR images, and sequential US changes in the patients with full thickness re-tears were demonstrated.
We also recognize that there are other elements that contribute to a successful clinical outcome in addition to tendon healing. We used a rehabilitation program that minimizes patient stretching and de-emphasizes an early, vigorous return of full motion. It is not clear what effect this approach had on our healing rates; but we believe this to be beneficial and currently prescribe a slower rehabilitation course for the vast majority of our patients. Despite these limitations, our data demonstrate a statistically significant higher healing rate with the MSC augmented suture bridge repair when compared to a single-row repair without MSCs. Whether or not this improved healing rate will translate into improved clinical outcomes is a subject for future investigation.
In conclusion, this study showed that significant improvement in imaging outcomes could be achieved by MSC augmentation of a surgical rotator cuff repair technique at a minimum six-month follow-up. Our study showed that the durability of these repairs also was different over time between the two groups, with greater protection in the MSC-treatment group. Our results support a long-term benefit of tendon integrity extending out to ten years, with 87 % of patients in the MSC-treatment group demonstrating tendon integrity compared to just 44 % of control group patients.
References
Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B et al (1999) Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 5:267–77
Barber FA, Coons DA, Ruiz-Suarez M (2007) Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy 23:355–360
Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG (2005) Arthroscopic repair of full-thickness tears of the supraspinatus: Does the tendon really heal? J Bone Joint Surg Am 87:1229–1240
Bigliani LU, Cordasco FA, McIlveen SJ, Musso ES (1992) Operative treatment of failed repairs of the rotator cuff. J Bone Joint Surg Am 74:1505–1515
Caplan AI (2005) Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11:1198–1211
Chang CH, Chen C, Su C, Liu H, Yu C (2009) Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc 17:1447–1453
Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D et al (1980) Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 56:289–301
Chen JM, Willers C, Xu J, Wang A, Zheng MH (2007) Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng 13:1479–91
Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH et al (2007) Bone marrow-derived mesenchymal stem cells influence early tendon healing in a rabbit Achilles tendon model. J Bone Joint Surg Am 89:74–81
Cohen S, Leshansky L, Zussman E, Burman M, Srouji S, Livne E et al (2010) Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A 16:3119–37
Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R (2012) Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc 20(2):373–377
Funakoshi T, Majima T, Iwasaki N, Suenaga N, Sawaguchi N, Shimode K et al (2005) Application of tissue engineering techniques for rotator cuff regeneration using a chitosan-based hyaluronan hybrid fiber scaffold. Am J Sports Med 33:1193–201
Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K (2004) The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86-A:219–224
Gerber C, Fuchs B, Hodler J (2000) The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 82:505–515
Geuze RE, Kruyt MC, Verbout AJ, Alblas J, Dhert WJ (2008) Comparing various off-the-shelf methods for bone tissue engineering in a large-animal ectopic implantation model: bone marrow, allogeneic bone marrow stromal cells, and platelet gel. Tissue Eng Part A 14:1435–1443
Goble EM, Somers WK, Clark R, Olsen RE (1994) The development of suture anchors for use in soft tissue fixation to bone. Am J Sports Med 22:236–239
Goutallier D, Bernageau J, Patte D (1989) L’evaluation par le scanner de la trophicite des muscles de la coiffe des rotateurs ayant une rupture tendineuse. Rev Chir Orthop Reparatrice Appar Mot 75(suppl 1):126–127
Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 405:14–23
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–7
Hernigou P, Homma Y, Flouzat-Lachaniette CH, Poignard A, Chevallier N, Rouard H (2013) Cancer risk is not increased in patients treated for orthopaedic diseases with autologous bone marrow cell concentrate. J Bone Joint Surg Am 95(24):2215–2221
Hoffmann A, Gross G (2006) Tendon and ligament engineering: from cell biology to in vivo application. Regen Med 1:563–574
Karas EH, Iannotti JP (1998) Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect 47:87–95
Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y et al (2006) Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg 15:371–7
Kovacevic D, Rodeo S (2008) Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res 466:622–633
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54. doi:10.1038/emm.2013.94
Murray DH, Kubiak EN, Jazrawi LM, Araghi A, Kummer F, Loebenberg MI et al (2007) The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg 16:251–254
Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H (2010) Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am 92:2776–2784
Riley G (2004) The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 43:131–42
Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL (2007) Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am 89:2485–97. doi:10.2106/JBJS.C.01627
Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM (2010) Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A 16:1549–1558
Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A et al (2003) Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res 287:289–300
Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M (2011) Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res 29:1351–60. doi:10.1002/jor.21400
Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D’Augusta D et al (2008) rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am 90:2206–2219
Acknowledgments
We thank Ted Sand and Richard Suzuki and the other members of Celling Biosciences for reviewing the final manuscript and for their help in translation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernigou, P., Flouzat Lachaniette, C.H., Delambre, J. et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. International Orthopaedics (SICOT) 38, 1811–1818 (2014). https://doi.org/10.1007/s00264-014-2391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2391-1