Abstract
Purpose
Osteosarcoma is the most common primary malignancy in orthopaedic surgery. Studies suggest that expression of VEGF and high vascularity within osteosarcoma may correlate with poor prognosis. The purpose of this study was to determine whether there was a correlation of VEGF expression with clinical tumour stage and metastasis.
Methods
This retrospective case series examined 54 cases of osteosarcoma patients who were treated during a ten-year period. Relevant clinical information included age, gender, tumour location, stage, adjuvant therapy, morbidity, mortality, and tumour subtypes. The clinical information was analysed for correlation of VEGF expression and tumour prognosis. Tumour sections were examined by routine H&E and by immunohistochemistry for VEGF, CD31, and the oncogenes c-myc and c-fos.
Results
There was a significantly positive correlation between VEGF expression and tumour stages among these cases (p < 0.01). The data also suggested a higher cancer recurrence and more frequent cases of remote metastasis in the high-VEGF group compared to the low-VEGF group. VEGF expression also positively associated with c-fos and c-myc expressions in the primary tumour sections.
Conclusion
The results of this study highlight the role of VEGF in angiogenesis and tumour burden. Data also suggest the influence of VEGF may associate with the elevations of c-fos and c-myc expression. The development of novel therapies to target the VEGF pathway in osteosarcoma may lead to improved survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is the most common primary malignant tumour seen by orthopaedic surgeons, affecting approximately 560 children and adolescents each year in the United States [1]. The incidence of osteosarcoma peaks during the second decade of life during periods of rapid skeletal growth. Osteosarcoma develops most commonly in the distal femur, proximal tibia or proximal humerus. More than 85 % of cases are categorised as primary osteosarcoma, arising de novo [1]. Osteosarcoma can be subcategorised based on clinical, radiographic, and histological features as either intramedullary (conventional, telangiectatic, small cell) or surface subtypes (parosteal, periosteal).
Despite intensive treatment involving adjuvant chemotherapy, wide excision of tumours, and amputation of the diseased limbs, the five year survival rate is 60 % to 78 % [2]. Thus, novel therapeutic strategies for osteosarcoma are needed. Studies have indicated that high metastatic potential and high recurrence rate of osteosarcoma are associated with high levels of vascularisation [3]. Vascular endothelial growth factor (VEGF) is a major angiogenic factor that induces endothelial cell proliferation and increases the permeability of the vascular endothelium [4, 5]. Enhanced VEGF gene expression has been reported in primary tumours from breast, lung, ovary, liver and colon [6–10]. In comparison with other types of cancers, there were limited clinical reports in the orthopaedic literature search that suggested the correlation of high VEGF and poor prognosis in osteosarcoma [11]. These studies showed a possible correlation between VEGF and some indications of clinical prognosis, while many other clinical situations such as tumour stage, recurrence, and remission rate are not conclusively reported. This study aimed to determine if there is a correlation between VEGF expression with tumour stage and metastasis.
Materials and methods
Research design
This retrospective case series study was approved by the University of Kansas School of Medicine-Wichita’s Human Subjects Committee. The participating orthopaedic tumour surgeon conducted a log search of the past ten years to identify osteosarcoma patients. Based upon this review, histology tissue blocks were identified and slides prepared by the participating musculoskeletal pathologist. For each identified osteosarcoma patient, one haematoxylin & eosin (H&E) stained slide and six unstained sections were prepared. The unstained slides underwent immunohistochemical staining for VEGF and oncogenes (c-fos and c-myc). Patient charts were reviewed by one clinical researcher and two medical students who abstracted relevant clinical information, including age, gender, primary tumour (osteosarcoma) location, stage at diagnosis, adjuvant therapy, morbidity, mortality and tumour subtype. Histology data and clinical outcomes were then analysed for correlations of VEGF and tumour prognosis.
Subject selection criteria and sample size justification
The software package PS Power and Sample Size Calculations (William D. Dupont and Walton D. Plummer, http://www.mc.vanderbilt.edu/prevmed/ps.htm) was used to calculate the required sample size for testing. We used an uncorrected chi-square statistic to analyse the null hypothesis. Prior data [12] suggested the probability of lung metastasis in VEGF-negative osteosarcoma was 0.15, whereas the incidence ratio of lung metastasis in VEGF-positive osteosarcoma was 0.82. At least 14 high and 14 low VEGF expressed cases were needed to be able to reject the null hypothesis with a probability (power) of 0.95. The type I error probability associated with this test of the null hypothesis was 0.05. Given the high and low VEGF expression ratio of 0.63 : 0.37 from previous data [12], a minimum of 40 clinical cases were needed. Pathologically confirmed osteosarcoma patients at the medical centre were then enrolled to participate in this study. Due to the relative rarity of the disease, no more than four to ten cases per year were expected. Therefore, a ten-year review period (2001–2011) was selected to capture the needed number of patients with a confirmed diagnosis of osteosarcoma and corresponding pathological specimens. Our accessible population from the participating tumour surgeon’s practice (H.R.) was 72. Of the 72 patients, 54 were included based on the availability of corresponding tissue blocks and completeness of charts.
Histology process and immunohistological (IHC) stains
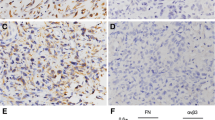
Tumour specimens in paraffin blocks were cut to 6-μm sections, and seven section-slides per specimen were prepared. One slide was stained with H&E for histological evaluation. IHC techniques were performed on the remaining sections to detect expression levels of VEGF, CD31, and oncogenes c-myc and c-fos. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Procedures were performed according to the instructions of antibody vendors and general protocol for IHC [13]. In negative control sections, the primary antibody was replaced by non-immune rabbit sera. Digital images of representative fields were captured and analysed using the Image-Pro+ software package (Media Cybernetics, Silver Spring, MD). The level of gene expression and localisation were then evaluated in six different fields and expressed as integrated optical density (IOD). Two investigators independently read all IHC-stained sections and graded the immunostains as 0 (negative), 1 (<20 % positive cells), 2 (<50 % positive cells), 3 (>50 % positive cells), and 4 (100 % positive) (Fig. 1). Another classification to distinguish the high and low expression of VEGF to correlate clinical data was set such that the specimens with less than 20 % of VEGF+ cells were the low-VEGF group, whereas specimens with more than 20 % VEGF-positive cells were the high-VEGF group.
Data analyses
Experimental databases were established to record and analyse the data generated by the experiments described in the protocol. Data were managed in Excel, and analyses of the data were conducted using appropriate statistical testing in SPSS (ver 16.0, SPSS, Chicago, IL). The histological data were combined with the clinical database for statistical analyses. To analyse the correlation between VEGF and oncogene expression levels with clinical prognosis indicators such as disease staging, metastasis, and tumour recurrence, bivariate correlations with Pearson’s correlation coefficient, Spearman’s rho, and Kendall’s tau-b were computed. Clinical primary tumour stages were converted to numerical readings for bivariate correlation analysis: sub-stage II-A converts to 2.1 and II-B to 2.5, etc. Other comparison parameters such as age and gender were analysed using a two-independent samples nonparametric test—Mann-Whitney U Test.
Source of funding
This study was supported by a Dean’s Pilot Research Grant (Level I) from University of Kansas School of Medicine-Wichita, a seed grant from Wichita Medical Research & Education Foundation, and a grant from Flossie E. West Memorial Foundation. The funds helped to reimburse travel expense between collaborators’ institutions for data collection and specimen transport, cover pathological process cost, and purchase staining reagents and laboratory supply.
Results
A total of 72 confirmed osteosarcoma cases treated between 2001 and 2011 were identified. Clinical data, without patient identifiers, were recorded to establish the database. Fifty-four matched histological tissue blocks were available from the Sarcoma Institute. Table 1 summarises the clinical data associated with low or high VEGF expression levels. The high VEGF group had more patients (n = 43) as compared to the low VEGF group (n = 11). Although there was a wide range of age in both the low and high VEGF groups, median ages for were similar at 21.3 years of age for the low VEGF and 19.3 years of age for the high VEGF groups. The location with the highest prevalence of tumour was the distal femur (n = 26), with the majority (n = 21) of the patients from the high VEGF group. There was a higher cancer recurrence in the high VEGF group (n = 13) as compared to the low VEGF group (n = 3), though this difference did not reach statistical significance (p = 0.09). The majority of metastases were also found in the high VEGF group (n = 21) compared to the low VEGF group (n = 2). Although the comparison using this method of analysis did not reach statistical significance (p = 0.07), further analysis using bivariate analysis suggested significant correlations between VEGF expression and tumour metastatical potentials (see Table 2).
Bivariate analysis was performed with results summarised in Table 2. Significantly positive Pearson correlations were observed between VEGF and CD31 (+0.367, p = 0.006), c-fos (+0.569, p < 0.001) and c-myc (+0.473, p < 0.001), with regression curve fit of f = 16.144 and p < 0.001. Figure 2 illustrates the correlation curve of VEGF vs. c-fos. Increased c-fos expression was associated with increased levels of VEGF. Also, there was a significantly positive correlation between VEGF expression and stage of tumour (+0.483, p < 0.001) and tumour metastasis (+0.314, p = 0.021). The correlation/regression analysis indicated a significantly positive correlation of VEGF and clinical osteosarcoma staging (Pearson correlation +0.324, p = 0.036; Fig. 3).
Vascular endothelial growth factor (VEGF) expression correlates with tumour stages \( \left( {{\text{f}} = {\text{y}}0 + {\text{a}} * {\text{x}}} \right) \). Tumour stages are converted to numerical numbers for statistical analysis purpose: stage II-A converts to 2.1, III-A to 3.1; where stage II-B converts to 2.5, III-B to 3.5, etc. Though VEGF expression at each level correlates with multiple stages of osteosarcoma, linear regression analysis overall does show a positive correlation between stage of osteosarcoma and VEGF expression (p < 0.01)
Discussion
Osteosarcoma is a highly malignant bone tumour that usually affects adolescents and young adults. Advances in multimodality treatments consisting of aggressive adjuvant chemotherapy and wide tumour resection have markedly improved the prognosis of patients with osteosarcoma [1]. Nearly half of the patients, however, suffer therapeutic failures [2]. Treatment of osteosarcoma patients, especially those who have acquired resistance to current chemotherapy protocols, remains a challenge. Although the precise mechanisms that regulate invasion of tumour cells into adjacent tissues, the microcirculatory system, and lymphogenic or haematogenic dissemination with subsequent extravasation and formation of secondary tumour foci are still unclear, it appears that high metastatic potential and high recurrence rate of these tumours are associated with high levels of vascularisation [3]. Indeed the growth, progression, and metastasis of all solid tumours are dependent on neovascularisation [14]. Tumour cells absolutely require a permanent supply of new blood vessels to nourish their growth and facilitate metastasis. Clinical studies suggest the density of intratumoural microvessels correlates well with the grade of invasiveness, the frequency of metastasis, and clinical prognosis in many types of cancer [15, 16].
Intratumoural microvessel angiogenic cascade can be divided into the pre-vascular phase and the vascular phase [17, 18]. Transition into the latter phase permits a rapid rate of tumour growth and increases metastatic potential. The development of tumour angiogenesis is believed to be dependent on the net balance between the actions of angiogenesis promoters and inhibitors. Pro-angiogenic factors are up-regulated in tumours, and such up-regulation has been linked to poor prognosis [19]. It appears the pro-angiogenic factors within solid tumours stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis. A large number of pro-angiogenic factors have been identified including basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor beta-1 (TGFβ1), transforming growth factor alpha (TGFα), and epidermal growth factor (EGF) [18]. Perhaps the best characterised pro-angiogenic factor is vascular endothelial growth factor (VEGF) which is relatively unique among growth factors in terms of its specificity for the vascular endothelium [4]. Not only is VEGF a diffusible endothelium-specific mitogen and angiogenic factor, it also increases vascular permeability [5] and stimulates/maintains neovascularisation in a variety of tumour types [20]. Indeed, enhanced VEGF gene expression has been identified in a number of malignant tumours from breast, lung, ovarian, liver, and colon cancer in comparison with normal tissue [6–10].
Unfortunately, there were few conclusive studies on the correlations of VEGF expression and osteosarcoma, except some that showed osteosarcoma patients who expressed high levels of VEGF but exhibited a poorer prognosis [11, 12]. Kaya et al., after examining 27 primary osteosarcomas and the correlation between expression of VEGF and microvessels, clinical-pathological variables, and survival of patients, suggest that patients with VEGF-positive osteosarcoma have a significantly higher lung metastasis rate and poorer chances of survival [12]. The sample size of Kaya et al., however, was relatively small (n = 27), and their study did not pursue the downstream molecular events. In this study, we examined and compared 54 cases of clinical osteosarcoma to correlate the VEGF expression levels in the primary tumours with the clinical stages, treatments and outcomes, tumour remission and recurrence, as well as metastasis and subject survival. Our data clearly indicate that over-expression of VEGF significantly correlated with the advance of the disease stage. In addition, elevated VEGF also suggested poorer prognosis such as more frequent incidence of metastasis and higher tumour recurrence rate, which appeared in agreement with the previous findings [11, 12].
With the expansion of molecular biology techniques, genetic alterations in the development and metastasis of malignant tumours have been observed. These alterations may result in increased or deregulated gene regulation. Although numerous oncogenes and tumour suppressor genes have been identified in osteogenic sarcomas, a select few seem to be expressed with relatively high incidence: c-myc proto-oncogene on Chromosome 8 encodes a transcription factor which is involved in the regulation of cell growth, DNA replication, and transcriptional regulation of specific target genes [21]. The c-fos proto-oncogene, a cellular homologue of v-fos, is associated with many biological processes ranging from transformation to cell-cycle progression and cell differentiation [22]. In particular, it seems that c-fos oncogene is involved in osteoblast and chondrocyte differentiation [22]. In osteosarcoma studies, c-myc and c-fos were suggested over-expressed in a relatively high percentage of relapsed tumours and metastases [22–25]. However, there was no report exploring the correlations of VEGF expression and these oncogenes. Data from this study have confirmed that the expression of c-fos and c-myc were highly correlated with the expression of VEGF, suggesting the influence of VEGF may associate with the c-fos and c-myc pathways in promoting tumour growth and advances. Further studies are warranted to explore further the potential molecular signalling pathways.
Since formation of solid tumours is angiogenesis-dependent, several strategies have been developed for targeting the VEGF pathway as a prospective anti-cancer therapy. Potential approaches for blocking VEGF action include inhibiting secretion of endogenous tumour VEGF, neutralising VEGF in the microcirculation, preventing VEGF binding to its receptor, and subsequent signal transduction. Takayma et al. [26] have surmised from a series of animal experimental data that suppression of tumour growth by inhibiting VEGF or its receptor can be achieved using (a) neutralising antibodies to VEGF; (b) a blocking antibody to VEGF receptor; (c) antisense oligonucleotides against VEGF; (d) an antisense VEGF expression plasmid; (e) a VEGF-diphtheria toxin conjugate; (f) a truncated VEGF receptor that inhibits the functioning of the wild-type receptor in a dominant negative fashion; and (g) a soluble form of VEGF receptor. We have also developed a mouse model of orthotopic osteosarcoma as a useful tool to evaluate the molecular interactions between alteration of VEGF effects and osteosarcoma progression [27]. Data from our laboratory has shown that virus-mediated soluble VEGF receptor-1 (sFlt-1) significantly halted experimental osteosarcoma growth [28]. These animal studies lend further support to the idea that VEGF plays a critical role in tumour angiogenesis, and indicate the potential of anti-VEGF treatment as a means of tumour suppression.
Conclusions
Results of this study suggest that there is a positive correlation with osteosarcoma staging and tumour metastasis. Further, osteosarcoma stage at diagnosis was positively correlated with metastatic potential and negatively affected disease remission. Data also suggest that VEGF expression positively associates with c-fos and c-myc expressions. Therefore, development of therapies targeting the VEGF pathway for osteosarcoma may lead to improved survival rates when combined with current treatment modalities.
There were limitations to this study, however. First, a larger sample size would have been desirable to increase the power of the study. The minimum power needed was reached for this study, but increased numbers would have been beneficial in showing stronger correlations. Second, given the limited number of cases, individual subtype analysis could not be performed. Therefore, subtype VEGF expression was not analysed.
References
Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ (2009) Osteosarcoma. J Am Acad Orthop Surg 17(8):515–527
Rosen G, Suwansirikul S, Kwon C, Tan C, Wu SJ, Beattie EJ Jr, Murphy ML (1974) High-dose methotrexate with citrovorum factor rescue and adriamycin in childhood osteogenic sarcoma. Cancer 33(4):1151–1163
Coomber BL, Denton J, Sylvestre A, Kruth S (1998) Blood vessel density in canine osteosarcomas. Can J Vet Res 62(3):199–204
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT (1989) Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246(4935):1309–1312
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246(4935):1306–1309
Sallinen H, Anttila M, Narvainen J, Koponen J, Hamalainen K, Kholova I, Heikura T, Toivanen P, Kosma VM, Heinonen S, Alitalo K, Yla-Herttuala S (2009) Antiangiogenic gene therapy with soluble VEGFR-1, -2, and -3 reduces the growth of solid human ovarian carcinoma in mice. Mol Ther 17(2):278–284. doi:10.1038/mt.2008.258
Jia ZZ, Jiang GM, Feng YL (2011) Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J (Chung-kuo i hsueh k'o hsueh tsa chih/Chinese Academy of Medical Sciences) 26(3):158–162
Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA (2009) Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther 8(7):1761–1771. doi:10.1158/1535-7163.MCT-09-0280
Tokunaga T, Oshika Y, Abe Y, Ozeki Y, Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Ueyama Y, Tamaoki N, Nakamura M (1998) Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer 77(6):998–1002
Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, Yang PC, Lee YC (2001) Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol 19(2):432–441
Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, Fukuda H, Nakano H, Abe S, Tateishi A, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y, Nakamura M (1999) Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer 35(7):1089–1093
Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F, Ishii S (2000) Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 6(2):572–577
Ben Josef E, Yang SY, Ji TH, Bidart JM, Garde SV, Chopra DP, Porter AT, Tang DG (1999) Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J Urol 161(3):970–976
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1(1):27–31
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J (1993) Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 143(2):401–409
Weidner N, Folkman J (1996) Tumoral vascularity as a prognostic factor in cancer. Important Adv Oncol 167–190
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86(3):353–364
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. Oncologist 5(Suppl 1):3–10
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18
Suzuki K, Hayashi N, Miyamoto Y, Yamamoto M, Ohkawa K, Ito Y, Sasaki Y, Yamaguchi Y, Nakase H, Noda K, Enomoto N, Arai K, Yamada Y, Yoshihara H, Tujimura T, Kawano K, Yoshikawa K, Kamada T (1996) Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res 56(13):3004–3009
Cole MD, McMahon SB (1999) The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene 18(19):2916–2924
van den Berg S, Rahmsdorf HJ, Herrlich P, Kaina B (1993) Overexpression of c-fos increases recombination frequency in human osteosarcoma cells. Carcinogenesis 14(5):925–928
Wu JX, Carpenter PM, Gresens C, Keh R, Niman H, Morris JW, Mercola D (1990) The proto-oncogene c-fos is over-expressed in the majority of human osteosarcomas. Oncogene 5(7):989–1000
Gamberi G, Benassi MS, Bohling T, Ragazzini P, Molendini L, Sollazzo MR, Pompetti F, Merli M, Magagnoli G, Balladelli A, Picci P (1998) C-myc and c-fos in human osteosarcoma: prognostic value of mRNA and protein expression. Oncology 55(6):556–563
Pompetti F, Rizzo P, Simon RM, Freidlin B, Mew DJ, Pass HI, Picci P, Levine AS, Carbone M (1996) Oncogene alterations in primary, recurrent, and metastatic human bone tumors. J Cell Biochem 63(1):37–50
Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N (2000) Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 60(8):2169–2177
Yang SY, Yu H, Krygier JE, Wooley PH, Mott MP (2007) High VEGF with rapid growth and early metastasis in a mouse osteosarcoma model. Sarcoma 2007:95628
Yin D, Jia T, Gong W, Yu H, Wooley PH, Mott MP, Yang SY (2008) VEGF blockade decelerates the growth of a murine experimental osteosarcoma. Int J Oncol 33(2):253–259
Author information
Authors and Affiliations
Corresponding author
Additional information
Meghan Patni and Elizabeth Rinehart contributed equally in this study
Rights and permissions
About this article
Cite this article
Lammli, J., Fan, M., Rosenthal, H.G. et al. Expression of Vascular Endothelial Growth Factor correlates with the advance of clinical osteosarcoma. International Orthopaedics (SICOT) 36, 2307–2313 (2012). https://doi.org/10.1007/s00264-012-1629-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1629-z