Abstract
Vascular endothelial growth factor (VEGF) has been linked with tumor invasion and metastasis. However, the role of VEGF expression in osteosarcoma remains controversial. By searching the PubMed, Embase, and Google Scholar databases, we conducted a meta-analysis to evaluate the pathological and prognostic significance of VEGF in osteosarcoma. Studies were pooled, and the odds ratio (OR) and its corresponding 95 % confidence interval (CI) were calculated. Nine relevant articles were included in this meta-analysis study. We performed pooled analysis with available data on the association between VEGF expression and age, gender, tumor stages IIB–III versus I–IIA, tumor recurrence, response to chemotherapy, and tumor metastasis. Our results revealed that VEGF expression might be closely associated with metastasis of osteosarcoma (OR 4.74, 95 % CI 2.53–8.87, P < 0.001). Furthermore, our findings also demonstrated that patients with grade IIB–III osteosarcoma showed a higher frequency of VEGF expression than those with grade I–IIA osteosarcoma (OR 5.33, 95 % CI 2.03–13.98, P = 0.001). We failed to find the association between VEGF expression and age (OR 0.82, 95 % CI 0.44–1.53, P = 0.539), gender (OR 1.33, 95 % CI 0.52–3.42, P = 0.553), tumor recurrence (OR 1.47, 95 % CI 0.56–3.86, P = 0.429), and response to chemotherapy (OR 1.26, 95 % CI 0.14–11.72, P = 0.839). In conclusion, VEGF is related to the grade and metastasis of osteosarcoma. It may play a significant role in clinical guidelines for the treatment and prognostic evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma, also known as osteogenic sarcoma or OGS, is the most common type of bone cancer among children, adolescents, and young adults [1, 2]. Over the past decades, the combination of multiagent neoadjuvant chemotherapy has improved 5-year survival from 20 % to about 70 % in patients without metastasis [3, 4]. Although there has been significant improvement in the long-term outcome of these patients, 25–50 % of patients subsequently develop metastatic disease, which remains the major cause of death [4]. High metastatic potential and high recurrence rate are considered to be associated with high levels of vascularization and rapid growth of these tumors [5].
Tumor growth depends on the proliferation of new blood vessels in their interior, which are directly and indirectly related to the release of several growth factors both by the primary tumor and by the resulting metastatic lesions [6]. Vascular endothelial growth factor (VEGF) is a major angiogenic factor that induces endothelial cell proliferation and increases the permeability of the vascular endothelium [7, 8]. Targeted disruption of the VEGF gene has indicated that VEGF is indispensable to new blood vessel formation and growth [9].
Vascular endothelial growth factor is generally considered to play an important role in neovascularization of tumors. VEGF expression has been shown to be correlated with vascular density in invasive ductal carcinoma and human epidermoid lung carcinoma [10, 11]. Enhanced VEGF gene expression has been reported in a number of malignant tumor cell lines as well as in primary tumors from breast, lung, ovarian, liver, and colon cancer in comparison with normal tissue [10–15].
Vascular endothelial growth factor plays an important role in tumor angiogenesis through promoting endothelial cell growth and migration, and it has been supposed to be a biomarker of prognosis in patients with osteosarcoma [16, 17]. There are many studies assessing the prognostic role of VEGF expression in osteosarcoma, and no consistent outcomes are reported [18–26]. To provide a comprehensive assessment of the pathological characteristics and prognostic role of VEGF expression in osteosarcoma, we performed a meta-analysis of published studies.
Materials and methods
Search strategy
We searched for relevant studies up to December 2014 through the PubMed, Embase, and Google Scholar database with the following terms and their combinations: “osteosarcoma/osteogenic sarcoma” and “vascular endothelial growth factor/VEGF.” All scanned abstracts, studies, and citations were reviewed. Moreover, references of the retrieved manuscripts were also manually cross-searched for further relevant publications. Meta-analysis does not involve ethical review.
Selection criteria
The inclusion criteria included: (1) the articles in which the association between VEGF expression and the clinicopathological significance of osteosarcoma was evaluated; (2) the articles in which the association of VEGF expression and prognosis in patients with osteosarcoma was evaluated; and (3) the studies which utilized immunohistochemical staining for tissue VEGF expression. The exclusion criteria included: (1) the studies which used the same population or overlapping database and (2) the studies of in vitro cell culture models.
Data extraction
All the available data were extracted from each study by two investigators independently according to the inclusion criteria listed above. The study characteristics were recorded as follows: (1) the first author, country, and article publication year; (2) the number of cancer cases for positive VEGF expression, which was measured by semiquantitatively assessing the percentage of tumor cells expressing VEGF, intensity of cell staining, and extent of staining. (3) The following data were collected from each study: age, gender, immunohistochemical technique, and rate of VEGF-positive expression.
Statistical analysis
We estimated the odds ratio (OR) for clinicopathological variables (age, gender, tumor stages IIB–III vs. I–IIA, tumor recurrence, response to chemotherapy, tumor metastasis). Tumor staging was based on the Enneking classification [27]. The heterogeneity of the studies was assessed using the Cochran’s Q test (considered significant for P < 0.10) and was quantified by the I 2 statistic. Both fixed effects (Mantel–Haenszel) and random effects (Der Simonian and Laird) models were used to combine the data. Relative influence of each study on the pooled estimate was assessed by omitting one study at a time for sensitivity analysis. Publication bias was evaluated by visual inspection of symmetry of Begg’s funnel plot and assessment of Egger’s test (P < 0.05 was regarded as representative of statistical significance). Statistical analyses were done in STATA software, version 12.0 (STATA Corp., College Station, TX, USA), and all tests were two-sided.
Results
Characteristics of the studies
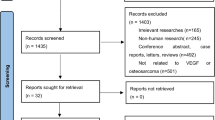
There were 181 papers relevant to the search words. Subsequently, 144 irrelevant articles were excluded. The remaining articles were systematically reviewed, and all 37 articles qualified for full-text reading. After full-text reading, 21 articles were deemed unsuitable and were therefore excluded, and 16 articles were identified to be included for qualitative analysis. In addition, another seven studies were excluded due to not present the usable data after a more careful assessment of the remaining articles. Finally, nine cohort studies composed of 383 OS samples were incorporated into the current meta-analysis. The flowchart of selection of studies and reasons for exclusion is presented in Fig. 1. The main characteristics of the eligible studies are given in Table 1.
Quantitative synthesis
All nine studies including 383 patients explored the association between VEGF expression and clinicopathological variables of osteosarcoma. We performed pooled analysis with available data on the association between VEGF expression and age, gender, tumor stages IIB–III versus I–IIA, tumor recurrence, response to chemotherapy, and tumor metastasis. We failed to find the association between VEGF expression and age (OR 0.82, 95 % CI 0.44–1.53, P = 0.539), gender (OR 1.33, 95 % CI 0.52–3.42, P = 0.553), tumor recurrence (OR 1.47, 95 % CI 0.56–3.86, P = 0.429), and response to chemotherapy (OR 1.26, 95 % CI 0.14–11.72, P = 0.839) (Figs. 2, 3 and 4). However, our results revealed that VEGF expression might be closely associated with metastasis of osteosarcoma (OR 4.74, 95 % CI 2.53–8.87, P < 0.001) (Fig. 4b). Furthermore, our findings also demonstrated that patients with grade IIB–III osteosarcoma showed a higher frequency of VEGF expression than those with grade I–IIA osteosarcoma (OR 5.33, 95 % CI 2.03–13.98, P = 0.001) (Fig. 3a).
Sensitivity analysis
We further conducted sensitivity analyses to determine whether review conclusions were affected by the choice of single study; the finding suggested that no single study had the effect on the pooled ORs in the current meta-analysis (Fig. 5).
Publication bias
Finally, the Egger’s regression test showed no evidence of asymmetrical distribution in the funnel plot in VEGF expression in gender (Begg’s test P = 0.266; Egger’s test P = 0.383) and tumor metastasis (Begg’s test P = 0.548; Egger’s test P = 0.262) (Fig. 6).
Discussion
Angiogenesis is essential for the growth of a tumor and its metastases [28]. VEGF is the most potent stimulator of angiogenesis with a specific mitogenicity for endothelial cells, which is crucial for both the growth of tumors and the progression with metastases [29]. Increased VEGF production has been shown to be important in the growth of various solid tumors in humans including osteosarcoma, gastric, esophageal, colorectal, renal, lung, and breast carcinomas [18, 30]. In osteosarcoma, patients with VEGF-positive tumors have poorer disease-free and overall survival compared with those with VEGF negative tumors [18, 31]. VEGF expression in pretreated osteosarcoma specimens is predictive of eventual development of pulmonary metastasis; further circulating VEGF levels by ELISA were found to be significantly higher in patients with osteosarcoma who had pulmonary metastasis [31]. Pathological characteristics and prognostic value of post-chemotherapy VEGF expression are largely unexplored. Therefore, in the current meta-analysis, we aimed to investigate whether VEGF expression may affect the pathological characteristics and prognosis in patients with osteosarcoma.
The results of our meta-analysis showed significant correlations of VEGF expression with stage of osteosarcoma (OR 5.33, 95 % CI 2.03–13.98, P = 0.001) and metastasis (OR 4.74, 95 % CI 2.53–8.87, P < 0.001) in osteosarcoma, implying that expression of VEGF protein may be a novel mediator in promoting tumor cell invasion and metastasis in osteosarcoma. Nevertheless, the precise mechanisms by which the enhanced expression of VEGF protein leads to an aggressive invasion and metastasis in the progression of osteosarcoma are still largely unknown. VEGF is a secreted mitogen and the dominant factor that regulates angiogenesis in both normal development and tumor growth [32]. During the process of hematogenous metastasis, it is suggested to play a pivotal role by participating in the regulation of angiogenesis [33]. This factor exerts its growth-promoting influence not on the tumor cells themselves but instead on the vascular endothelial cells, promoting both proliferation and new vessel formation. It is well demonstrated by Kaya et al. [18] that the expression of VEGF in primary osteosarcoma is correlated with an increase in local microvessel density in the tumor tissue, development of pulmonary metastasis, and poor prognosis for patients with osteosarcoma, which suggests that VEGF secreted by osteosarcoma cells elicits angiogenesis, which critically contributes to the development of pulmonary metastasis of osteosarcoma.
Vascular endothelial growth factor expression and survival of osteosarcoma have been investigated by several meta-analyses [34–36]. Compared with their work, we focus on the association of VEGF expression and pathological characteristics and prognosis of osteosarcoma, while they only analyzed survival of osteosarcoma. Recently, Rossi et al. [37] showed that the increase in VEGF expression after preoperative chemotherapy in patients positively correlated with relapse of osteosarcoma, lung metastases, and survival. Unfortunately, this meta-analysis showed no remarkable correlations between VEGF expression and relapse of osteosarcoma and response to neoadjuvant chemotherapy. These negative results might be due to that only three eligible studies with heterogeneity were analyzed. Therefore, more precise estimates of these relationships would be possible if more researches were included.
Meanwhile, some limitations in this meta-analysis should be noticed: First, the immunohistochemical method could affect the prognostic value due to the different detection antibodies and the application of different cutoff values for determining high VEGF levels. Second, there might be some biases if studies other than English were excluded. Third, our meta-analysis failed to obtain original data from the included studies, which may limit further evaluation of the potential role of VEGF expression in the progression and prognosis of osteosarcoma. Fourth, the relatively small sample sizes in the included studies result in that even very powerful prognostic factors may not have become significant.
In conclusion, despite the limitations of this meta-analysis, our study confirmed that the expression of VEGF might be closely associated with metastasis of osteosarcoma. In addition, patients with grade IIB–III osteosarcoma showed a higher frequency of VEGF expression than those with grade I–IIA osteosarcoma. Further studies with larger dataset and well-designed models are required to validate our findings.
References
Aung L, Tin AS, Quah TC, Pho RW. Osteogenic sarcoma in children and young adults. Ann Acad Med Singapore. 2014;43:305–13.
Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin N Am. 1997;44:973–89.
Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–61.
Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6.
Coomber BL, Denton J, Sylvestre A, Kruth S. Blood vessel density in canine osteosarcoma. Can J Vet Res. 1998;62:199–204.
Ribatti D, Vacca A, Dammacco F. The role of the vascular phase in solid tumor growth: a historical review. Neoplasia. 1999;1:293–302.
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–12.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9.
Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42.
Lee AH, Dublin EA, Bobrow LG, Poulsom R. Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol. 1998;185:394–401.
Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumor cell proliferation in human epidermoid lung carcinoma. Br J Cancer. 1996;73:931–4.
Berse B, Brown LF, Van De Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–20.
Boocock CA, Charnock-Jones DS, Sharkey AM, et al. Expression of vascular endothelial growth factor and its receptors and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–16.
Suzuki K, Hayashi N, Miyamoto Y, et al. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996;56:3004–9.
Tokunaga T, Oshika Y, Abe Y, et al. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77:998–1002.
Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012;8:961–87.
Portal-Nuñez S, Lozano D, Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol. 2012;27:559–66.
Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–7.
Jung ST, Moon ES, Seo HY, Kim JS, Kim GJ, Kim YK. Expression and significance of TGF-beta isoform and VEGF in osteosarcoma. Orthopedics. 2005;28:755–60.
Huang Y, Lin Z, Zhuang J, Chen Y, Lin J. Prognostic significance of alpha V integrin and VEGF in osteosarcoma after chemotherapy. Onkologie. 2008;31(10):535–40.
Mizobuchi H, García-Castellano JM, Philip S, Healey JH, Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin Orthop Relat Res. 2008;466(9):2052–9.
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011;20(1):13–9.
Lin F, Zheng SE, Shen Z, et al. Relationships between levels of CXCR4 and VEGF and blood-borne metastasis and survival in patients with osteosarcoma. Med Oncol. 2011;28:649–53.
Lammli J, Fan M, Rosenthal HG, et al. Expression of vascular endothelial growth factor correlates with the advance of clinical osteosarcoma. Int Orthop. 2012;36:2307–13.
Becker RG, Galia CR, Morini S, Viana CR. Immunohistochemical expression of vegf and her-2 proteins in osteosarcoma biopsies. Acta Orthop Bras. 2013;21(4):233–8.
Baptista AM, Camargo AF, Filippi RZ, Oliveira CR, Azevedo Neto RS, Camargo OP. Correlation between the expression of VEGF and survival in osteosarcoma. Acta Orthop Bras. 2014;22(5):250-5.
Jawad MU, Scully SP. In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system. Clin Orthop Relat Res. 2010;468:2000–2.
Folkman J. Seminars in medicine of the Beth Israel Hospital, Boston: clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–63.
Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–73.
Handa A, Tokunaga T, Tsuchida T, et al. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int J Oncol. 2000;17:291–5.
Kaya M, Wada T, Kawaguchi S, et al. Increased pre-therapeutic serum vascular endothelial growth factor in patients with early clinical relapse of osteosarcoma. Br J Cancer. 2002;86:864–9.
Oshika Y, Nakamura M, Tokunaga T. Expression of cell-associated isoform of vascular endothelial growth factor 189 and its prognostic relevance in non-small cell lung cancer. Int J Oncol. 1998;12:541–4.
Yin D, Jia T, Gong W, et al. VEGF blockade decelerates the growth of a murine experimental osteosarcoma. Int J Oncol. 2008;33:253–9.
Zhuang Y, Wei M. Impact of vascular endothelial growth factor expression on overall survival in patients with osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:1745–9.
Yu XW, Wu TY, Yi X, et al. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60.
Qu JT, Wang M, He HL, Tang Y, Ye XJ. The prognostic value of elevated vascular endothelial growth factor in patients with osteosarcoma: a meta-analysis and systemic review. J Cancer Res Clin Oncol. 2012;138:819–25.
Rossi B, Schinzari G, Maccauro G, et al. Neoadjuvant multidrug chemotherapy including high-dose methotrexate modifies VEGF expression in osteosarcoma: an immunohistochemical analysis. BMC Musculoskelet Disord. 2010;11:34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Han, G., Wang, Y., Bi, W. et al. Effects of vascular endothelial growth factor expression on pathological characteristics and prognosis of osteosarcoma. Clin Exp Med 16, 577–584 (2016). https://doi.org/10.1007/s10238-015-0382-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0382-1