Abstract
Purpose
The aims of this study were to systematically review the medical literature, in order to find controlled studies about microfracture in the treatment of patients with full-thickness cartilage lesions of the knee, to statistically combine these studies in order to determine a best estimate of the average treatment effect, and to gather information to detect cartilage-specific and patient-specific factors that might have an influence on the clinical outcome.
Methods
We searched four electronic databases for controlled clinical trials or controlled prospective observational studies. We pooled before/after-data of study arms using the term microfracture.
Results
We calculated an overall best estimate of 1.106, with [0.566; 1.646] as 95% confidence interval of the mean standardized treatment effect for a representative patient population.
Conclusions
Our meta-analysis revealed a clinically relevant improvement of the postoperative clinical status as compared to the preoperative status. An increase of 22 overall KOOS points may provide a rough estimate for the mean expected treatment effect achieved by microfracturing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage lesions are a common pathology of the knee joint. In a retrospective analysis of 25,124 knee arthroscopy patients chondral lesions were found in 60% of the patients [1]. A total of 7% of all patients analysed under the age of 40 and 9% under the age of 50 years showed one to three localized grade III or IV cartilage defects according to Outerbridge et al. [2]. Full-thickness articular cartilage defects only have limited regenerative potential. Thus, spontaneous healing is unlikely [3–5]. Untreated full-thickness cartilage lesions are usually associated with significant pain and swelling. Moreover, patients have an increased risk of subsequent osteoarthritis [6–10] which is a major cause of disability and represents a significant socioeconomic burden [9, 11, 12]. Conservative therapy includes physical measures (physiotherapy, weight loss, and bracing) as well as medical treatment of cartilage lesions, e.g. oral administration or intra-articular injection of hyaluronic acid or chondroprotective agents (such as D-glucosamine sulphate, chondroitin sulphate, diacerein) [13–16]. Unfortunately, none of these therapies can heal cartilage defects, but only relieve symptoms and improve knee function [14, 17, 18]. Surgery is primarily indicated for patients with grade III or IV cartilage defects according to Outerbridge. Surgical techniques include reparative marrow-stimulation techniques, i.e. migration of bone marrow cells into the cartilage defect, as well as restorative techniques using autografts, allografts, or synthetic material [19, 20]. These techniques have been shown to significantly relieve symptoms and improve function [13] and generally provide better results in the treatment of defects in the femoral condyles rather than in the patellofemoral compartment [16]. However, up to now no treatment option has proven to be superior to others in terms of efficacy and safety [21].
Microfracture treatment is a single-stage arthroscopic procedure that has gained popularity over the past two decades due to its minimally invasive approach, technical simplicity, limited surgical morbidity, and low costs [6, 23]. Because of these benefits and the fact that this technique does not rule out other cartilage repair procedures that might be needed in future [23], microfracture has become the treatment of choice for patients with knee cartilage defects of grade III or IV according to Outerbridge [24, 25]. Out of 150,000–200,000 US Americans who undergo knee surgery due to cartilage lesions every year, an estimated 60,000 are treated with this technique [26]. Moreover, microfracture has been shown to be the preferred method for the treatment of articular cartilage defects in recreational and professional athletes (including high-impact sports such as basketball, American football, soccer, and rugby) [27].
Numerous reviews about the microfracture technique have already been published, but to our knowledge no quantitative analysis of this technique has been performed so far. Being able to establish an average difference of the pre- and post-operative score values for this technique would be helpful for patients and clinicians. Therefore, the objective of our study was to systematically review medical literature in order to find controlled studies about microfracture in the treatment of patients with full-thickness cartilage lesions of the knee and statistically combine these studies in order to determine a best estimate of the average treatment effect. Furthermore, information was gathered to detect cartilage-specific and patient-specific factors that might have an influence on the clinical outcome.
Methods
We searched the electronic databases MEDLINE, EMBASE, CINAHL, and Cochrane Central Register of Controlled Trials. Our literature search was completed on 1 May 2011. As “microfracture” has been the generally accepted term for this technique since its introduction by Steadman [28] in 1980, we decided to use this search term. No language restrictions were applied. For the evaluation of clinical outcomes we used clinical scores, because their subjective variables referring to symptoms and function are highly associated with patient satisfaction [29, 30]. Our eligibility criteria are presented in Table 1.
First of all, two clinicians separately analysed the studies derived from our database research on the basis of the study title or abstract, respectively. Studies failing to meet the selection criteria (original paper, microfracture of the knee, outcome evaluation) and duplicates were excluded. Subsequently, full-text versions of the remaining 38 papers were obtained for detailed evaluation. Two reviewers then independently extracted all relevant information about outcome-related demographic data. Furthermore, they determined all eligible studies and independently evaluated their internal validity (risk of bias). For controlled trials we assessed the method of randomization, concealment of allocation, blinding, drop-out rates, as well as method of statistical analysis. For observational studies we used the criteria proposed by Deeks et al. [31]. Studies with a high risk of bias were excluded from further analyses and discrepancies were resolved by consensus. Finally, data on study characteristics and study design, patient age, defect size, etiology and localization, duration of symptoms, previous and concomitant surgeries, clinical follow-up, as well as pre- and postoperative clinical score values were extracted.
Statistical analysis
Because control procedures across included studies were heterogeneous, we could not perform a meta-analysis of the treatment effects of microfracture as compared to the control procedures. In order to determine a best estimate of the average expected treatment effect, we pooled the before/after-data of study arms using microfracture. Due to the fact that studies used different scales in the measurement of functional improvements, we standardized treatment effects using paired standardized mean differences. We assumed a pre/post-test correlation of 0.5.
Results
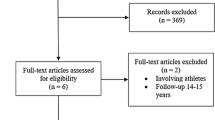
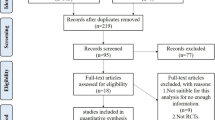
Figure 1 shows the selection process for eligible studies. Out of 1,030 citations obtained from electronic literature search, 38 original papers were suitable to determine cartilage- and patient-specific factors that might have an influence on the clinical outcome.
Reparative microfracture does not induce growth of normal hyaline cartilage. The quality of the repair tissue consisting of type-I, type-II, and type-III collagen [32] varies from fibrocartilage [33] alone to a mixture of fibrocartilage and hyaline-like cartilage [34–39]. Bae et al. [34], Gill et al. [40, 41], Miller et al. [42], Pässler et al. [43] and Steadman et al. [38, 44, 45] presume that the original defect filling will become stable over time and perform the function of hyaline cartilage, whereas Gudas et al. [33] and Gobbi et al. [35] express the opinion that the hybrid tissue does not support weight bearing in the long term. A decrease in score values was observed after 18 [46, 47] and 24 months [36, 37, 48, 49], respectively. However, according to Blevins et al. no deterioration of results was observed within three years after surgery [28].
Several studies conclude that microfracture is not effective for the treatment of large lesions [38, 48, 50, 51]. Miller et al. [42] reported that significantly less improvement could be achieved after treatment of defects larger than 400 mm² as compared to smaller lesions. According to Gudas et al. [33, 52], patients with small lesions (< 200 mm²) had a significantly better treatment effect than those with large defects (≥ 200 mm²). Asik et al. [53] reported equivalent results, as their relevant Spearman coefficient between defect size and Lysholm score was −0.538 (p < 0.001).
Asik et al. [53] and Steadman et al. [38] reported significantly better treatment effects for patients under the age of 35 years as compared to older patients and showed that Lysholm score values decrease with age. Asik et al. [53] observed a Spearman coefficient of −0.623 (p < 0.001). Steadman et al. [38] found a Pearson coefficient of −0.28 (p < 0.018). Furthermore, a multivariate analysis (R² = 0.193) by Steadman et al. [38] revealed age as the sole moderator with a regression coefficient of −0.299 (p = 0.011). According to Gudas et al. [33] and Knutsen et al. [37], patients younger than 30 years of age, and according to Kreuz et al. [46], patients younger than 40 years of age, respectively, had a significantly better clinical and functional outcome than older patients. However, no significant correlation was found between clinical scores and patient age in the studies of Miller et al. [42] and Kon et al. [51].
While Gill et al. [41, 54], Miller et al. [42], Mithoefer et al. [49] and Steadman et al. [38] found no influence of defect localization on the clinical outcome, Gobbi et al. [35] and Kreuz et al. [47] observed the best results in chondral lesions on the femoral condyles and consistently poor results at the patella. Poorer outcomes were observed in patients with degenerative defects as compared to traumatic lesions [35] and patients with traumatic lesions had significantly better clinical results than those with osteochondrosis dissecans [52]. In patients with higher preoperative activity levels (Tegner score [55] >4) significantly better clinical results were observed [28, 37]. Professional athletes had a higher repair cartilage fill volume [28] which was found to play a critical role in the postoperative functional improvement [56]. Furthermore, shorter preoperative duration of symptoms (<12 months) also significantly affected the clinical outcome [48]. Athletes without prior surgical intervention were more likely to return to high-impact sports than those who had undergone previous knee surgery [49]. Finally, it has been shown that it is more difficult to regenerate cartilage of hyaline-like quality when the patient has had previous knee surgery or a chronic defect [57]. Surprisingly, no statistically significant differences were observed between patients with and without anterior cruciate ligament [28, 35] or meniscus repair [35].
Five papers, i.e. four randomized controlled trials and one cohort study (the allocation of patients was determined by health care and insurance policy), met our eligibility criteria for statistical analysis. These papers are presented in Table 2. They include data of 187 patients between 15 and 60 years of age with chondral defects of 1–10 cm² and a follow-up period of two to five years. Concomitant surgery refers to anterior cruciate ligament reconstruction and surgical repair of meniscal tears. Osteoarthritis and valgus or varus deformities of more than 5° were exclusion criteria for all five papers. Thus, neither subgroup analysis nor regression analysis could be performed due to the small number of controlled studies.
All patients were treated with the microfracture technique as described by Steadman et al. [58]. After surgery, they followed similar rehabilitation protocols, i.e. full weight-bearing was not allowed for four to eight weeks post-operatively (see Table 3). For the evaluation of the surgical outcome the Lysholm Score [59] was applied by Knutsen et al. and Basad et al. However, Gudas et al. and Kon et al. used the IKDC score [60] (2000 IKDC Subjective Knee Evaluation Form, Patients’ Part), whereas Saris et al. used the KOOS [61] questionnaire. Saris determined the overall KOOS rating as the average of sub-scores “function in daily living”, “pain”, “symptoms/stiffness”, and “quality of life”. Sub-score “sports” was excluded from the overall KOOS analysis, as patients were significantly limited in sports activities so that hardly any data were available for this sub-domain. Clinical knee scores evaluate comparable parameters, but rate them differently. Therefore, score values of one particular patient can vary considerably depending on the system chosen [62–65]. For a better comparability of the individual treatment effects, the individual paired standardized mean differences were calculated and combined to the overall best estimate of 1.700 with [0.889; 2.511] as 95% confidence interval measured in units of standard deviation of the difference scores [66]. All data are presented in Fig. 2.
Due to the fact that the results of Gudas et al. differ significantly from those of Basad et al., Knutsen et al., Kon et al. and Saris et al. we excluded them from our further analysis, resulting in an overall best estimate of 1.106 with [0.566; 1.646] as 95% confidence interval (see Fig. 3).
In any case, both analyses report a mean standardized effect size of more than 0.8, which has to be considered as “large” according to Cohen [67].
As we wanted to convert the summarized effect back into the respective scales of the numerical scores, we contacted the authors of the five relevant papers and asked for the standard deviations referring to the differences in the pre- and postoperative score values. Unfortunately, we only received one reply by D.B.F. Saris so that we could not calculate a clinically meaningful best estimate of the treatment effect achieved by microfracture.
Discussion
Our statistical analysis summarized five controlled studies about the microfracture technique. The forest plot in Fig. 2 shows that the paired standardized mean differences of four studies are comparable, whereas the results of Gudas et al. are significantly better. Notably, their patients belong to a clearly defined homogenous target group. All patients were either highly competitive sportsmen (40%) or well-trained and athletic people (60%) under the age of 40 years with condylar lesions of less than 4 cm². Including these athletes in the analysis of a diverse patient population would unjustifiably increase the mean expected treatment effect resulting in higher expectations that might not be fulfilled by the microfracture technique. Without the patients of Gudas et al., the best estimate of the paired mean standardized difference decreases from 1.700 to 1.106, which in our opinion, seems to be the relevant value for a representative patient population.
In order to provide an approximate clinically significant value of the mean expected treatment effect by microfracture, we performed a rough calculation by multiplying the standard deviation of the difference scores (20.16) measured as overall KOOS points with the standardized best estimate (1.106). According to the definition of the paired standardized mean difference [66], 22 overall KOOS points with a 95% confidence interval of [11; 33] represent a rough estimate of the mean expected treatment effect. As eight to ten KOOS points are suggested for a minimal perceptible clinical improvement (the difference on the measurement scale associated with the smallest change in the health status noticeable by the patient) [68], our rough estimate of the lower limit of the 95% confidence interval suggests at least a clinically significant improvement is achieved by the microfracture technique.
Limitations of our meta-analysis include the fact that failures (patients requiring revision surgery due to persistent or recurrent symptoms using the same or another surgical technique) were weighed differently by the authors. While Basad et al. excluded data of failures from the analysis a priori, they were considered by Gudas et al., Knutsen et al., Kon et al. and Saris et al. Their last recorded clinical follow-up score value before revision surgery was considered as their final clinical score value. Moreover, only Knutsen et al. and Saris et al. started their evaluation with all patients randomized to microfracture and did not exclude those who did not return for post-operative evaluation (see Table 2). Because deterioration of initial functional improvement has been reported between 18 and 24 months after microfracture [36, 37, 46–49], the fact that the final outcome evaluation of the five controlled studies was not performed at the same time point must also be considered a limitation, as well as the similar but not identical rehabilitation protocols. Interestingly, Steadman’s [45] physiotherapy protocol includes the use of a continuous passive motion machine (any impact on the outcome could not be proven [69]) already in the recovery room. For lesions on the femoral condyle Steadman insists on crutch-assisted touch-down weight bearing for six to eight weeks with only exceptional bracing. In contrast, weight bearing as tolerated is allowed for patients with patellofemoral lesions treated with the microfracture technique. However, these patients have to wear a brace for at least eight weeks to prevent excessive shear forces on the maturing marrow clot.
We are aware that our rough calculation should be interpreted cautiously. Comprehensive, well-designed, long-term multicenter studies are required to evaluate clinical outcomes across study populations, including subgroup analysis to identify the predicting factors that can lead to functional deterioration after microfracture. Clearly defined indications would help to find out which patients will benefit most from this technique and which patients will probably not achieve permanent improvement. By allocating the latter to an alternative treatment option early failures would be avoided.
Conclusions
A calculation of 1.106 as the appropriate mean standardized treatment effect to be expected by means of meta-analysis of 187 patients (between 15 and 60 years of age with full-thickness chondral defects of 1–10 cm²) revealed a clinically relevant improvement of the post-operative clinical status as compared to the pre-operative status. An increase of 22 overall KOOS points may provide a rough estimate for the mean expected treatment effect achieved by microfracturing.
References
Widuchowski W, Widuchowski J, Trzaeka T (2007) Articular cartilage defects: study of 25,124 knee arthroskopies. Knee 14:177–182
Outerbridge HK, Outerbridge AR, Outerbridge RE (1995) The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am 77:65–72
Kaneshiro N, Sato M, Ishihara M, Mitani G, Sakai H, Kikuchi T, Mochida J (2007) Cultured articular chondrocytes sheets for partial thickness cartilage defects utilizing temperature-responsive culture. Eur Cell Mater 13:87–92
Buckwalter JA, Lane NE (1997) Athletics and osteoarthritis. Am J Sports Med 25:873–881
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75:542–553
Safran MR, Seiber K (2010) The evidence for surgical repair of articular cartilage in the knee. J Am Acad Orthop Surg 18(5):259–266
Buckwalter JA (2002) Articular cartilage injuries. Clin Orthop Relat Res 402:21–37
Curl WW, Krome J, Gordon ES, Rushing J, Patterson-Smith B, Poehling GG (1997) Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy 13:456–460
Davies-Tuck ML, Wluka AE, Wang Y, English DR, Giles GG, Cicuttini FM (2008) The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthr Cartil 16:337–342
Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ (2000) Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med 133:321–328
Merx H, Dreinhöfer KE, Günther KP (2007) Socioeconomic relevance of osteoarthritis in Germany. Z Orthop Unfall 145(4):421–429
Woolf A, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81(9):646–656
Henn RF, Gomoll AH (2011) A review of the evaluation and management of cartilage defects in the knee. Phys Sportmed 39(1):101–107
Fritz J, Janssen P, Gaissmaier G, Schewe B, Weise K (2008) Articular cartilage defects in the knee—basics, therapies and results. Injury 39S1:S50–S57
Kirkley A, Webster-Bogart S, Lichtfeld R, Amendola A, MacDonald S, McCalden R, Fowler P (1999) The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am 81(4):539–548
Gomoll AH, Farr J, Gillogly SD, Kercher JS, Minas T (2010) Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am 92(14):2470–2490
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G (2006) Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2:CD005328
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G (2006) Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2:CD005321
Cole BJ, Pascual-Garrido C, Grumet RC (2009) Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am 91:1778–1790
Farr J, Cole BJ, Dhawan A, Kercher JS, Sherman S (2011) Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res 469(10):2696–2705. doi:10.1007/s11999-010-1764-z
Magnussen RA, Dunn WR, Carey LC, Spindler KP (2008) Treatment of focal cartilage defects in the knee. Clin Orthop Relat Res 466:952–962
Gill TJ, Asnis PD, Berkson EM (2006) The treatment of articular cartilage defects using the microfracture technique. J Orthop Sports Phys Ther 36(10):728–738
Williams JR 3rd, Harnly HW (2007) Microfracture: indications, technique, and results. AAOS Instructional Course Lectures 56:419–428
Bekkers JEJ, Inklaar M, Saris DBF (2009) Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med 37:148S–155S
Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K (2010) Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage 1(2):145–152. doi:10.1177/1947603510366718
Agnvall E (2007) Joint initiatives. The Washington Post, 4 December 2007
Mithoefer K, Gill TJ, Cole BJ, Williams RJ, Mandelbaum BR (2010) Clinical outcome and return to competition after microfracture in the athlete’s knee: an evidence-based systematic review. Cartilage 1(2):113–120. doi:10.1177/1947603510366576
Blevins FT, Steadman JR, Rodrigo JJ, Silliman J (1998) Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics 21:761–768
Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterret WI, Hawkins RJ (2002) Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Surg (Am) 9(84):1560–1572
Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterret WI, Hawkins RJ (2002) Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Surg Am 9(84):1560–1572
Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG (2003) Evaluating non-randomised intervention studies. Health Technol Assess 7(iii-x):1–173
Frisbie DD, Trotter GW, Powers BE, Steadman JR, Howard RD, Park RD, McIlwraith CW (1999) Arthroscopic subchondral bone plate microfracture technique augments healing of large osteochondral defects in the radial carpal bone and medial femoral condyle of horses. J Vet Surg 28(4):242–255
Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ (2006) Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc 14:834–842
Bae DK, Yoon KH, Song SJ (2006) Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy 22(4):367–374
Gobbi A, Nunag P, Malinowski K (2005) Treatment of chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc 13:213–221
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigson TC et al (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E et al (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86A(3):455–464
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 19(5):477–484
Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ (2003) The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg 16:83–86
Gill TJ, MacGillivray JD (2001) The technique of microfracture for the treatment of articular cartilage defects in the knee. Oper Tech Orthop 11:105–107
Gill TJ (2000) The treatment of articular cartilage defects using microfracture and debridement. Am J Knee Surg 13:33–40
Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG (2004) Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg 17(1):13–17
Pässler HH (2000) Die Mikrofrakturierung zur Behandlung von Knorpeldefekten. Zentralbl Chir 125:500–504
Rodrigo JJ, Steadman JR, Silliman JF, Fulstone HA (1994) Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg 7:109–116
Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391(Suppl):362–369
Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P et al (2006) Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 22(11):1180–1186
Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N (2006) Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 14:1119–1125
Mithoefer K, Williams RJ, Warren RF, Potter H, Spock C, Jones E et al (2005) The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 87:1911–1920
Mithoefer K, Williams RJ, Warren RF, Wickiewicz TL, Marx RG (2006) High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med 34:1413–1419
Mithoefer K, Williams RJ, Warren RF (2006) Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am 88:294–304
Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M (2009) Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Am J Sports Med 37:33–41
Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, Smailys A (2005) A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 21(9):1066–1075
Asik M, Ciftci F, Sen C, Erdil M, Atalar A (2008) The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy 24:1214–1220
Gill TJ (2000) The role of the microfracture technique in the treatment of full-thickness chondral injuries. Oper Tech Sports Med 8:138–140
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR (2009) Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37:2053–2063. doi:10.1177/0363546508328414
Saris DB, Dhert WJ, Verbout AJ (2003) Joint homeostasis: the discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br 85(7):1067–1076
Steadman JR, Rodkey WG, Singleton SB, Briggs KK (1997) Microfracture technique for full thickness chondral defects: technique and clinical results. Operat Tech Orthop 7:300–304
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10:150–154
ICRS (2000) IKDC subjective knee evaluation form. www.cartilage.org. Accessed 22 September 2011
KOOS (2001) KOOS questionnaire. www.koos.nu. Accessed 22 September 2011
Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ (1995) Critical analysis of knee ligament rating systems. Am J Sports Med 23:660–667
Labs K, Paul B (1997) To compare and contrast the various evaluating scoring systems after anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg 116:92–96
Bollen S, Seedhom BB (1991) A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med 19(2):189–190
Peters G, Wirth CJ, Kohn D (1997) Vergleich von Scores und Bewertungsschemata bei Knieinstabilitäten. Z Orthop 135:63–69
Borenstein M, Hedges L, Higgins J, Rosenstein H (2009) Introduction to meta-analysis. Wiley, Chichester
Cohen J (1969) Statistical power analysis for the behavioral sciences (1st edition). Academic, New York
Roos EM, Lohmander LS (2003) The Knee Injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 1(1):64
Marder RA, Hopkins G, Timmerman LA (2005) Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy 21(2):152–158
Acknowledgement
We would like to thank Daniel B.F. Saris for providing unpublished data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negrin, L., Kutscha-Lissberg, F., Gartlehner, G. et al. Clinical outcome after microfracture of the knee: a meta-analysis of before/after-data of controlled studies. International Orthopaedics (SICOT) 36, 43–50 (2012). https://doi.org/10.1007/s00264-011-1364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-011-1364-x