Abstract
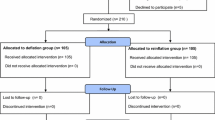
We included 46 total knee arthroplasties (43 patients) in a prospective, randomised study, dividing them into two groups: group A (23 knees, 21 patients) in which the ischaemia was released prior to wound closure allowing control of bleeding and group B (23 knees, 22 patients) releasing the tourniquet after suturing and bandaging. We compared the haemoglobin before surgery and at 24 and 48 h postoperatively, the total blood loss and the transfusions that were needed. Student's t-test was used to analyse the data. The results we obtained were as follows: preoperative haemoglobin in group A was 14.21 g/dl and group B 14.28 g/dl; haemoglobin at 24/48 h for group A was 10.04/10.1 g/dl and group B 10.28/10.3 g/dl; total blood loss was 743.2 cc for group A and 692.5 cc for group B; the mean number of blood units transfused were 2 in group A and 1.8 in group B. No statistical differences were found in the data analysed, but one of the complications in group B was major blood loss right after surgery that needed reintervention. We assume that this could have been avoided if the tourniquet had been released beforehand. We conclude that releasing ischaemia prior to wound closure does not demonstrate a statistical difference, but like other authors, we found clinical advantages suggesting the need of further study of this situation.
Résumé
Nous avons inclus 46 prothèses totales du genou (43 patients) dans une étude prospective randomisée avec deux groupes de patients. Un premier groupe : groupe A, 23 genoux, 21 patients avec lâchage du garrot avant la fermeture avec contrôle du saignement et un groupe B, 23 genoux, 22 patients, avec lâchage du garrot après la suture et le pansement. Ont été comparés le taux d’hémoglobine préopératoire à 24 et 48 heures postopératoires, les pertes totales sanguines et la nécessité de transfusion. Les résultats obtenus ont été les suivants. En préopératoire dans le groupe A, le taux d’hémoglobine était à 14.21 g/dl et dans le groupe B de 14.28 g/dl ; l’hémoglobine à 24/48 heures pour le groupe A était à 10.04 et 10.01 g/dl respectivement et dans le groupe B 10.28 et 10.3 g/dl respectivement ; les pertes sanguines étaient de 743.2 cc pour le groupe A et de 692.5 cc pour le groupe B et le nombre d’unités transfusées était de 2 dans le groupe A et de 1.8 dans le groupe B. Il n’y a pas de différences significatives entre les deux séries, en dehors d’une complication dans le groupe B, un saignement majeur nécessitant une réintervention. Nous pouvons avoir l’assurance que cette complication aurait été évitée si le garrot avait été levé avant la fermeture. Nous pouvons conclure que le relâchement du garrot après la fermeture ne permet pas d’avoir une différence significative entre les deux séries, néanmoins, nous pensons, ainsi que d’autres auteurs, qu’il est plus avantageux sur le plan clinique de relâcher le garrot avant la fermeture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most orthopaedic surgeons use ischaemia for total knee arthroplasties with the obvious advantage of a bloodless field, making it easier to place the implants and obtain a better cement-bone interface [1, 19]. The use of a tourniquet for long periods of time is associated with nerve paralysis and more postoperative pain [2]; this is why new methods are being investigated to allow a clean surgical field without the complications caused by the use of ischaemia for a long period of time [4]. The pressure used in the tourniquet should be enough to stop bleeding in the surgical field [11], but not excessive, which would increase postoperative pain [15, 21].
Epinephrine-augmented hypotensive epidural anaesthesia and tranexamic acid are some alternatives used to avoid ischaemia, but they are still under investigation, and their use has not been generalised [5, 9, 13]. Tranexamic acid acts by binding to one of the enzymes at the start of the coagulation cascade inhibiting the fibrinolytic system resulting in less blood loss. It is of concern that this inhibition might cause an increase in thrombosis [17].
Haemostasis is important in total knee arthroplasties due to the potential for major blood loss. The purpose of this study was to demonstrate the benefits of an early release of ischaemia in these operations.
Patients and methods
This was a prospective, randomised study that took place from December 2004 to December 2005. We included 43 patients (46 total knee arthroplasties), 13 males (30%) and 30 females (70%), dividing them into two groups: group A (23 knees, 21 patients), in which the ischaemia was released prior to wound closure allowing control of bleeding, and group B (23 knees, 22 patients) releasing the tourniquet after suturing and bandaging. The patients were assigned in random order to each group according to the moment of intervention. Inclusion criteria were patients with the diagnosis of knee arthrosis undergoing a primary total knee arthroplasty; we excluded patients with a revision knee arthroplasty, arthrosis caused by a previous fracture, rheumatoid arthritis and patients with anticoagulation therapy 6 months prior to intervention. Every patient signed an informed consent before being included in the study.
Three surgeons participated in the procedures. One gram of cephalothin was given IV at anaesthesia induction and continued every 8 h for the next 24 h. Clindamycin was used for patients allergic to beta-lactam agents, giving 600 mg IV at anaesthesia induction, followed by the same dosage every 12 h for the next 24 h. For pain control we used 40 mg of parecoxib administered IV every 12 h combined with ketorolac 30 mg IV every 8 h or as an alternative clonixinate of lisine 100 mg IV every 6 h. Anticoagulation prophylaxis was carried out with 40 mg of enoxaparin administered subcutaneously every 24 h and compressive stockings until active mobilisation of the patient.
Student's t-test was used to analyse the data.
Operative technique
Before surgery a tourniquet cuff was placed on the upper half of the thigh and the limb was exsanguinated by elevation and compressive bandaging. The cuff was then inflated 100 mmHg above median arterial pressure. The knee was exposed through a midline skin incision and a deep medial parapatellar approach with a conventional capsulotomy. The prostheses that were implanted were: Scorpio (Stryker, Mahwah, NJ), Sulzer Medica (Protek, Austin, TX) and Depuy (Johnson & Johnson, Warsaw, IN); the sizes are mentioned in Table 1.
Before surgical wound closure, a large suction drain was placed intrarticularly. When the drainage was less than 20 cc in 24 h the suction drain was removed. The transoperative blood loss, surgical time and amount of the drainage were recorded. We compared the haemoglobin before surgery and at 24 and 48 h postoperatively, the total blood loss and the transfusions that were needed. Transfusions were given to patients with haemoglobin under 9 g/dl.
Results
The two groups were similar in age, preoperative haemoglobin (P = 0.2), prostheses and total blood loss (P = 0.2) (Table 2). The time of removal of the drain did not demonstrate statistical diferences between the two groups.
Preoperative haemoglobin was similar, in group A 14.21 g/dl and group B 14.28 g/dl, with a statistical value of P=0.2 (Table 2).
The average total blood loss (transoperative blood loss + postoperative drainage) obtained was of 743.2 cc (range 270–1,485) for group A and 692.5 cc (range 218–1,830) for group B, with a statistical value of P=0.2 (Table 2).
Haemoglobin at 24 h for group A was an average of 10.04 g/dl (range 7.6–13.9) and group B 10.28 g/dl (range 7.4–12.4), without finding a statistical difference of P=0.2 (Table 3). For haemoglobin at 48 h, the average was 10.1 g/dl (range 7.4–14.0) for group A and 10.3 g/dl (range 8.2–12.9) for group B without a statistical difference (P = 0.2) (Table 3).
The mean number of blood units transfused were 2 (range 0–5) in group A and 1.8 (range 0–4) in group B; no statistical difference was found (P = 0.2) (Table 3).
Complications
One of the complications in group A was a patient with a flexion contraction at 3 weeks, which was resolved after a second operation. In group B one patient needed reintervention 2 h after surgery because of a blood loss of 1,200 cc, treated by coagulating two medium calibre vessels that were still bleeding. Another patient in group B had a deep wound infection 10 days after the initial surgery, treated by surgical lavage and antibiotic therapy.
Discussion
In a study by Barwell [2], there was no significant difference in operating time nor decrease in haemoglobin at 48 h postoperatively with the early release of ischaemia; but they did demonstrate less postoperative pain, better range of motion and fewer wound complications. Abdel-Salam [1] did not find any differences in operating time or blood loss when comparing the use or not of a tourniquet; but they did observe less postoperative pain in patients without the use of a tourniquet.
Burkart [3], Widman [20] and Hersekli [8] found that releasing ischaemia prior to wound closure did not have any benefits when comparing blood loss and the need of transfusion. Schuh [18] found the same results, but observed an increase in deep vein thrombosis in patients that did not have the tourniquet released for haemostasis.
In a publication by Gutiérrez-Alamo [6], releasing ischaemia early allowed them to check the integrity of blood vessels and to control any major haemorrhage.
Henderson [7] found that releasing the tourniquet after wound closure decreases the operative blood loss, having this blood in the postoperative drainage and increasing the possibility of reinfusion. As a result, the use of bank blood is reduced, decreasing the risk of infection and the length of stay in the hospital [14, 16].
In our study no statistical differences were found in the postoperative haemoglobin, the total blood loss and the transfusions that were needed between the group with early release of the tourniquet and the group with release after suturing and bandaging. One of the complications in group B was major blood loss right after surgery that needed reintervention. We assume that this could have been avoided if the tourniquet had been released beforehand.
We conclude that releasing ischaemia prior to wound closure has no statistical effect compared to release after suturing and bandaging, but as with other authors, we found clinical advantages in the early release group, suggesting the need of further study of this situation.
References
Abdel-Salam A, Eyres KS (1995) Effects of tourniquet during total knee arthroplasty: a prospective randomized study. J Bone Joint Surg [Br] 77(B):250–253
Barwell NJ, Anderson G, Hassan A, Rawlings I (1997) The effects of early tourniquet release during total knee arthroplasty: a prospective randomized double-blind study. J Bone Joint Surg [Br] 79(B):265–268
Burkart BC, Bourne RB, Rorabeck CH, Kirk PG, Nott L (1994) The efficacy of tourniquet release in blood conservation after total knee arthroplasty. Clin Orthop Rel Res 299:147–152
Christodoulou AG, Ploumis AL, Terzidis IP, Chantzidis P, Metsovitis SR (2004) The role of timing of tourniquet release and cementing on perioperative blood loss in total knee replacement. Knee 11(4):313–317
Cid J, Lozano M (2005) Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: result of a meta-analysis of randomized controlled trials. Transfusion 45(8):1302–1307
Gutiérrez-Alamo (2000) Isquemia y hemostasia en artroplastía total de rodilla. Revista Digital de la Sociedad Española de Rodilla (9)
Henderson MS, Newman JH, Hand CG (1998) Early tourniquet release during total knee arthroplasty. J Bone Joint Surg [Br] 80(B):372
Hersekli MA, Akpinar S, Ozkoc G, Ozalay M, Uysal M, Cesur N, Tandogan RN (2004) The timing of tourniquet release and its influence on blood loss after total knee arthroplasty. Int Orthop 28(3):138–141
Hynes M, Calder P, Scott G (2003) The use of tranexamic acid to reduced blood loss during total knee arthroplasty. Knee 10(4):375–377
Iorio R, Healy WL (2001) Tourniquet use during total knee arthroplasty did not reduce total blood loss. J Bone Joint Surg [Am] 83(A):1282–1284
Ishii Y, Matsuda Y (2005) Effect of tourniquet pressure on perioperative blood loss associated with cementless total knee arthroplasty. J Arthroplasty 20(3):325–329
Jorn LP, Lindstrand A, Toksvig-Larsen S (1999) Tourniquet release for hemostasis increases bleeding: a randomized study of 77 knee replacements. Acta Orthop Scand 70(3):265–267
Kiss H, Raffl M, Neumann D, Hutter J, Dorn U (2005) Epinefrine-augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Rel Res 436:184–189
Majkowski RS, Currie IC, Newman JH (1991) Postoperative collection and reinfusion of autologous blood in total knee arthroplasty. Ann R Coll Surg Engl 73(6):381–384
Manen BF, Novellas CM, Angeles CM (2002) Effect of ischemic tourniquet pressure on the intensity of postoperative pain. Rev Esp Anestesiol Reanim 49:131
Newman JH, Bowers M, Murphy J (1997) The clinical advantage of autologous transfusion: a randomized, controlled study after knee replacement. J Bone Joint Surg [Br] 79(B):630–632
Orpen NM, Little C, Walker G, Crawfurd E (2006) Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomized controlled trial of 29 patients. Knee 13(2):106–110
Schuh A, Hausel M, Salminen S (2003) Effect of tourniquet use on blood loss in total knee arthroplasty. Zentralbl Chir 128(10):866–870
Wakankar HM, Nicholl JE, Koka R, D’Arcy JC (1999) The tourniquet in total knee arthroplasty: a prospective, randomized study. J Bone Joint Surg [Br] 81(B):30–33
Widman J, Isacson J (1999) Surgical hemostasis after tourniquet release does not reduce blood loss in knee replacement: a prospective randomized study of 81 patients. Acta Orthop Scand 70(3):268–270
Worland RL, Arredondo J, Angeles F (1997) Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty 12:848
Author information
Authors and Affiliations
Corresponding author
Additional information
No benefits in any form have been or will be received from a commercial party related directly or indirectly to this article.
Rights and permissions
About this article
Cite this article
Hernández-Castaños, D.M., Ponce, V.V. & Gil, F. Release of ischaemia prior to wound closure in total knee arthroplasty: a better method?. International Orthopaedics (SICO 32, 635–638 (2008). https://doi.org/10.1007/s00264-007-0376-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-007-0376-z