Abstract
Background
As a result of the growing use of immune checkpoint inhibitors (ICIs) for treating malignancy, immune-related adverse events (irAEs) have been increasingly reported. Higher body mass index (BMI) has been highlighted as a potential risk factor for the development of irAEs. However, there are no meta-analyses summarizing the association between BMI and irAEs in patients on ICI therapies.
Methods
PubMed, MEDLINE, EMBASE, Cochrane and grey literature were searched up to January 2020. Odds ratios (ORs) 95% and confidence intervals (CIs) were summarized using the random-effects model. Heterogeneity test, subgroup and sensitivity analyses were conducted. The protocol was registered on PROSPERO (number registration: CRD42020168790).
Results
Five studies (n = 1937) met eligibility criteria for inclusion. Being overweight or obese was associated with an increased odds of developing irAEs (OR 2.62, 95% CI 1.70–4.03, P ≤ 0.00001, I2 = 53%). In subgroup analyses, higher BMI was associated with irAEs in patients using anti-CTLA-4 single agents or in combination with anti-PD-1/PD-L1 (OR 1.87, 95% CI 1.17–2.98, P = 0.009, I2 = 0%) and in patients using anti-PD-1/PD-L1 (OR 3.22, 95% CI 2.06–5.01, P = 0.00001, I2 = 32%) monotherapy. The increased odds of irAEs in patients with higher BMI was comparable (test for subgroup differences, P = 0.72, I2 = 0%) between studies with adjusted OR (OR 2.21, 95% CI 1.44–3.38, P = 0.0003, I2 = 4%) and unadjusted OR (OR 2.65, 95% CI 1.08–6.50, P = 0.03, I2 = 66%).
Conclusion
Our meta-analysis provides evidence of a relationship between higher BMI (overweight–obesity) and increased risk of irAEs in patients on ICI therapies. Further research is needed to strengthen this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) have been described as an effective therapy for many different types of cancer, particularly non-small cell lung carcinoma (NSCLC), melanoma, renal cell carcinoma (RCC) and squamous cell cancer of the head and neck among others [1,2,3,4]. A cross-sectional study reported that as of 2018, 46.3% of US cancer patients were considered eligible for treatment with ICIs [5]. The increasing use of these agents has revealed a wide spectrum of immune-related adverse events (irAEs), inflammatory events that take place as a result of unleashing the typical blockades of immune system [1]. These include toxic effects along the gastrointestinal tract, cardiorespiratory system, endocrine system, skin, nervous system and musculoskeletal system among others [1, 6,7,8,9].
It is evident that the use of ICIs allows the immune system to circumvent the typical checkpoints, resulting in dysregulated inflammation and causing irAEs. However, it remains unclear which patients will develop these irAEs and in which organ systems. Previous studies have described biomarkers, genetic variations, changes in B cell populations and the presence of IgG autoantibodies which are associated with irAEs in patients being treated with ICIs therapies [10,11,12,13].
A number of investigators have explored the role of body composition on the immune response with a range of findings. Based on extensive evidence, obesity is considered a low-grade systemic inflammatory state and has been linked to a large number of autoimmune diseases [14]. Increased markers of inflammation such as C-reactive protein, leptin, TNF-α and IL-6 have been found in the serum of obese and overweight patients [14]. Moreover, adipokines, which are produced by adipose tissue, are involved in immune regulation and leptin, a hormone secreted primarily by adipocytes upregulated in obese individuals, both stimulates pro-inflammatory cytokines and disrupts the Th17/Treg balance, increasing Th17 cells that are involved in the pathogenesis of immune-related conditions [1, 14, 15]. This baseline inflammatory state may explain the predisposition for the development of irAEs. Preclinical studies have indeed shown that PD-1-mediated T cell dysfunction is partly influenced by the presence of leptin in mice with obesity [16]. Interestingly, multiple groups have found an improvement in progression-free and overall survival in obese patients treated with PD-(L)1 checkpoint inhibitors, suggesting enhanced PD-1 mediated T cell dysfunction and overall decreased regulation of inflammation in response to checkpoint blockade [16, 17].
Observational studies exploring the association between higher BMI and irAEs have been published in the last few years, the first couple of which did not identify a positive correlation. First, a prospective study in metastatic melanoma patients examining the relationship between the change in BMI and toxicity in patients receiving anti-CTLA-4, anti-PD-1 or combination therapy revealed a positive association between BMI and rate of irAEs. However, this relationship was not statistically significant [18]. Subsequently, a prospective study looking at patients being treated with nivolumab indicated no significant association between BMI and irAEs [19].
In contrast with these findings, a number of subsequent studies found a positive association between increased BMI and the development of irAEs. A multicenter retrospective study looking at stage IV cancer patients treated with single agent anti-PD-1/PD-L1 reported that patients with elevated BMI (overweight or obesity) were more likely to have irAEs compared with non-overweight patients and this association was statistically significant (P ≤ 0.0001) [20]. Similarly, three retrospective studies analysed the association between BMI and irAEs in patients on ICIs therapy and found a significantly increased risk of irAEs in patients with higher BMI (overweight or obesity) [21, 22, 23]. Likewise, in a cohort of 68 patients with melanoma being treated with anti-PD-1 agents, the mean BMI among those patients who developed toxicities was higher than in those patients who did not (27.9 kg/m2 vs. 24.7 kg/m2) (P = 0.04) [24]. In addition, in a recent post hoc analysis of individual participant data from clinical trials in patients with NSCLC who were treated with atezolizumab [25], an association between higher BMI and skin-related irAEs was found (HR 1.47; 95% CI 1.2–2.0 for overweight).
In light of the lingering ambiguity over the impact of body weight on irAEs in patients treated with ICIs and the absence of a meta-analysis on this topic, we aimed to conduct a systematic review and meta-analysis exploring the relationship between BMI and irAEs in patients on ICI therapies.
Methods
Search strategy
A systematic review of studies was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Statement [26] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for observational studies [27]. The protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/) under the registration number CRD42020168790. After developing the clinical question and translating it into a well-defined systematic review question based on the PICOS format (Patients, Interventions, Comparators, Outcomes and Studies), a manual search of medical databases was performed including PubMed, MEDLINE, EMBASE and Cochrane Library in English from inception until January 2020. Grey literature was also searched through review of repositories, websites OpenGrey, GetNet International and abstracts of major international congresses. The search was conducted using the following PICOS format: P: adult participants (age > 18 years) with overweight or obesity (body mass index ≥ 25 kg/m2); I: on immune checkpoint inhibitor therapies; C: control or comparator group low and normal weight (body mass index < 25 kg/m2); O: immune-related adverse events; S: observational studies.
The results were supplemented by a manual search of the bibliographies of the shortlisted review and original study articles. In addition, a number of field experts were approached to identify additional viable studies from grey literature. Two independent investigators separately screened the titles and abstracts for eligible studies (YGP and OS). Disagreements were resolved by consensus among all authors. Additionally, a manual search of abstracts was conducted from major medical conferences from 2010 to 2019 (Cancer Research UK, The British Association for Cancer Research, The European Association for Cancer Research, American Association for Cancer Research, American Society of Clinical Oncology, The European League Against Rheumatism, Endocrine Society Annual Meeting).
Eligibility criteria
Studies were included if: (1) they involved humans; (2) ≥ 18 year-old participants were enrolled; and (3) participants had received at least one dose of immune checkpoint inhibitor therapies (CTLA-4 or PD-1/PD-L1). Studies were excluded if: (1) they were non-human studies (cell culture, animal models); (2) they were case reports, editorials, comments, letters, reviews, meta-analyses or interventional studies; (3) they were duplicate; (4) they were written in languages other than English or studies without English translated versions; or (5) the data were incomplete on clinical outcome statistical measures (odds ratios, 95% CIs) or the outcome measures were unable to be calculated with the available data.
Outcome measures
The primary outcome of this systematic review and meta-analysis was to identify and quantify the relationship between BMI and irAEs in patients on ICI therapies.
Study selection
Two reviewers independently screened the studies according to the titles and abstracts (YGP and OS). If the articles were potentially eligible, full texts were retrieved and screened. Conflicts in study selection at this stage were resolved by discussion between the researchers, referring back to the original article in consensus among all authors.
Data extraction and synthesis
The selected studies were reviewed and the data were independently extracted by two researchers (YGP and OS). Discrepancies between them were resolved by consensus among all authors. Numeric and texted data were extracted from the eligible articles as follows: author, publication year, country/region, study type, sample size, age and outcomes. The study authors were contacted as needed to obtain detailed data. When more than one study was published from the same cohort, only the largest sample size study was included in the meta-analysis to avoid overlapping populations. Data were extracted into a bibliographic database using Microsoft® Office Excel® version 14.0 software (Microsoft, Redmond, WA, USA).
Study quality assessment
The Newcastle–Ottawa quality assessment scale was used to assess the quality of the observational studies, evaluating three items: patient selection, comparability of study groups and assessment of outcomes [28]. Studies scoring 7–9 points were considered to have high quality, 4–6 of moderate quality and ≤ 3 of low quality. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment was used to rate the overall quality of the evidence [29]. Studies were not excluded a priori based on quality reporting assessment. Two researchers independently evaluated the risk of bias (YGP and OS). Disagreements were resolved by consensus among all authors.
Data synthesis and analysis
A narrative synthesis of the findings from the included studies was structured around target population characteristics and exposure. The effect measures for the outcome were summarized. Adjusted odds ratios (ORs), where reported in studies, were used for analysis to account for confounding variables; if the ORs and 95% CIs were not reported directly, we used data from a 2 × 2 table to recalculate crude estimates. Differences were considered statistically significant when P < 0.05. Heterogeneity between the studies in effect measures was assessed using both the χ2 test and the I2 statistic, where I2 is the proportion of total variation attributable to between-study variability according to the Cochrane Handbook for Systematic Reviews and Interventions recommendation heterogeneity [30]. I2 values of < 30%, 30–60%, 60–75% and > 75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively. The sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates based on (A) OR adjustment (adjusted OR vs unadjusted OR), (B) age (median age ≤ 65 years and > 65 years) and (C) type of ICI: anti-PD-1/PD-L1 or anti-CTLA-4 (single or in combination with anti-PD-1/PD-L1). In this analysis, a P value for differences between subgroups of < 0.10 was considered statistically significant. Random-effect meta-analysis described by DerSimonian and Laird [31] was adopted to calculate summary OR and 95% confidence intervals (CI).
A sensitivity analysis by excluding one study at a time was conducted. Publication bias was assessed by visual inspection of a funnel plot. All analyses were performed with the Review Manager (RevMan) software, Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Since this study was a meta-analysis of published studies, institutional review board approval was not required.
Results
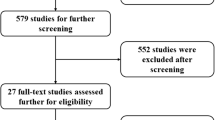
In the preliminary literature search, 372 studies were identified. 207 articles remained after the duplicates were removed. A title and abstract review was performed on each of the remaining 207 studies, with 196 excluded at this first pass stage for not meeting the PICOS criteria. A total of 11 articles were eligible for full‐text screening. Five full-text publications met the selection criteria and were included in the meta-analysis (n = 1937). A PRISMA flow diagram of the screening and selection process can be found in Fig. 1. Supplementary table S1 shows the search strategy.
Characteristics of included studies
Four studies included in the meta-analysis had a retrospective design [20,21,22,23] and one was prospective [19]. The patients included in the meta-analysis come from five countries (Italy, Ireland, South Korea, France and the USA). The mean age was 61.6 years and the mean proportion of male patients was 0.641. Four studies reported cases of NSCLC [19, 20, 22, 23]; three with melanoma [20, 22, 23]; two with urothelial carcinoma [22, 23]; and one with gastric cancer [22], hepatocellular carcinoma [23], RCC [20] and lymphoma [22], among others. Regarding the type of ICIs, two studies reported the use of anti-CTLA-4 (single or combination with anti-PD-1/PD-L1) [21, 23] and three studies reported the use of single agent anti-PD-1/PD-L1 [19, 20, 22]. Two studies informed median follow-up time, ranging from 48 days [21] to 8.7 months [23]. Regarding pre-existing conditions, one study [23] reported 10.8% of patients with a background of an autoimmune disease. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 in all included studies. Four studies [19, 20, 21, 23] categorized BMI according to the World Health Organization (WHO) classification: underweight (BMI < 18.5 kg/m2); normal (18.5 ≤ BMI ≤ 24.9 kg/m2); overweight (25 ≤ BMI ≤ 29.9 kg/m2); and obesity (BMI ≥ 30 kg/m2). One study [22] was carried out in an Asian population with BMI categorized into four groups according to the proposed classification in an adult Asian population presented by the WHO: underweight (BMI < 18.5 kg/m2); normal (18.5 kg/m2 ≤ BMI < 23 kg/m2); overweight (23 kg/m2 ≤ BMI < 25 kg/m2); and obese (BMI ≥ 25 kg/m2). Three studies reported adjusted OR [20,21,22], one study reported unadjusted OR [19] and one study [20] did not report OR and thus the crude OR was calculated using the available data. Of note, included studies reported only dichotomous exposure assessment categorized as BMI ≥ 25 kg/m2 vs. BMI < 25 kg/m2 [19,20,21, 23] or BMI ≥ 25 kg/m2 vs. BMI 18.5–22.9 kg/m2 [22] when calculating the outcome measures (OR and 95% CI). Table 1 shows the description of the characteristics of the included studies. Additional characteristics are summarized in Supplementary table S2.
The quality of included studies assessed by the Newcastle–Ottawa scale for each study is summarized in Supplementary table S3a and presented as percentages across all studies in Supplementary table S3b. Among included studies, four were considered high quality (score 7–9) [19,20,21,22], whereas one study [23] was of moderate quality (score 6). The GRADE quality of evidence suggested that there was moderate quality of evidence in the included studies (Supplementary table S4).
Description of excluded studies
There were a number of different reasons why six studies did not meet eligibility criteria and were excluded from our analysis: two studies did not link BMI with irAEs; one study was a post hoc analysis of randomized clinical trials; one study did not provide specific data about patients with and without irAEs; one study had insufficient data to calculate clinical outcome statistical measures and the authors did not respond via e-mail; and one study consisted of a cohort already included in the meta-analysis and in that case, only the largest sample size study was considered in the meta-analysis to avoid overlapping populations. Supplementary table S5 describes the excluded studies.
Body mass index and risk of irAEs
On the basis of the pooled estimate across the five studies, patients with higher BMI (overweight–obesity) were associated with increased odds of developing irAEs (pooled OR 2.62, 95% CI 1.70–4.03, P ≤ 0.00001, I2 = 53%) (Fig. 2).
Subgroup analysis based on OR adjustment (studies with adjusted OR vs. unadjusted OR) revealed that the increased odds of irAEs in patients with higher BMI (overweight–obesity) was comparable (test for subgroup differences, P = 0.72, I2 = 0%) between studies with adjusted OR (pooled OR 2.21, 95% CI 1.44–3.38, P = 0.0003, I2 = 4%) and unadjusted OR (pooled OR 2.65, 95% CI 1.08–6.50, P = 0.03, I2 = 66%) (Fig. 3).
Subgroup analyses based on age (median age ≤ 65 years or > 65 years) and type of ICI: anti-PD-1/PD-L1 or anti-CTLA-4 (single or in combination with anti-PD-1/PD-L1) showed that the increased odds of irAEs in patients with higher BMI (overweight–obesity) was comparable (test for subgroup differences, P = 0.90, I2 = 0%) between participants with median age ≤ 65 years (pooled OR 2.53, 95% CI 1.41–4.54, P = 0.002, I2 = 0%) and over 65 (pooled OR 2.66, 95% CI 1.31–5.42, P = 0.007, I2 = 84%) (Supplementary figure S1). The subgroup analysis also revealed that the increased odds of developing irAEs in patients with higher BMI (overweight–obesity) was comparable (test for subgroup differences, P = 0.10, I2 = 63.5%) between the studies that used anti-CTLA-4 agents (single or in combination with anti-PD-1/PD-L1) (pooled OR 1.87, 95% CI 1.17–2.98, P = 0.009, I2 = 0%) and in the studies with anti-PD-1/PD-L1 agents (pooled OR 3.22, 95% CI 2.06–5.01, P = 0.00001, I2 = 32%) (Supplementary figure S2).
The subgroup analysis of the studies that classified BMI according to the categories: underweight (BMI < 18.5), normal (18.5 ≤ BMI ≤ 24.9), overweight (25 ≤ BMI ≤ 29.9) and obesity (BMI ≥ 30) showed an increased odds of developing irAEs in patients with higher BMI (overweight–obesity) (pooled OR 2.40, 95% CI 1.41–4.09, P = 0.001, I2 = 64%) (Supplementary figure S3).
One study [20] (n = 976) compared the rate of irAEs grades 3 and 4 between overweight–obese patients and non-overweight (7.6% vs. 5.3%) and reported that the difference was not statistically significant (P = 0.13). Sensitivity analyses performed by excluding one study at a time indicated that one study [20] contributed the most to the variability among the included studies, while the analysis of the remaining studies [19, 21,22,23] demonstrated statistical homogeneity (P ≤ 0.0002, I2 = 0%). Supplementary table S6 shows the sensitivity analysis in detail. There was no evidence of publication bias based on examination of funnel plot symmetry (Supplementary figure S4).
Discussion
To the best of our knowledge, this is the first meta-analysis to quantify the association between BMI and irAEs in patients on ICI therapies. Our study demonstrated that elevated BMI (overweight-obesity) is significantly associated with the development of irAEs (OR 2.62, 95% CI 1.70–4.03, P ≤ 0.00001, I2 = 53%) in patients being treated with ICIs therapies. This association was stable across different subgroups including adjusted OR, age, ICI type and BMI classification. The results of our study are clinically meaningful, suggesting the negative impact of elevated BMI on ICI treatment and highlighting the role of high body weight as a potential modifiable risk factor that must be considered and monitored for safety in clinical practice. In line with this evidence, Rassy et al. [32] has highlighted the importance of an assessment of patient's weight and inflammatory profile before the initiation of ICIs in cancer patients.
The exact mechanism by which BMI influences the development of irAEs is not completely understood. This effect is likely related to the association of obesity with low-grade systemic inflammation resulting in an excessive risk of irAEs [31, 33, 34]. There are a multitude of interwoven pathways which account for this relationship. Adipose tissue macrophages accumulate in subcutaneous and visceral fat depots and take on a pro-inflammatory phenotype, secreting pro-inflammatory cytokines such as TNF-α and IL-6 [35]. In addition, the accumulation of fat results in increased infiltration of pro-inflammatory CD8 + T cells into adipose tissue and the depletion of adipose Tregs [36, 37]. Furthermore, adipose tissue overexpresses nucleotide-binding domain, leucine rich-containing family, pyrin domain-containing-3 (Nlrp3) leading to caspase-1 activation and subsequently a cascade of inflammation resulting from the secretion of IL-1β and IL-18 [38]. Alternatively, overexposure to treatment may occur in overweight patients due to an increased dose calculation based on mass weight without a parallel increase in distribution volume resulting in a possible toxicity [31].
Although the methodology of this meta-analysis was rigorous, several limitations should be acknowledged. Firstly, the small number of studies suitable for inclusion may affect the reliability of conclusions. Secondly, our meta-analysis included only observational studies and the baseline differences among study groups cannot be entirely avoided. Thirdly, the included studies presented dichotomous categories of BMI (above or below median weight) and did not analyse the impact of obesity as a specific category (BMI ≥ 30 kg/m2) when estimating the outcome measures (OR and 95% CI) to fully elucidate possible differences among weight classes. Finally, information regarding race, disease progression, history of previous treatments, pre-existing autoimmune diseases and follow-up was not available from all of the studies even though some of the included studies adjusted for these variables in the outcome OR.
Despite these limitations, our study has a number of strengths. This is the first meta-analysis integrating the evidence for the association between BMI and irAEs in patients on ICI therapies. In addition, our meta-analysis included cohorts from diverse geographical and ethnic groups (Europe, USA, Asia). Moreover, the quality of the included studies was high, the heterogeneity was moderate and there was no evidence of publication bias. Furthermore, the assessment of adjusted and unadjusted ORs allowed us to evaluate the influence of confounders on the summary estimate. Finally, we acknowledge the moderate heterogeneity (I2 = 53%) among the studies mainly stemming from different ICI regimens and follow-up procedures and from differences in ethnicity, comorbidities and baseline characteristics on the study populations. In an attempt to reduce heterogeneity, we carried out subgroup analyses significantly improving the accuracy of our outcomes.
Our study has important implications for clinical practice. Our findings illustrate a role for the evaluation of body weight in the monitoring of patient safety during treatment with ICIs and suggest that elevated BMI can serve as an important tool to be used in clinical practice in predicting risk of irAEs. Moreover, our results imply that BMI should be considered as a potential effect modifier and therefore included as a stratification factor in further ICIs trials.
In conclusion, our systematic review and meta-analysis provides evidence for the presence of a significant relationship between higher BMI (overweight and obesity) and an increased risk of developing irAEs in patients on ICI therapies. Further prospective cohort studies are needed to strengthen the association between obesity and irAEs.
References
Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, Kim H, Lee J (2019) Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep 9(1):1–8
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480:480–489
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2:e192535
Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A (2017) Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol 69:687–699
Abdel-Rahman O et al (2017) Immune-related musculoskeletal toxicities among cancer patients treated with immune checkpoint inhibitors: a systematic review. Immunotherapy 9:1175–1183
Kuswanto WF et al (2018) Rheumatologic symptoms in oncologic patients on PD-1 inhibitors. Semin Arthritis Rheum 47:907–910
Mooradian MJ, Nasrallah M, Gainor JF, Reynolds KL, Cohen JV, Lawrence DP, Miloslavsky EM, Kohler MJ, Sullivan RJ, Schoenfeld SR (2019) Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience. InSeminars Arthritis Rheum 48(6):1127–1132
Manson G, Norwood J, Marabelle A, Kohrt H, Houot R (2016) Biomarkers associated with checkpoint inhibitors. Ann Oncol 27(7):1199–1206
Marschner D, Falk M, Javorniczky NR, Hanke-Müller K, Rawluk J, Schmitt-Graeff A, Simonetta F, Haring E, Dicks S, Ku M, Duquesne S (2020) MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 5(6):e132334. https://doi.org/10.1172/jci.insight.132334
Das R et al (2018) Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128(2):715–720
Ali OH, Bomze D, Ring S, Berner F, Fässler M, Diem S, Abdou MT, Hammers C, Emtenani S, Braun A, Cozzio A (2020) BP180-specific IgG is associated with skin adverse events, therapy response and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol 82(4):854–861. https://doi.org/10.1016/j.jaad.2019.08.045
Harpsøe MC, Basit S, Andersson M et al (2014) Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 43(3):843–855. https://doi.org/10.1093/ije/dyu045
Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y (2019) Obesity in autoimmune diseases: not a passive bystander. InMosaic Autoimmun 13:343–372
Wang Z, Aguilar EG, Luna JI et al (2018) Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. https://doi.org/10.1038/s41591-018-0221-5
Naik A, Monjazeb AM, Decock J (2019) The obesity paradox in cancer, tumor immunology and immunotherapy: potential therapeutic implications in triple negative breast cancer. Front Immunol 10:1940
Donnelly D, Bajaj S, Yu J, Hsu M, Balar A, Pavlick A, Weber J, Osman I, Zhong J (2019) The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immunother Cancer 7(1):222
Hirsch L, Bellesoeur A, Boudou-Rouquette P, Arrondeau J, Thomas-Schoemann A, Kirchgesner J, Gervais C, Jouinot A, Chapron J, Giraud F, Wislez M (2020) The impact of body composition parameters on severe toxicity of nivolumab. Eur J Cancer 124:170–177
Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, Tinari N (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 7(1):57
Daly LE, Power DG, O'Reilly Á, Donnellan P, Cushen SJ, O'Sullivan K, Twomey M, Woodlock DP, Redmond HP, Ryan AM (2017) The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 116(3):310–317
Eun Y, Kim I, Kim H et al (2018) SAT0715 Risk factors of immune-related adverse events in patients treated with anti-pd-1 antibody pembrolizumab. Ann Rheum Dis 77:1205
Leiter A, Jia R, Carroll E, Brooks D, Ben Shimol J, Eisenberg E, Galsky M, Gallagher E (2019) SAT-094 overweight and obesity associated with immune-related adverse events in patients on immune checkpoint inhibitor therapy. J Endocr Soc 3(Supplement_1):SAT-094
Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, Chanal J, Arrondeau J, Franck N, Alexandre J, Blanchet B (2017) Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs 35(4):436–441
Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ (2020) Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol 6(4):512–518
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Wells GA, Shea B, O’Connell D et al (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Dec 2019
Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Rassy EE, Ghosn M, Rassy NA, Assi T, Robert C (2018) Do immune checkpoint inhibitors perform identically in patients with weight extremes? Immunotherapy 10:733–736
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB (1999) Elevated C-reactive protein levels in overweight and obese adults. JAMA 282:2131–2135
Guijian L, Jinchuan Y, Rongzeng D, Jun Q, Jun W, Wenqing Z (2013) Impact of body mass index on atrial fibrillation recurrence: a meta-analysis of observational studies. Pacing Clin Electrophysiol 36(6):748–756
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15(8):914
Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q (2011) Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS ONE 6(1):e16376
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17(2):179
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, analysis and validation were performed by Yuli Guzman-Prado, Jennifer Ben Shimol and Ondrej Samson. The first draft of the manuscript was written by Yuli Guzman-Prado and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yuli Guzman-Prado declares that she has no conflict of interest. Jennifer Ben Shimol declares that she has no conflict of interest. Ondrej Samson declares that he has not conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guzman-Prado, Y., Ben Shimol, J. & Samson, O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunol Immunother 70, 89–100 (2021). https://doi.org/10.1007/s00262-020-02663-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02663-z