Abstract
Background
This systematic review and meta-analysis aimed to evaluate the association between pretreatment body mass index (BMI) and clinical outcomes in cancer patients treated with immune checkpoint inhibitors (ICIs).
Methods
Systematical searches of PubMed, Embase, and the Cochrane Library databases were carried out. Studies reporting on the association between BMI and outcomes of ICIs were included. The intended outcomes included overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and immune-related adverse events (irAEs). Quantitative analyses and dose–response meta-analyses were performed under random effect models.
Results
Twenty-two eligible studies involving 5686 cancer patients treated with ICIs were identified.
Compared to those with lower BMI, patients with higher BMI obtained a significant benefit on OS (HR = 0.698, 95% CI 0.614–0.794, P < 0.001; I2 = 45.9%) and PFS (HR = 0.760, 95% CI 0.672–0.861, P < 0.001; I2 = 37.9%). Most stratified analyses for OS and PFS also showed similar pooled risk estimates. For an increment of every 5 kg/m2 in BMI, the risk for death reduced by approximately 15.6% (HR = 0.844, 95% CI 0.752–0.945, P = 0.003). Moreover, patients with higher BMI had a remarkably better ORR (OR = 0.468, 95% CI 0.263–0.833, P = 0.010; I2 = 73.6%) than that of those with lower BMI. However, no statistically significant differences were found in the incidence of any grade irAEs (P = 0.073) and ≥ 3 grade irAEs (P = 0.105) between higher and lower BMI.
Conclusion
Higher BMI is significantly associated with improved outcomes in patients treated with ICIs. Further large-scale prospective research is warranted to better illuminate the association between BMI and outcomes from ICIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, cancer immunotherapy based on immune checkpoint inhibitors (ICIs) has become an increasingly attractive approach for diverse malignancies [1, 2]. With the emerging clinical trials in ICIs, several monoclonal antibodies targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1) or PD ligand 1 (PD-L1), which achieved encouraging anti-tumor activity, have been gradually approved for the treatment of multiple cancers. Despite the durable responses of ICIs reported in previous studies, only a limited number of patients can benefit from these agents. If not selected, the response rates seem unsatisfactory for several cancer entities, such as non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC), with a response rate lower than 20% [3, 4] and 30% [5, 6], respectively. Moreover, immune-related adverse events (irAEs) vary greatly across individual patients, with a small proportion of them suffering from severe irAEs, especially when combination regimens are used. Therefore, there is an urgent need to discover robust predictive biomarkers for clinical outcome and toxicities of ICIs, to identify the subgroups who can benefit from these immunotherapeutic agents.
So far, several candidate biomarkers have been recognized to be associated with clinical outcomes for ICIs, such as the expression of PD-L1 protein, tumor-infiltrating lymphocytes(TILs), tumor mutational burden(TMB) and microsatellite instability (MSI) [7]. Among them, PD-L1 expression by immunohistochemistry on tumor cells was the first biomarker associated with treatment response to PD-1 inhibitors [8]. Numerous studies have generally shown better objective response and longer overall survival of PD-L1 positive patients, compared with the PD-L1 negative subgroup [4, 9, 10]. Other trials, however, yielded contradictory results, in which some patients whose tumor was PD-L1- negative could also achieve clinical benefit with anti-PD-1/PD-L1 therapies [3, 11,12,13,14]. A meta-analysis also demonstrated that PD-L1 expression alone was not yet sufficient in selecting patients for PD-1/PD-L1 blockade therapy [15]. Based on these findings, the prognostic value of PD-L1 expression by immunohistochemistry alone for routine clinical use remains to be established.
More recently, substantial efforts are ongoing to elucidate the potential role of patient-associated factors such as age, sex, and body mass index (BMI) in the prediction of clinical outcomes from immunotherapy. Traditionally, it has been reported that high BMI is significantly associated with a higher risk of incidence and death for multiple cancers [16, 17]. However, recent clinical data demonstrate that obesity, defined by increased BMI (≥ 30 kg/m2), is associated with improved outcomes of cancer patients treated with targeted therapy or ICIs, which may be supported by the contrasting/paradoxical impact of obesity on cancer immune responses [18,19,20,21]. Notably, higher BMI was also significantly related to a higher occurrence of irAEs [22]. Despite a growing body of evidence that indicates a favorable efficacy with ICIs in patients with higher BMI, however, contradictory findings have also been reported [23, 24]. From these studies, it can be concluded that several factors, including heterogeneity in cancer type, age, and BMI threshold, make it hard to define the predictive value of BMI for outcomes after ICIs therapy.
Although there was one pooled analysis exploring the impact of obesity on the outcomes of ICIs for cancer patients, the analysis was limited by the relatively inadequate power and the small number of primary studies included [25]. Herein, with recently accumulated evidence, we performed a more comprehensive systematic review and meta-analysis to evaluate the association between BMI and clinical outcomes in cancer patients treated with ICIs.
Methods
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [26].
Search strategy and selection criteria
We carried out the systematic review of literature by searching PubMed, Embase, and the Cochrane Library databases from the inception of each database until 18th, May 2020 with no language restrictions. The main keywords for the literature search included cancer, body mass index, overweight, obesity, PD-1, PD-L1, CTLA-4, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, ipilimumab, and tremelimumab. The complete search strategy was available in Supplementary: Table S1. In addition, the reference lists of relevant studies were also searched with hand.
Two investigators (HZC and DQW) independently performed the search and selected articles for eligibility. If there were any disagreements, the study would be re-evaluated by a third investigator (YXT). Full-text publications of original prospective or retrospective studies were included. The main criteria for eligibility were as following:(1) the studies in which cancer patients were treated with anti-CTLA-4, anti-PD-1/PD-L1, or combination therapy; (2) the studies where the association between baseline BMI and clinical outcomes of ICIs was evaluated; (3) the studies in which any of clinical endpoints such as objective response rate(ORR), progression-free survival (PFS), overall survival (OS) or irAEs were reported; (4) the studies from which the related data could be extracted directly or calculated indirectly; (5) the studies that were written in English. Studies were excluded if they were reviews, case reports, comments, letters, editorials, animal studies or conference abstracts. Other studies were also removed if they lacked sufficient information. When the same study population appeared in multiple publications, or patient cohort was overlapping between different articles, the most updated and complete studies were included.
Data extraction and quality assessment
Two investigators (HZC and DQW) independently performed study data collection, which included: (1) characteristics of studies included (first author, publication year, area, type of studies, sample size, follow-up time); (2) characteristics of patients (age, sex, disease, study drugs, BMI categories); (3) treatment outcomes (OS, PFS, ORR, and irAEs). Hazard ratios (HRs) and their corresponding 95% confidence intervals(CIs) for PFS or OS were also extracted from original studies. When results in both univariate and multivariate analyses were available, we preferred results in the multivariate analysis. If the information needed was not reported, or not available after contacting the authors, the study was discarded.
Since all the studies included were non-randomized observational studies, the Newcastle–Ottawa Scale(NOS) criteria were adopted to evaluate the quality of studies [27]. The total scores ranged from 0 to 9 points, with a score of lower than five indicating poor quality, five to seven indicating medium quality, and higher than seven representing high quality.
BMI definition and categories
BMI was defined as the ratio of weight in kilograms divided by squared height in meters. According to the classification of World Health Organization, four BMI categories were defined: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–25.0 kg/m2), overweight (BMI 25–30.0 kg/m2), and obesity (BMI ≥ 30.0 kg/m2). Since not all the identified studies compared the differences between the four groups for outcomes and the cut-off values of BMI varied, we compared the effect of higher BMI with lower BMI on outcomes of ICIs for convenience. The higher BMI group was defined by a BMI value of ≥ cut-off in each study, otherwise it was identified as the lower BMI group. When studies reported more than two BMI categories, the results for each BMI category were collected. When performing the pooled analysis, we only included one comparison for each study, selected as the comparison between the highest with the lowest category of BMI available in the study.
Statistical analysis
The impacts of BMI on the clinical outcomes of ICIs measured in terms of OS, PFS, ORR, and irAEs for patients were analyzed. HR with 95% CI was used for the pooled analyses of OS and PFS, and odds ratio (OR) was selected as the effect size of ORR and irAEs. Categorical meta-analyses were conducted by comparing the higher BMI with a lower BMI. Besides, we also evaluated the association between different comparative models of BMI categories (overweight vs normal weight, obese vs normal weight, underweight vs normal weight, obese/overweight vs normal/underweight) and the intended outcomes (OS and PFS).
In addition, the dose–response meta-analysis was performed to assess the association of BMI with OS and PFS. The HRs of OS and PFS for every 5 kg/m2 increment in BMI were estimated by generalized least-squares for trend estimation [28]. The mean or median of each BMI category per study was collected, if not available, the midpoint of the upper and lower boundaries in each BMI category was estimated. When the lowest or highest category was open-ended, the same interval as the adjacent category was used to estimated midpoints. If the lowest BMI category was not considered as the reference group in studies, the method of Hamling et al. was chosen to transform the data [29].
Statistical heterogeneity among studies was evaluated using Cochran’s Q test and the inconsistency index(I2). Heterogeneity was regarded as low (I2 < 25%), moderate (25% ≤ I2 < 50%), and high (I2 > 50%) [30]. Considering the inherent clinical heterogeneity between studies included in this analysis, we selected a random effect model according to the method of DerSimonian and Laird [31]. Sensitivity analyses and subgroup analyses were carried out to explore the potential sources of heterogeneity. These subgroups involved sex of patients (male vs. female), type of cancer (melanoma vs. NSCLC vs RCC vs multiple cancers), type of ICIs (anti-PD-1/PD-L1 vs. anti-CTLA4) and study regions (Europe vs. America vs Asia vs others). Funnel plots, Egger’s tests, and Begg’s tests were used to examine publication bias [32, 33]. All the statistical analyses were conducted on Stata version 15.0. Two-sided P < 0.05 was considered statistically significant.
Results
Systematic search and study characteristics
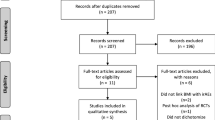
In total, 613 publications were identified through the initial literature search, and 459 were retained after 154 duplicated records were removed. After screening for titles and abstracts, 406 studies were excluded because of irrelevant topics, conference abstracts, reviews, letters, case reports or insufficient information. After reviewing the remaining 57 articles via the full-text view, 35 were further removed, due to data duplication, conference abstracts or unavailable data. Ultimately, 22 studies published between 2017 and 2020 were included in the final meta-analysis [18,19,20,21, 23, 24, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. The flow chart of the literature selection was illustrated in Supplementary Fig 1.
All eligible studies were retrospective studies of cohorts or clinical trials. Overall, there were 5686 patients with cancer included in our analysis, with a sample size ranging from 35 to 1434 per study. The median age ranged from 61.7 to 70 years (13 studies provided the data), with a slight majority of patients (65.3%) being male. Seven studies were conducted in Europe, six were conducted in Asia, five were from the USA, one and three were from Australia and multiple areas, respectively. Among these studies, six studies included patients with melanoma, eight with NSCLC, three with RCC, and the remaining five studies with other types or multiple cancers. All patients enrolled were at advanced or metastatic settings. With regards to the types of ICIs used, 17 studies focused on anti-PD-1/PD-L1 monotherapy, only one on anti-CTLA-4 monotherapy, and four studies reported anti-PD-1/PD-L1 monotherapy or in combination with anti-CTLA-4 therapy. The cut-off values of BMI varied among various studies, ranging from 18.5 to 35 kg/ m2, and the most common cut-off value was 25 kg/m2 or 30 kg/m2. The major characteristics of all included studies were summarized in Table 1.
The results of Newcastle–Ottawa Scale for quality assessment were shown in Table 1. Sixteen studies were considered as having a high quality, with a score of eight points. Six studies had medium quality, with a score of ranging from 6 to 7 points.
Association between BMI and overall survival
19 out of all included studies, covering 5447 patients, evaluated the impact of BMI on OS. As shown in Fig. 1a, patients in higher BMI groups obtained a significant benefit on OS compared with those in lower BMI group (HR = 0.698, 95% CI 0.614–0.794, P < 0.001), with a moderate level of heterogeneity (I2 = 45.9%, P = 0.012) between the studies. The sensitivity analysis, which was carried out by removing one study at each time, showed that the pooled results were not significantly changed by any single study (Supplementary: Figure S1).
Subgroup analyses for OS were further carried out. When stratifying by cancer type, significantly positive impact of higher BMI on OS was observed in patients with melanoma (HR = 0.700, 95% CI 0.506–0.968, P = 0.031; I2 = 40.6%, P = 0.011), NSCLC (HR = 0.803, 95% CI 0.736–0.877, P < 0.001; I2 = 0%, P = 0.817) and multiple malignancies (HR = 0.491, 95% CI 0.397–0.607, P < 0.001; I2 = 0%, P = 0.649). However, no such impact was found in patients with RCC (P = 0.377), probably due to the limited number of studies pooled (n = 3). When stratifying by sex, significantly better OS was achieved in the higher BMI group compared to the lower BMI group for the subgroups of male patients (P < 0.001), but not in females (P = 0.211). In addition, subgroup analyses by types of ICIs and study regions revealed that no significant association between BMI and OS was observed in patients treated with anti-CTLA-4 therapy (P = 0.238), or patients from America (P = 0.289). Detailed results of subgroup analyses for OS were summarized in Table 2.
Association between BMI and progression-free survival
PFS data was available in 17 studies involving 5162 patients. According to a random-effect model on the basis of the heterogeneity test (I2 = 37.9%, P = 0.049), higher BMI was also associated with improved PFS, with a pooled HR of 0.760 (95% CI 0.672–0.861, P < 0.001) (Fig. 1b). The stability of the result was assessed by sensitivity analysis, which revealed that the results were stable (Supplementary: Figure S2).
Table 2 summarized the results of subgroup analyses for PFS. Similarly, subgroup analyses did not find any evidence of a significant association between BMI and PFS in patients with melanoma (P = 0.220), patients treated with anti-CTLA-4 therapy (P = 0.252), or patients from America (P = 0.390). When stratifying by sex, higher BMI was significantly associated with better PFS in males (HR = 0.627, 95% CI 0.454–0.867 P = 0.005; I2 = 44.8%, P = 0.142), whereas no significant association was found in females (P = 0.698).
Association between different BMI categories and OS or PFS
The association between different comparative models of BMI categories and the intended outcomes (OS and PFS) was further examined. As shown in Fig. 2, compared with normal weight patients, the pooled HRs for OS were 0.652 (95% CI 0.496–0.859, P = 0.002; I2 = 70.9%, P = 0.002) for overweight patients, 0.617 (95% CI 0.477–0.797, P < 0.001; I2 = 48.4%, P = 0.060) for obese patients, and 2.087(95% CI 1.113–3.913, P = 0.022; I2 = 77.0%, P = 0.013) for underweight patients. Significantly better OS was also found in obese/underweight patients compared with normal/underweight patients (HR = 0.638, 95% CI 0.515–0.790, P < 0.001), with a moderate level of heterogeneity (I2 = 33.0%, P = 0.165).
In terms of PFS, when compared with normal weight patients, patients who were overweight (HR = 0.796, 95% CI 0.668–0.947, P = 0.010; I2 = 47.8%, P = 0.074) or obese (HR = 0.788, 95% CI 0.644–0.963, P = 0.020; I2 = 46.1%, P = 0.072) achieved significantly longer PFS. Nevertheless, being underweight was significantly associated with increased risk for disease progression, compared to being normal weight (HR = 1.834, 95% CI 1.208–2.782, P = 0.004; I2 = 32.7%, P = 0.226). In addition, compared with those were normal/underweight, obese/underweight patients showed a 25% lower risk for disease progression (HR = 0.755, 95% CI 0.662–0.862, P < 0.001), without any heterogeneity (I2 = 0%, P = 0.634) (Supplementary Fig. 2).
Dose–response meta-analysis
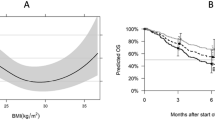
Five and four studies for OS and PFS, respectively, were included in dose–response analyses. A positive association between BMI increase and OS was observed. For an increment of every 5 kg/m2 in BMI, the risk for death reduced by approximately 15.6% (HR = 0.844, 95% CI 0.752–0.945, P = 0.003). However, the pooled results showed there was no linear association between BMI increase(5 kg/m2) and PFS (HR = 0.956, 95% CI 0.873–1.046, P = 0.325). The results of the dose–response analyses were presented in Supplementary Fig. 3a, b.
Association between BMI and objective response rate or adverse events
Only three of the 22 studies including 1306 patients provided ORR data. The pooled OR for ORR was 0.468 (95% CI 0.263–0.833, P = 0.010) based on a random effect model, which indicated patients with higher BMI had a significantly better ORR than that of those with lower BMI (Supplementary Fig. 4). There was evidence of high heterogeneity (I2 = 73.6%, P = 0.023).
Furthermore, the association between BMI and the incidence rates of irAEs was evaluated in six studies involving 2713 patients. As shown in Supplementary Fig. 2, compared with patients in lower BMI group, patients in higher BMI group tended to experience a higher frequency of any grade irAEs, although the result was not statistically significant (OR = 2.025, 95% CI 0.937–4.374, P = 0.073). However, the potential sources of heterogeneity should be taken into account according to the I2 statistic and Cochran’s Q test (I2 = 91.9%, P < 0.001). Similarly, the pooled risk estimates of three studies showed that BMI was not significantly associated with the incidence of ≥ 3 grade irAEs (OR = 1.243, 95% CI 0.955–1.617, P = 0.105), with no heterogeneity (I2 = 0.0%, P = 0.416) (Supplementary Fig. 4).
Publication bias
The funnel plot indicated no publication bias in all the pooled analyses (Supplementary: Figure S3). Besides, the Begg’s and Egger’s test also revealed no evidence of substantial publication bias for OS (Begg’s test: P = 0.450, Egger’s test: P = 0.112) and PFS (Begg’s test: P = 0.263, Egger’s test: P = 0.219). Similarly, no significant publication bias was found in other meta-analyses.
Discussion
This meta-analysis focuses on the effect of pretreatment BMI on clinical outcomes of cancer patients treated with ICIs. The results of our categorical meta-analysis revealed that compared to those with lower BMI, patients with higher BMI showed markedly improved OS and PFS, with 30% and 24% lower risk for mortality and disease progression, respectively. The pooled results for most subgroup analyses, which involved sex of patients, type of cancer, type of ICIs, and study regions, were not observably influenced. The limited number of studies on ORR also suggested the existence of a positive association. Our findings suggest that BMI may be a promising predictive biomarker for outcomes in cancer patients following ICIs.
The association between different comparative models of BMI categories and the intended outcomes was also examined in the current study. Compared with those who were normal-weight, both overweight and obese patients showed a statistically significant OS benefit, with 35% and 38% lower risk for mortality, respectively. Regarding PFS, being overweight or obese was associated with a lower risk for disease progression, compared to being normal weight. In addition, we also evaluated the contribution of underweight to survival. With normal weight as a reference category, both significantly inferior OS and PFS were found in underweight patients, though only three studies were pooled. Since BMI categories varied greatly across the included studies, the relationship between BMI and survival was further examined using the dose–response meta-analysis, indicating that an increment of each 5 kg/m2 in BMI corresponded to a 15.6% lower risk of mortality. Considering the findings above and the lack of publication bias, we confirmed that higher BMI was associated with better OS and PFS for patients with cancer who received ICIs treatment.
Previously, BMI has already been proved to be a potential indicator for improved survival in patients with NSCLC or RCC following targeted therapy, chemotherapy or surgery [50,51,52]. In consistent with these findings, subsequent evidence indicates that high BMI is associated with superior survival outcomes in cancer patients following ICIs [18,19,20]. It appears that the association between BMI and outcomes in cancer patients is complicated. The survival advantage from high BMI, which can be conferred within other treatment interventions, may be not specific to ICIs. Whether the presence of “obesity paradox”, wherein obesity is related to increased risk of cancers but shows a survival benefit, is influenced by different treatment strategies remains unclear. The biological mechanisms behind the positive association between BMI and ICIs are also not well understood. It is possible that patients with higher BMI may have better nutritional status, thus potentially increasing immune response [53]. In addition, preclinical data indicated that obesity could lead to T cell aging, tumor progression, a higher level of PD-1 expression, and an exhausted T-cell dysfunction, which was partly due to leptin production. However, the PD-1-mediated T cell dysfunction and increased PD1 expression made tumors more sensitive to checkpoint blockade, allowing survival benefit for patients treated with anti-PD1 therapy in the setting of obesity [21]. It is still unclear whether other non-immune factors contribute to the effect of obesity on checkpoint inhibition. Taken together, the biological basis of the relationships between BMI and ICIs outcomes remains unclear, hence further investigations are needed to elucidate these mechanisms. In addition, BMI should be considered as a stratification factor in a prospective randomized study with non-ICI control arm, to better define its role in checkpoint inhibitors therapy.
Interestingly, significant sex-associated differences in the influence of BMI on outcomes in the context of immunotherapy, have been previously described. It appeared that the significantly positive impact of high BMI on outcomes was observed across males, but not among females [20, 44]. However, inconsistent results have also been reported [18]. A recent pooled analysis conducted by Xu et al. confirmed the significant association between obesity and improved survival in cancer patients following ICIs, irrespective of sex [25]. Indeed, the relationship between sex, BMI, and immunotherapy is complex, and the underlying mechanisms of sex-related differences remain ambiguous. Gender-based differences in hormones, body mass composition or muscle mass may contribute to different BMI effects [54, 55]. Contrary to the previous pooled analysis [25], stratified analyses in our study demonstrated that the association between BMI and outcomes varied by sex, in which higher BMI was associated with OS and PFS benefit in male patients rather than females. These conflicting results may be explained by the different studies included between these two analyses. The small sample included, with only three studies available in both analyses, may be another reason contributing to the inconsistent results. In consideration of the inadequate statistical power, more robust evidence that focuses on the association between BMI and response to immunotherapy in the context of sex is required.
Notably, despite the limited sample size, our study observed that patients with higher BMI had a significantly higher ORR, implying the positive impact of overweightedness or obesity on the efficacy of ICIs. On the other hand, we also found that patients in higher BMI group tended to have a higher risk of any grade irAEs (P = 0.073). However, with the considerably obvious heterogeneity among different studies observed, caution must be applied. Furthermore, no significant differences were found in the frequency of ≥ 3 grade irAEs between higher BMI and lower BMI group. To some extent, these findings were not in line with the previous pooled analysis conducted by Xu et al. in which overweight or obesity patients developed a significantly higher incidence of adverse events [25]. Compared with their study, we updated the search and included two recent studies [19, 48], which may be the underlying reason for the discrepancy. Given these non-duplicate results, further investigations with larger samples are warranted to evaluate the association between BMI and ORR or the incidence of irAEs.
Finally, several important limitations in the present study need to be considered. The major limitation lies in the fact that all included studies were retrospective in nature, leading to some inevitable sources of bias. Another limitation is that some confounding risk factors across studies, such as age, sex, treatment, cancer type, BMI cutoff value, etc., might influence the association between BMI and ICIs. Nonetheless, to minimize these impacts, HRs obtained from multivariate analyses were favored in our study, and stratified analyses by several important factors were performed. Moreover, the sample sizes were relatively small in several meta-analyses, which limited the power of our analysis. For instance, only three and six studies with ORR and irAEs data available, respectively, were included in the analyses. We were unable to conduct subgroup analyses for ORR because of the insufficient sample size, thus failing to explore the potential sources of heterogeneity. Similar concerns also existed in the dose–response analyses and in several subgroup analyses. Therefore, these results must be interpreted with caution, and further research is required to provide more definitive evidence.
Conclusion
In conclusion, the results of this systematic review and meta-analysis supported that higher BMI prior to the treatment of ICIs was significantly associated with improved OS and PFS in cancer patients receiving ICIs, regardless of the different comparative models of BMI categories. Most stratified analyses also showed similar pooled risk estimates. In addition, the limited number of studies on ORR also suggested the existence of a positive association. Regarding adverse events, no statistically significant differences were found in the incidence of any grade and ≥ 3 grade irAEs between higher and lower BMI. Further large-scale prospective research is warranted to better illuminate the association between BMI and outcomes from ICIs. Besides, considerably more work regarding the biological mechanisms underlying these associations will be worthwhile.
Data availability
All data and material analyzed during this study are included in this article.
References
Ghahremanloo A, Soltani A, Modaresi SMS, Hashemy SI (2019) Recent advances in the clinical development of immune checkpoint blockade therapy. Cell Oncol (Dordr) 42:609–626
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028
McDermott DF, Sosman JA, Sznol M et al (2016) Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol 34:833–842
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17:e542–e551
Taube JM, Klein A, Brahmer JR et al (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20:5064–5074
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373:23–34
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Daud AI, Wolchok JD, Robert C et al (2016) Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in Melanoma. J Clin Oncol 34:4102–4109
Gettinger SN, Horn L, Gandhi L et al (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33:2004–2012
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532
Shen X, Zhao B (2018) Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 362:k3529
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638
Reeves GK, Pirie K, Beral V et al (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335:1134
Cortellini A, Bersanelli M, Buti S et al (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immuno Ther Cancer 7(1):57
Kichenadasse G, Miners JO, Mangoni AA et al (2019) Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol 6(4):512–518
McQuade JL, Daniel CR, Hess KR et al (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19(3):310–322
Wang Z, Aguilar EG, Luna JI et al (2019) Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 25:141–151
Cortellini A, Bersanelli M, Santini D et al (2020) Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer 128:17–26
Bergerot PG, Bergerot CD, Philip EJ et al (2019) Targeted therapy and immunotherapy: effect of body mass index on clinical outcomes in patients diagnosed with metastatic renal cell carcinoma. Kidney Cancer 3:63–70
Dumenil C, Massiani MA, Dumoulin J et al (2018) Clinical factors associated with early progression and grade 3–4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS ONE 13:e0195945
Xu H, Cao D, He A, Ge W (2019) The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol 74:105745
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6:40–57
Hamling J, Lee P, Weitkunat R, Ambühl M (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27:954–970
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
De Giorgi U, Procopio G, Giannarelli D et al (2019) Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res 25:3839–3846
Donnelly D, Bajaj S, Yu J et al (2019) The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immuno Ther Cancer 7(1):222
Heidelberger V, Goldwasser F, Kramkimel N et al (2017) Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs 35:436–441
Ichihara E, Harada D, Inoue K et al (2020) The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer 139:140–145
Katayama Y, Shimamoto T, Yamada T et al (2019) Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med 9(1):102
Kondo T, Nomura M, Otsuka A et al (2019) Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int J Clin Oncol 24:323–327
Labadie BW, Liu P, Bao R et al (2019) BMI, irAE, and gene expression signatures associate with resistance to immune-checkpoint inhibition and outcomes in renal cell carcinoma. J Trans Med 17(1):386
Magri V, Gottfried T, Di Segni M et al (2019) Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manage Res 11:8201–8207
Martini DJ, Kline MR, Liu Y et al (2020) Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 126:575–582
Minami S, Ihara S, Tanaka T, Komuta K (2020) Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World J Oncol 11:9–22
Naik GS, Waikar SS, Johnson AEW et al (2019) Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J ImmunoTher Cancer 7(1):89
Popinat G, Cousse S, Goldfarb L et al (2019) Sub-cutaneous Fat Mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology 8:e1580128
Qi Y, Liao D, Fu X et al (2019) Elevated platelet-to-lymphocyte corresponds with poor outcome in patients with advanced cancer receiving anti-PD-1 therapy. Int Immunopharmacol 74:105707
Richtig G, Hoeller C, Wolf M et al (2018) Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS ONE 13:e0204729
Rogado J, Romero-Laorden N, Sanchez-Torres JM et al (2020) Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology 9:1751548
Shiroyama T, Suzuki H, Tamiya M et al (2018) Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non–small cell lung cancer. Cancer Med 7:13–20
Albiges L, Hakimi AA, Xie W et al (2016) Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol 34:3655–3663
Dahlberg SE, Schiller JH, Bonomi PB et al (2013) Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 8:1121–1127
Sepesi B, Gold KA, Correa AM et al (2017) The Influence of body mass index on overall survival following surgical resection of non-small cell lung cancer. J Thorac Oncol 12:1280–1287
Lee CS, Devoe CE, Zhu X et al (2020) Pretreatment nutritional status and response to checkpoint inhibitors in lung cancer. Lung Cancer Manag 9:LMT31
Naik GS, Waikar SS, Johnson AEW et al (2019) Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer 7:89
Schneider G, Kirschner MA, Berkowitz R, Ertel NH (1979) Increased estrogen production in obese men. J Clin Endocrinol Metab 48:633–638
Funding
This work was financially supported in part by the China National Major Project for New Drug Innovation (2017ZX09304015) and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-001).
Author information
Authors and Affiliations
Contributions
YKS, HZC, and DQW designed the study. HZC and DQW performed the systematic search. HZC, DQW, QFZ, YXT, and YZ selected eligible articles and conducted the quality assessment. HZC and DQW analyzed, interpreted the data, and drafted the manuscript. YKS revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Wang, D., Zhong, Q. et al. Pretreatment body mass index and clinical outcomes in cancer patients following immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother 69, 2413–2424 (2020). https://doi.org/10.1007/s00262-020-02680-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02680-y