Abstract
Chemotherapy is the preferred treatment for advanced stage gastric cancer (GC) patients, and developing chemoresistance is a tremendous challenge to efficacy of GC treatment. The treatments of anti-tumor chemo-agents recruit more tumor-associated macrophages (TAMs) which are highly implicated in the chemoresistance development, but the underlying molecular mechanism is unclear. Here, we demonstrate that hypoxia-inducible factor 1α (HIF1α) in GC cells is activated upon 5-fluorouracil (5-FU) treatment and results in much more accumulation of M2-type TAMs which protect tumor cells from chemo-agents. Mechanistically, in the GC cells under the 5-FU treatment, reactive oxygen species is accumulated and then induces the activation of HIF1α signaling to drive the expression of high-mobility group box 1, which leads to more macrophage’s infiltration into GC tumor. In turn, the recruited TAMs exhibit tumor-protected M2-type phenotype and promote the chemoresistance of GC cells via producing growth differentiation factor 15 (GDF15) to exacerbate the fatty acid β-oxidation in tumor cells. Blocking GDF15 using antibody or inhibiting FAO of tumor cells by etomoxir efficiently gave rise to the tumor cell sensitivity to 5-FU. Therefore, our study demonstrates a novel insight in understanding the cross talking between tumor cells and immune microenvironment and provides new therapeutic targets for clinic treatments of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the second common cause of cancer-related death globally and causes a growing heavy health burden in China [1]. For chemotherapy to GC patients in advanced stage, 5-fluorouracil (5-FU)-based treatments are well preferred as the first-line therapy worldwide [2, 3]. However, the development of intrinsic or acquired chemoresistance is a tremendous challenge to efficacy of GC treatment.
The mechanism of chemoresistance developed in GC is definitely complicated and not well elucidated. Many genetic or microenvironmental factors contribute to the development of chemoresistance in GC [4]. In response to the hypoxic environment inside many solid tumors, HIF1α translocates to the nucleus under hypoxia to form a functional heterodimer with HIF1β and transactivates a series of genes involved in angiogenesis, invasion, energy metabolism, tumor growth and poor prognosis of gastric cancer [5]. Accumulative clinical studies reveal the significant association between HIF1α expression and prognosis of gastric cancer [6,7,8]. HIF1α is closely correlated to both overall and disease-free survivals in GC after gastrectomy [9], and higher expression of HIF1α is considered as a promising independent prognostic biomarker [10]. It is interesting to note that OXPHOS damage leading to elevated ROS could enhance the stability of HIF1α even in normoxia via blocking the activity of prolyl hydroxylase domain protein (PHD), which hydroxylates HIF1α to promote its proteasomal degradation [11]. Meanwhile, the activation of HIF1α signaling in tumor cells displays the high correlation with the infiltration of TAMs, which play an important role in aggressive behavior and chemoresistance of gastric cancer [12,13,14], but the molecular mechanism still remains unclear.
As the most abundant immune cells in the microenvironment of solid tumors, TAMs preferentially accumulate in hypoxic area and polarize into two specific cell types, resembling as classically activated macrophages (M1 type) and alternatively activated macrophages (M2 type) [15]. In contrast to anti-tumor M1-type macrophages, M2-type macrophages exert a tumor-supporting role by producing anti-inflammatory substrates, cytokines and growth factors to suppress the host immune response and engage in the activation of growth enhancing signaling pathways in tumor cells [16].
Accumulative studies report that the treatments of anti-tumor chemo-agents (i.e., 5-FU) lead to the recruitment of circulating monocytes to the tumor sites and the tumor-infiltrating macrophages [17, 18]. It remains obscure how tumor cells under anti-tumor therapies cross talk with TAMs, however. In the present study, we report that HIF1α signaling activated by 5-FU drives the expression of damage-associated molecular patterns (DAMPs) HMGB1, which induce more macrophages infiltration in gastric cancer tumor. Infiltrated macrophages display tumor-protective M2-type phenotype and secret high levels of GDF15 to enhance the chemoresistance of tumor cell by promoting fatty acids β-oxidation. Overall, our current study demonstrates a novel insight in understanding the cross talking between gastric tumor cells and macrophages and provides new therapeutic targets for clinic treatments of gastric cancer.
Materials and methods
Cell assays
Cell culture
Human gastric cancer cell line AGS cells were obtained from ATCC (#ATCC® CRL-1739™). Cells were cultured in Ham’s F-12 K (Kaighn’s) medium (#21127022, Thermo Fisher Scientific) supplemented with 10% v/v fetal bovine serum (Gibico, Thermo Fisher) and maintained at 37 °C in a humidified atmosphere of 5% CO2. 5-FU and VCR were purchased from Shanghai Xudong Haipu Pharmaceutical Co. Ltd. (Shanghai, China). For cell treatments, 5-FU or VCR was added into medium at the concentrations of 0.1 mg/ml or 0.1 nmol/l, respectively.
For derivation of stably HIF1a mutant overexpression cell lines, lentiviruses expressing human HIF1a mutants (HIF1a-ΔODD, HIF1a-R27G) were prepared based on the constructs of pLenti-HIF1a-ΔODD-CMV-MCs-SV-puro, pLenti-HIF1a-R27G-CMV-MCs-SV-puro or blank vector. AGS cells were infected with the indicated lentivirus by applying 4–6 cfu/cell of virus in a total of 5 ml of culture medium containing 4 μg/ml of polybrene for 48 h. Then, the cells were maintained in the medium with 2 μg/ml puromycin and stable clones were isolated by the puromycin selection.
For GDF15 treatment, murine recombinant GDF15 proteins (#8944-GD-025, R&D Systems) were added into the culture medium of AGS cells at a concentration of 100 ng/ml for indicated time. For blocking intracellular fatty acid oxidation, etomoxir (#ab144763, Abcam) was added into the cell medium at a concentration of 1 μmol/l.
Murine BMDM preparation
Murine bone marrow-derived macrophages (BMDMs) were prepared as previously described [19]. In brief, bone marrow cells were isolated from the femurs and tibias of 8-week-old male C57BL/6J mice. Subsequently, the isolated cells were maintained in L929-cell-derived differentiation medium for 7 days to be induced to differentiate into mature macrophages.
Conditional media preparation and transwell assay
For the preparation of conditional media, control AGS cells or cells stably expressing HIF1a mutants were treated with 0.1 mg/ml 5-FU for 6 h and then washed with PBS for three times before the addition of fresh serum-free medium. Following another 24-h culture, the culture media were collected and centrifuged at a speed of 3000g for 20 min to get rid of the debris.
Infiltration ability of macrophages was evaluated by transwell assays which were performed by the using of transwell plate (#CLS3422, Corning) according to the manufacturer’s protocol. Briefly, 5 × 103 BMDMs suspended in serum-free medium were added to the top chamber and condition media (CM) as indicated were added to the bottom chamber. After 2-h coculture, the infiltrated macrophages were subsequently fixed by methanol and then subjected to nuclear staining by DAPI. For quantification, the cells were counted under a microscope in randomly selected five predetermined fields.
Colony formation
Sensitivity of cells to 5-FU was assessed by colony-formation assay as previously described [20]. In brief, 5 × 102 single-cell suspended AGS cells were seeded into each well of 6-well plates (Corning) together with or without 100 TAMs freshly isolated from xenograft tumors of control group. Subsequently, the cells were treated with vehicle or 5-FU (0.1 mg/ml) for 24 h. After the drug removed, the cells were washed with PBS and then cultured in fresh medium with the addition of 360 ng anti-GDF15 neutralizing antibodies [21] (#AF957, R&D Systems, Minneapolis, MN) for another 14 days, during which the cell medium was replaced every 4 days. The colonies were then fixed with 4% PFA for 30 min followed with 0.1% crystal violet staining.
Animal experiments
Male BALB/C nude mice (4-week old) were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China). The mice were maintained on a 12-h light/dark cycle in a temperature-controlled environment (~ 22 °C) and given free access to water and food. The mice were randomly assigned into different groups for indicated assays.
For xenograft tumor implantation, AGS cells suspensions (5 × 105 cells) were subcutaneously injected into the dorsal flank of per male athymic nude mouse (~ 5 weeks old) in a laminar flow cabinet via 22-gauge needle. Tumor volume in nude mice was measured by touch and digital calipers every other day. As soon as the mean tumor size reaches 10 mm, the mice carrying xenograft tumor were administered intravenously with vehicle or 5-FU solution (200 μl; 0.18 mg/dose solved in 25% cremophor; 25 mg/kg/week; Shanghai Xudong Haipu Pharmaceutical), or 5-FU in combination with anti-HMGB1 Ab (#CABT-B8909, CD Creative Diagnostics; 2 mg/kg/mouse per time) three times a week. All experiments in this study were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Fudan University.
The isolation of tumor-associated macrophages from xenograft tumors
TAMs were isolated about 3 weeks after tumor implantation as previously described [22]. In brief, dissected solid tumors were disaggregated by stirring in HBSS containing 1 mg/ml collagenase A (#10103578001, Sigma) and 0.1 mg/ml DNase I (#03724751103, Roche Diagnostics) in 37 °C water bath for 1 h. Then, the digestion suspension was filtered through a 100-µm cell strainer (BD FalconTM, Singapore) into a 50 ml falcon tube. After centrifuged and incubated with RBC lysis buffer (#00430054, eBioscience), cells were then filtered with 40-mm cell strainer and then washed twice with flow buffer (Dulbecco’s phosphate buffered saline [#D8537, Sigma] containing 2% FBS). For macrophages sorting, primary antibodies were added to the cells after incubation with blocking buffer containing anti-mouse CD16/CD32 Fc Block 1:200 for 15 min at 4 °C. The cells were then washed once and resuspended in flow buffer before sorted by a BD Biosciences FACSAria cytometer. The primary antibodies and the working concentrations are as follows: CD45-PerCP/Cyanine5.5 1:400 (Biolegend, clone 30-F11, #103132), CD11b-Pacific Blue 1:200 (Biolegend, clone M1/70, #101224), F4/80-PE (Biolegend, clone BM8, #123110). For ROS detection, AGS cells were treated by 5-FU (1 mg/ml) or VCR1 (0.1 nmol/l) for 24 h and subjected to DCFDA staining using DCFDA/H2DCFDA cellular ROS assay kit (#ab113851, Abcam) according to the manufacturer’s instructions prior to flow cytometry analysis. All the flow cytometry results were analyzed, and plots were generated with FlowJo.
Quantitative real-time PCR
qRT-PCR was performed as previously described [23]. Briefly, the samples of tissues or cells were fixed and lysed by TRizol reagent (#15596026, Invitrogen, Carlsbad, CA, USA) and then prepared for total RNA extraction according to the manufacturer’s instructions. RNA contents were measured by Nano2000 and 2 μg of total RNA each sample was then used for cDNA synthesis by the using of M-MLV reverse transcriptase following the manufacturer’s instructions. Indicated mRNA levels were determined by quantitatively real-time PCR using SYBR Premix Ex Taq (ABI) and RPS18 of human and mouse that were used as internal control, respectively.
Immunoblotting
Immunoblotting was conducted as previously described [24]. Briefly, the samples of cells or tissues were homogenized for dissecting in RIPA lysis buffer before centrifuging. The supernatant was collected with the adding of SDS-PAGE loading buffer and then incubated in 100 °C for 10 min. The proteins in the gel were transferred to nitrocellulose membrane after separated by the using of SDS-PAGE. Following the incubation of the membrane with 5% BSA for 1 h in room temperature, membranes were, respectively, subjected to an overnight incubation with the primary antibodies at 4 °C and then incubated in horseradish peroxidase-conjugated secondary antibodies. The primary antibodies and the working concentrations are as follows: anti-HIF1α 1:500 (#NB100-105, Novus Biologicals) and anti-β-actin 1:5000 (#A1978, Sigma). Proteins were detected by enhanced chemiluminescence assay (Thermo Fisher Scientific).
Luciferase reporter assay
HRE luciferase reporter plasmid was obtained from Addgene (#26731). For generating HMGB1 promoter luciferase reporter, the pGL3 basic plasmid was constructed with the insertion of the promoter of human HMGB1 gene, corresponding to the region of − 2000 to + 100 with respect to the putative transcription start site (TSS, denoted nucleotide + 1). The designed plasmids were transfected into AGS cells in combination with Renilla plasmids, and luciferase activities were measured using Dual-Luciferase Assay Kit (#E1910, Promega) according to the manufacturer’s instructions. Renilla activity was used as the internal control.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described [25]. AGS cells were transfected with plasmids and treated with vehicle or 5-FU as indicated. Subsequently, the cells were cross-linked with 1% formaldehyde in PBS for 10 min at 37 °C and quenched in 125 mM glycine in PBS for 5 min at 4 °C. The cells were then lysed in Farnham lysis buffer (5 mM PIPES pH 8.0, 85 mM KCl, 0.5% NP-40, 1 mM DTT and protease inhibitor cocktail [Sigma, #P8340]) to obtain nuclear material. Nuclear pellets were collected by centrifugation and resuspended in lysis buffer (5 mM Tris–HCl pH 7.9, 1% SDS, 10 mM EDTA, 1 mM DTT and protease inhibitor cocktail [Sigma, #P8340]) and incubated on ice for 10 min. The chromatin fragmentation was performed at 4 °C by ultrasonic to generate chromatin fragments of 100–1000 bp in length. The soluble chromatin fragments were pre-cleared using Protein G Sepharose 4 Fast Flow (#17-0618-01, GE Healthcare Bio-sciences) for 1 h at 4 °C. The pre-cleared samples were incubated with anti-HIF1α antibody ((#NB100-105, Novus Biologicals) at 4 °C for overnight before the addition of Protein G Sepharose 4 Fast Flow (#17-0618-01, GE Healthcare Bio-sciences) at 4 °C for 2 h to capture the antibody–protein–DNA complexes. After eluted using elution buffer (100 mM NaHCO3, 1% SDS), the IP material was digested with RNase (#11119915001, Roche) and proteinase K (#EO0491, Thermo Fisher Scientific) before the purification and concentration of the immunoprecipitated genomic DNA by ChIP DNA Clean & Concentrator kit (#D5201, Zymo Research). ChIP-isolated DNA was subjected to qPCR (ChIP-qPCR). The primers for the qPCR are as follows: for − 1623 to − 1505, forward: 5′-ccggtggccgcgcggccgaggag, reverse: 5′-gagcctgacaaaatgggggcggc; for − 2478 to − 2313, forward: 5′-tgacattttgcctctcggcttct, 5′-ctactgtgttaaataaccagtactt.
Seahorse analysis
FAO assays were conducted by the using of Seahorse XF24e extracellular flux analyzer (Agilent Technologies, Santa Clara, CA, USA) according to the manufacture’s instruction. In brief, AGS cells were plated in XF cell culture microplates and cultured in normal medium for 4 h which were then replaced with substrate-limited medium for 16 h more culture. FAO assay medium was added before the assay followed with the addition of XF palmitate-BSA FAO substrate (#102720-100, Agilent Technologies). The oxygen consumption rate (OCR) was detected by Mito stress tests protocols of Seahorse XF24e extracellular flux analyzer.
IC50 determination
Cell viability was determined by the using of the CellTieter-Glo luminescent cell viability assay (#7570, Promega) according to the manufacturer’s instructions. In brief, AGS cells were grown in 96-well plates and then treated with 5-FU at serial concentrations of 0, 0.5, 1, 5, 10, 50, 100, 200 and 500 ug/ml for 48 h. After 48-h treatment, the cells were incubated with CellTiter-Glo reagent for 10 min at room temperature. The luminescence value was measured using luminometer. The 50% inhibitory concentration (IC50) for 5-FU, which is defined as the 5-FU concentration which causes a 50% reduction in luminescence relative to the untreated control, is determined directly from semilogarithmic dose–response curves.
Statistical analysis
All experiments presented in this paper have been repeated more than three times. Data are presented as the mean ± standard error of means (SEM). Statistical analysis (Graphpad Prism 8.0) was performed using the two-tailed unpaired Student’s t test after a demonstration of homogeneity of variance with the F test or one-way or two-way ANOVA for more than two groups. Statistical significance was set as p < 0.05.
Results
Elevated ROS accumulation activates HIF1α signaling in gastric cancer cells upon chemotherapeutic agent treatment
5-FU, as one of the main chemotherapeutic drugs against cancer, can block the synthesis of the pyrimidine thymidine to cause cellular damages to tumor cells [26]. We hypothesized that 5-FU treatments could cause the production of reactive oxygen species (ROS). To this end, 2′,7′-dichlorofluorescin diacetate (DCFDA) was applied to stain the cells which have intracellular ROS accumulation. Utilizing flow cytometry to determine DCFDA signal, we found that the addition of 5-FU robustly gives rise to the frequency of ROS-positive cells in gastric cancer cell line AGS cells (Fig. 1a). Additionally, vincristine (VCR), another chemotherapy agent, also increases the percentage of AGS cells which carries high ROS levels (Fig. 1a). It is well appreciated that high levels of ROS are linked to the accumulation of HIF1α proteins [11, 27]. Consistently, the HIF1α protein levels appear to be much higher when the AGS cells are treated by 5-FU or VCR (Fig. 1b). To determine whether HIF1α signaling is activated by the chemo-agents’ treatments, luciferase reporter assays were conducted to evaluate the transcriptional activity of HIF1α protein. Intriguingly, the addition of both 5-FU and VCR dramatically exacerbates the transcriptional activity of hypoxia response element (HRE), which is main binding site for HIF1α (Fig. 1c). It is worthy to notice that the transcriptional activities of HRE induced by 5-FU or VCR display in a time-dependent manner, strongly suggesting that the activation of HIF1α signaling is not directly caused by chemo-treatment (Fig. 1c).
Chemo-agents activate HIF1α signaling via inducing ROS accumulation in gastric cancer cells. a Representative images of flow cytometry analysis of intracellular ROS. AGS cells were treated by 0.1 mg/ml 5-FU or 0.1 nmol/l VCR for 24 h and then stained with DCFDA for the ROS analysis by flow cytometry. ROS-positive cells were quantified. n = 5 for each group. b Representative images of immunoblotting of HIF1α and β-actin (internal control) in the cell extracts of AGS cells 24 h after treatments by VCR or 5-FU. c Luciferase activities were determined in AGS cells treated by 5-FU or VCR for indicated time. d Luciferase activities were determined in AGS cells treated with vehicle, 5-FU, 5-FU combined with NAC, VCR, or VCR combined with NAC, respectively. For c, d AGS cells were transfected with reporter constructs within which luciferase expression is driven by the activated HRE element. The expression of luciferase is driven by the HRE element. Luciferase activity is presented as fold change after normalizing values of Luc to Renilla (RLU). The values of vehicle control group were set as “1”. All data are shown as mean ± SEM. *p < 0.05; **p < 0.01; or ***p < 0.001 by one-way ANOVA
To test whether ROS is required for the activation of HIF1α signaling during 5-FU and VCR treatment, we introduced N-acetyl-l-cysteine (NAC) to block ROS accumulation in GC cells. Impressively, the addition of NAC efficiently impairs the activation of HIF1α in AGS cells upon the 5-FU and VCR treatment, respectively (Fig. 1d). Together, these data reveal that the production of ROS during chemotherapy treatments results in a pseudo-hypoxia in gastric tumor cells and then activates the HIF1α signaling.
HIF1α-driven HMGB1 exacerbates the macrophage accumulation
Anticancer agents (i.e., 5-FU) treatment was reported to amplify the recruitment of tumor-associated macrophages (TAMs) [17, 18]. Linking to the cellular damages caused by therapeutic agents, we hypothesized that the damage-associated molecular patterns (DAMPs) released by injured tumor cells induce the macrophages infiltration into tumors. To this end, we tested the expression levels of HMGB1, one of the major DAMPs, in gastric tumor cells. The expression levels of HMGB1 mRNA were robustly amplified after the adding of VCR (~ 16-fold) or 5-FU (~ 35-fold, Fig. 2a). To determine whether HIF1α signaling is involved in regulating HMGB1 in the setting of 5-FU treatment, we introduced siRNA to abrogate endogenous HIF1a, or two HIF1a mutants to modulate HIF1α signaling: (1) HIF1a-ΔODD, the truncation without oxygen-dependent degradation (ODD) domain renders the stability of HIF1α and enables its transcription activity even in normoxia; and (2) HIF1a-R27G, the point mutation with replacing Arg-27 with glycine to impair its DNA binding capacity [28]. Intriguingly, the abrogation of HIF1a dramatically represses the expression of HMGB1 which is increased upon 5-FU treatment (Fig. 2b). Even with the presence of 5-FU, enforced expression of HIF1a-ΔODD mutant still upregulates HMGB1 mRNA levels, indicating that HIF1α is sufficient to activate HMGB1 transcription in AGS cells (Fig. 2c). Consistently, blocking DNA binding of HIF1α by overexpressing HIF1a-R27G efficiently impairs HMGB1 expression which is activated by 5-FU treatment (Fig. 2c), supporting that HIF1α signaling is required in regulating HMGB1 expression during 5-FU treatment. To confirm that the upregulated HMGB1 could be secreted by AGS cells, we used ELISA to determine the content of extracellular HMGB1 proteins. In agreement with mRNA changes, 5-FU robustly increases the HMGB1 contents in cell culture (Fig. 2d). Moreover, in the presence of 5-FU treatment, ectopic HIF1a-R27G significantly reduces the HMGB1 production, while HIF1a-ΔODD overexpression obviously raises HMGB1 contents in AGS cell culture (Fig. 2d).
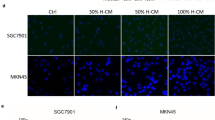
HIF1α drives HMGB1 expression in 5-FU-treated gastric cancer cells to exacerbate macrophage infiltration. a qRT-PCR analysis of HMGB1 mRNA levels of AGS cells in the presence of 5-FU (0.1 mg/ml), VCR (0.1 nmol/l) or vehicle control for 24 h. b qRT-PCR analysis of HMGB1 mRNA levels of AGSs in the presence of 5-FU or vehicle. The AGS cells were transfected with siRNA targeted at HIF1a (siHIF1a) or negative control (NC) prior to 5-FU or vehicle treatment. c, d, AGS cells were transfected with empty vector (control) or constructs expressing human HIF1a mutants HIF1a-ΔODD or HIF1a-R27G. (c) qRT-PCR analysis of HMGB1 mRNA levels of 5-FU-treated cells and d ELISA determined the HMGB1 protein levels in the cell culture of AGS cells after treated by vehicle or 5-FU. e AGS cells were transfected with reporter constructs within which luciferase expression is driven under HMGB1 full-length promoter (2000 bp upstream of TSS) or HRE-truncated promoter in combination with empty vector (control) or constructs expressing HIF1a-ΔODD or HIF1a-R27G, respectively. Luciferase activity is presented as fold change after normalizing Luc values to Renilla activity (RLU). The values of vehicle control group were set as “1”. f ChIP assays were conducted by the using of IgG or anti-HIF1α antibody in cell lysis from AGS cells which were transfected with control vector or constructs expressing HIF1a-ΔODD or HIF1a-R27G followed with treatments of 0.1 mg/ml 5-FU or vehicle. qRT-PCR analyses of immunoprecipitated DNA were performed using the primers which were designed to amplify the indicated region of the HMGB1 promoter. g Representative images of transmembraned macrophages counterstained with DAPI. Transwell assays of macrophages were conducted in the presence of conditioned media derived from the cell culture of AGS cells which were previously subjected to indicated treatments. All data are shown as mean ± SEM. *p < 0.05; **p < 0.01; or ***p < 0.001 by unpaired two-tailed Student’s t test or one-way ANOVA
Based on these findings, we hypothesized that HIF1α directly activates the transcription of HMGB1 in gastric cancer cells. After searching the genomic sequence in the upstream of human HMGB1 transcription starting site (TSS), we found the hypoxic response elements (HRE) “ACGTGC” in HMGB1 promoter region [29], suggesting the DNA binding site for HIF1α (Fig. 2e). To test whether HIF1α could directly drive the transcriptional activity of HMGB1 promoter, we cloned the 2 kB upstream sequence of human HMGB1 TSS and constructed a luciferase reporter driven by HMGB1 promoter (Fig. 2e). Compared to vehicle, the addition of 5-FU highly elevates the luciferase activity in AGS cells (Fig. 2e). Inconsistent with the above results (Fig. 2c, d), enforced expression of HIF1a-ΔODD mutant apparently gives rise to the transcriptional activity of HMGB1 promoter, while overexpressed HIF1a-R27G impairs the effects caused by 5-FU (Fig. 2e). To confirm that HRE is required for HIF1α-regulated HMGB1 promoter activity, we generated a truncated HMGB1 promoter luciferase reporter construct within which HRE is deleted. Impressively, the deletion of HRE in HMGB1 promoter almost abolishes the upregulation of luciferase activity induced by 5-FU treatment or HIF1a-ΔODD overexpression (Fig. 2e). To further investigate whether HIF1α directly binds to HMGB1 promoter, we conducted chromatin immunoprecipitation (ChIP) in the cell extracts of AGS cells by the using of anti-HIF1α antibodies. Impressively, 5-FU treatment or HIF1a-ΔODD overexpression dramatically enhances the capacity of anti-HIF1α Ab in precipitating the genomic region from -1623 to -1505 in the upstream of HMGB1 TSS, within which HRE is included (Fig. 2f). In contrast to these, either 5-FU treatment or HIF1a-ΔODD overexpression does not alter the anti-HIF1α Ab precipitating capacity to the promoter region of − 2478 to − 2313 in the upstream of HMGB1 TSS (Fig. 2f). Together, these data strongly support our hypothesis that activated HIF1α drives the transcription of HMGB1 in AGS cells upon 5-FU treatment.
To explore the physiological function of HMGB1 derived from gastric tumor cell, we applied a transwell system to evaluate macrophage infiltration (Fig. 2g). Intriguingly, the conditioned media derived from 5-FU-treated AGS cells, which are transfected with control vector or HIF1a-ΔODD mutant, induces much more macrophages infiltration relative to that of vehicle-treated AGS cells (Fig. 2g). In contrast to these, blocking HIF1α signaling by HIF1a-R27G overexpression in tumor cells obviously attenuates macrophages infiltration capacity upon conditioned media (Fig. 2g). To determine that HMGB1 is required for the macrophage infiltration induced by conditioned media, we utilized antibodies to neutralize the HMGB1 protein in conditioned media and siRNA to silence endogenous HMGB1 mRNA. Much less macrophage infiltration was observed when conditioned media were previously incubated with anti-HMGB1 Ab or derived from siHMGB1-transfected AGS cells (Fig. 2f).
HIF1α is required for macrophage accumulation of gastric cancer
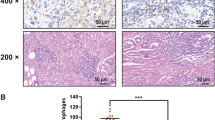
To explore the role of HIF1α signaling in regulating tumor-associated macrophages (TAMs) in vivo, we used xenograft tumor mouse model in which AGS cells are subcutaneously implanted in nude mice. Relative to the vehicle-treated control group, the treatment of 5-FU apparently exacerbates the number of F4/80-positive macrophages (Fig. 3a), as well as the ratio of CD11b+F4/80+ macrophages in CD45+ immune cells (Fig. 3b, c) in the xenograft tumor. Meanwhile, the amounts of both CD68+ cells and CD11b+F4/80+ macrophages are increased in the HIF1a-ΔODD-expressing tumor in which HIF1α signaling of tumor cells is constitutively activated (Fig. 3a–c). Consistently, the tumor derived from HIF1α-R27G-expressing AGS cells displays obviously reduced contents of both F4/80+ macrophages and CD45+CD11b+F4/80+ immune cells (Fig. 3a–c). Next, we analyzed the genes expression levels in the tumors to assess the phenotype of TAMs. Compared to vehicle group, tumors derived from either wild type or HIF1a-ΔODD-expressing AGS cells display much higher mRNA levels of HMGB1 during the 5-FU treatment (Fig. 3d). Moreover, HMGB1 expression levels is relatively lower in the HIF1a-R27G-expressing tumors (Fig. 3d). In agreement with our ex vivo results, mRNA levels of pan-macrophage markers genes ANGRE1 (coding F4/80 protein) and CD68 change in the same pattern as HMGB1 in tumors, indicating the closely correlation of HMGB1 expression and macrophages accumulation (Fig. 3d). According to the critical role of alternatively activated macrophages in promoting the tumor progression [16], we also assessed the marker genes expression for macrophage alternative activation in tumors. Intriguingly, with the increased macrophages contents, these alternative activation marker genes (IL-10, CCL17, CCL18, CCL22, MRC1, ARG1) expression levels are robustly increased in the 5-FU-treated tumors of control and HIF1a-ΔODD groups, but not in the tumors of HIF1a-R27G group (Fig. 3e). To explore the function of HMGB1 in regulating TAMs infiltration in response to 5-FU treatment, we administered the mice with anti-HMGB1 Ab to neutralize the HMGB1 proteins in the body. Without impacting the expression of HMGB1, the treatment of anti-HMGB1 Ab results in dramatic reductions of 5-FU-promoted macrophages contents (Fig. 3a–d) and impairs the expression levels of genes involved in macrophage alternative activation (Fig. 3e). As expected, the treatment of 5-FU causes obviously smaller tumors which derived from control or HIF1a-ΔODD-expressing AGS cells (Fig. 3f). Impressively, relative to control tumors, the tumors derived from HIF1a-R27G-expressing cells or treated by anti-HMGB1 Ab exhibit much less in size after the chemotherapy of 5-FU (Fig. 3f), suggesting that the insensitivity of tumor to 5-FU is dependent on HIF1α activity and HMGB1 secretion.
HIF1α signaling is required for recruiting and activating TAMs by 5-FU-treated GC. AGS cells were transfected with lentivirus to derive new cell lines which stably express GFP (control) or HIF1α mutants (HIF1a-ΔODD or HIF1a-R27G), respectively. 5 × 106 cells per cell line were implanted into nude mice at flank to induce tumor formation (n = 6 per group). The mice were subsequently i.p. administered with vehicle or 5-FU, or 5-FU in combination with anti-HMGB1 Ab as indicated. a Representative images of immunostaining of the xenograft tumors sections for F4/80. b Representative images of flow cytometry analysis of macrophages in xenograft tumor using anti-CD45, anti-F4/80 and anti-CD11b flow antibodies. TAMs were gated as CD45+CD11b+F4/80+ cells. c The frequencies of TAMs in CD45+ cells of each group were quantified. d qRT-PCR analysis of mRNA levels of HMGB1 and macrophage markers (ANGRE1 and CD68) in xenograft tumor. e qRT-PCR analysis of mRNA levels of indicated genes in xenograft tumor. f Tumor sizes of each group. All the data are shown as mean ± SEM. *p < 0.05; **p < 0.01; or ***p < 0.001 by one-way ANOVA
TAMs enhance the chemoresistance of GC via secreting GDF15
High TAMs content is reported to be closely correlated with the chemoresistance of GC [30]. To investigate the role of TAMs in regulating the tumor cell chemoresistance, we applied a cells coculture system in which AGS cells are cultured together with freshly sorted TAMs from xenograft tumors (Fig. 4a). Relative to vehicle group, the AGS cells display much more chemoresistance when cultured with TAMs from 5-FU-treated tumors of control and HIF1a-ΔODD groups (Fig. 4a). In contrast to these, TAMs from 5-FU-treated tumors of HIF1a-R27G or anti-HMGB1 group do not show any impacts on the sensitivity in response to 5-FU (Fig. 4a). GDF15 has been implicated in the resistance to chemotherapeutic drugs in many types of cancers, including gastric, lung, prostate, colon, ovarian and breast cancer [31, 32]. Particularly, increased GDF15 expression is found in gastric cancer and it is worth to note that GDF15 levels are even higher in non-improvement patients when compared to that of improvement patients, reflecting the closed correlation between chemoresistance of GC and GDF15 [33]. Various cell types within tumors could express GDF15, including tumor cells, macrophages and fibroblast stromal cells [34]. Interestingly, we found apparently heightened Gdf15 expression in infiltrated TAMs sorted from 5-FU-treated tumors of control group and HIF1a-ΔODD group (Fig. 5b), while 5-FU treatment does not impact the GDF15 expression in AGS cells (Fig. S1a), suggesting that TAMs produce GDF15 to confer to chemoresistance of GC cells. To this end, we treated AGS cells with recombinant GDF15 and found that the addition of GDF15 robustly gives rise to the chemoresistance of GC cells in response to 5-FU (Fig. 4c). Furthermore, the coculture with TAMs does not lead to any obvious impacts on the colony-formation ability of AGS cells (Fig. 4d). Surprisingly, the treatment of 5-FU loses its effects on suppressing the colony-formation ability of AGS cells when TAMs are present (Fig. 4d). Neutralizing GDF15 proteins by anti-GDF15 Ab significantly impairs the colony-formation ability of TAMs-cocultured AGS cells (Fig. 4d), indicating that GDF15 is essential for the macrophage-promoted chemoresistance of GC cells. To test this, we used anti-GDF15 Ab to neutralize the proteins in the conditioned media derived from sorted TAMs. Impressively, the addition of Ab efficiently attenuates the chemoresistance to 5-FU of AGCs which are culturing in conditional media derived from TAMs of 5-FU-treated tumors of control or HIF1a-ΔODD groups (Fig. 4e).
Accumulated TAMs elevate the chemoresistance of GC cells via secreting GDF15. a IC50 of 5-FU was determined in AGS cells which were cocultured with TAMs isolated from xenograft tumors as indicated. The tumors were derived from AGS cell lines expressing GFP (control) or HIF1α mutants, and then, the mice were treated with vehicle or 5-FU, or 5-FU in combination with anti-HMGB1 Ab prior to the sorting of macrophages. The sorted TAMs were cocultured with AGS cells in the transwell plates as indicated. b Colony-formation assays were performed using AGS cells with or without the presence of sorted TAMs followed the treatments of vehicle, 5-FU or 5-FU in combination with anti-GDF15 Ab. c IC50 of 5-FU was determined in AGS cells treated with vehicle or recombinant murine GDF15. d qRT-PCR analysis of mRNA levels of Gdf15 in TAMs isolated from the xenograft tumors as indicated. e IC50 of 5-FU was determined in AGS cells which were maintained in indicated conditioned media. Conditioned media were derived from TAMs which were isolated from indicated xenograft tumors and then incubated with IgG or anti-GDF15 Ab. All the data are shown as mean ± SEM. *p < 0.05; **p < 0.01; or ***p < 0.001 by unpaired two-tailed Student’s t test or one-way ANOVA
GDF15 enhances the FAO of tumor cells to reduce chemo-sensitivity as well as promote ROS production. a, b Analysis of mitochondrial FAO in vehicle- or 100 ng/ml GDF15-treated AGS cells by the using of the Seahorse Bioscience XF24e extracellular flux analyzer. c qRT-PCR analysis of FAO-associated genes expression in AGS cells treated with vehicle or 100 ng/ml GDF15. d–f AGS cells were treated with vehicle, 100 ng/ml GDF15 or 100 ng/ml GDF15 in combination with FAO inhibitor etomoxir (ETO, 1 μmol/l). d IC50 of 5-FU was determined in AGS cells. e ROS-positive AGS cells were analyzed using flow cytometry. f Luciferase activity using HRE reporter was determined in AGS cells. g Schematic model. Upon the chemotherapy of 5-FU, ROS is accumulated and activates HIF1α signaling to drive HMGB1 transcription in AGS cells. The increased HMGB1 exacerbates the infiltration of M2-type TAMs, which in turn secret GDF15 to enhance FAO and reduce chemo-sensitivity of 5-FU in gastric tumor cells. All data are shown as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 by unpaired two-tailed Student’s t test or one-way ANOVA
The FAO enhanced by GDF15 promotes GC chemoresistance and sustains ROS production
Given that fatty acid oxidation (FAO) elevates the chemoresistance of gastric cancer cells [20], we explored whether the GDF15 could enhance the β-oxidation of fatty acids in GCs. To this end, we utilized Seahorse assays to determine the intracellular oxidative phosphorylation activity in GCs. The addition of recombinant GDF15 obviously upregulates the mitochondria oxidative phosphorylation levels of GC indicated by oxygen consumption rate (OCR), strongly suggesting the enhanced FAO after GDF15 treated (Fig. 5a, b). In support of this, we also found that GDF15 significantly heightens the expression levels of FAO-associated genes, including CD36, ACSL1, PGC1a, CPT1a, CPT1b and CPT2 (Fig. 5c). Additionally, we utilized siRNA and chemical inhibitors to block AKT and ERK pathways to explore whether GDF15 exerts its functions via AKT and ERK pathways. Interestingly, the impacts of GDF15 on FAO-associated genes expression or chemoresistance of tumor cells are not altered with the abrogation of AKT and ERK by siRNA (Fig. S2a-b) or the adding of LY2780301 (AKT inhibitor) and FR180204 (ERK inhibitor) (Fig. S2c-d), respectively. To test whether the FAO activity promoted by GDF15 is essential for the chemoresistance in response to 5-FU, we used CPT1α inhibitor etomoxir (ETO) to block mitochondria FAO. The ETO addition apparently lowers the IC50 of 5-FU in AGS cells which is robustly elevated by GDF15 treatment (Fig. 5d).
The TCA cycle along with the electron transport chain (ETC) increases ROS production in mitochondria and further contributes to HIF1a stabilization [35]. Interestingly, while promoting mitochondria FAO, GDF15 also exacerbates ROS generation in AGS cells (Fig. 5e) and subsequently the activation of HIF1α signaling (Fig. 5f). Meanwhile, blocking FAO by ETO sufficiently attenuates the ratio of ROS-positive cells (Fig. 5e) as well as HIF1α activity (Fig. 5f). These data indicate the positive feedback from TAMs via secreting GDF15 to enhance the ROS production and sustain HIF1α signaling activation.
Discussion
Enhancing the efficiency of anti-tumor therapy is the most challenge in clinical oncology. In the clinical treatments for gastric tumor, drug resistance in surviving cancer cells is a major challenge to efficacy. The mechanism of GC developing chemotherapy resistance is complicated and not well understood. It is well appreciated that the therapeutic sensitivity of tumors is significantly impacted by the complex interaction of cancer cells with different components of the tumor microenvironment, particularly with immune cells [30]. In our present study, we demonstrate a novel cross talking between tumor cells and tumor-associated macrophages which promotes chemoresistance of gastric cancer cells. In response to chemotherapeutic agents, HIF1α signaling is dramatically activated by the accumulation of ROS and subsequently drives the production of HMGB1. Tumor cell-derived HMGB1 exacerbates the infiltration of macrophages and gives rise to the contents of M2-type macrophages. In turn, accumulative TAMs produce GDF15 to promote the fatty acid β-oxidation of tumor cells and enhance the gastric tumor cells chemoresistance in response to 5-FU (Fig. 5g).
It is well appreciated that increasing ROS is implicated in more HIF1a proteins and higher transcriptional activity via repressing PHDs [36]. Linking to the raised cellular oxygenation level for their maximal efficacy, some chemotherapeutic agents (i.e., 5-FU and VCR) could induce HIF1α expression and transcriptional activity under nonhypoxic conditions, strongly suggesting that the production of ROS contributes to the activation of HIF1α signaling in tumor cells under the context of chemotherapy [37, 38]. According to this, it is not surprising to see that high levels of stabilized and functional HIF1α proteins are observed in AGS cells even without any hypoxia (Fig. 1). Though other contributors cannot be excluded in current study, our data indicate that ROS accumulation is required for HIF1α signaling activation in GC cells in response to 5-FU in the normoxia setting (Fig. 1). It is reported by Liu et al. [39] that chemo-agent-resistant VCR-treated gastric cell line SGC7901 cells display a high level of HIF1α protein under nonhypoxic condition. Mechanistically, Liu et al. found that MGr1-Ag/37LRP is implicated in VCR-inducing transcription HIF1α, which plays a key role in chemoresistance of gastric tumor cells. Further studies are needed to clarify the molecular mechanisms underlying in (1) how chemotherapeutic agents cause the generation of ROS; (2) which molecule(s) mediate(s) the function of ROS on HIF1α signaling (MGr1-Ag/37LRP or PHDs?).
Macrophages accompanied with other myeloid cells generally accumulate in tumors sites under chemotherapy where chemotherapy-damaged cells are localized and the tumor-protective function of TAMs is found in a growing number of studies in vivo and in vitro for some anti-tumor agents, including doxorubicin, platinum compounds, 5-FU, gemcitabine and paclitaxel [17, 18]. Notably, HIF1α is also well appreciated in regulating the biological behaviors of both tumor cells and infiltrated immune cells, including polarization of tumor-associated macrophages (TAMs) [4]. In current study, we found that the activated HIF1α in 5-FU-treated tumor cells drives the expression of HMGB1, which promotes the infiltration of macrophages in vitro and in vivo (Figs. 2, 3). Interestingly, 5-FU treatment was reported to give rise to HMGB1 expression in colon carcinoma cells to induce a striking attraction of leukocytes in an orthotropic model of colon carcinomatosis in vivo and in monocyte migration assays in vitro [40], supporting our study that HMGB1 expression upregulated by 5-FU is required for TAMs accumulation in gastric cancer under chemotherapy.
M2-type macrophages are known to have strong tumor-supporting functions and mediate chemoresistance via secreting growth factors, establishing a local immunosuppressive microenvironment as well as regulating the biological behavior of tumor cells to protect them from the cytotoxic effects of chemotherapy [18, 30, 41]. Notably, we found that the infiltrated TAMs upon 5-FU treatment exhibit a phenotype of tumor-supportive M2-type macrophage which efficiently lower the sensitivity of AGS cells to 5-FU and are essential for chemoresistance of gastric cancer (Fig. 4). It is interesting that the infiltrated macrophages in tumor upon tumor cell-derived HMGB1 exhibit protumoral M2-type macrophages (alternatively activated macrophage) (Fig. 3). Although HMGB1 is usually considered as an alarming factor and causes inflammatory responses, its roles in macrophage activation are controversial, especially in the context of tumor microenvironment. Rojas et al. [42] reported that HMGB1 heightens the M2 activation of macrophages and enhances their protumoral activities via a RAGE-dependent mechanism, indicating that HMGB1 may play a role in promoting macrophage alternative activation within tumor. Alternatively, other factors derived from tumor cells may contribute to M2 activation of macrophage upon 5-FU treatment. For instance, it was reported that tumor cells under chemotherapy also produce high levels of prostaglandin E2 (PGE2) and IL-6, which skew the differentiation of monocytes toward M2-like macrophages and lead to chemoresistance [17]. In the future, many more studies are needed to clarify the molecular mechanisms underlying the macrophage activation in gastric tumors under chemotherapy. In mechanisms, we discovered that TAMs inside the tumors release high levels of GDF15 which confer to the less sensitivity of tumor cells in response to 5-FU via exacerbating the levels of fatty acid β-oxidation in gastric cancer cells (Figs. 4, 5). As a member of the transforming growth factor beta (TGFβ) family, GDF15 is a stress response cytokine expressed by a variety of tissues and cells, including liver, lung, kidney, placental trophoblasts and macrophages [43, 44]. GDF15 expression and serum levels are elevated in anticancer therapies including chemotherapy and ionizing irradiation [43]. The roles played by GDF15 in cancer, however, are limited and controversial. Some data suggest that GDF15 has tumor promoting activity, while other results suggest the opposite [45]. In our current study, we reveal that GDF15 derived from macrophages could impair the chemo-sensitivity of GC cells to 5-FU in a paracrine manner, supporting the tumor-protective role for GDF15 in gastric cancer under chemotherapy. According to the physiological function of GDF15 in regulating cellular metabolism, we found that after the treatment of GDF15, GC cells undergo metabolic reprogramming and shifts to using more fatty acids as fuel (Fig. 5). Compared to carbohydrate, the oxidation of fatty acids in mitochondria provides 2.5-fold ATP for cells. Our data indicate that the 5-FU-enhanced FAO of GC cells is required for the development of chemoresistance since inhibiting FAO by adding etomoxir efficiently raises 5-FU sensitivity of tumor cells (Fig. 5).
In summary, our current study uncovers a novel mechanism underlying the chemoresistance development in GC cells, which tumor cells cross talk with TAMs via secreting factors. These results demonstrate a novel insight in understanding how immune microenvironment forms and the impacts of immune cells on tumor progression in the setting of chemotherapy and provide new therapeutic targets for clinic treatments of gastric cancer.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics. CA Cancer J Clin 66(4):271–289. https://doi.org/10.3322/caac.21349
Digklia A, Wagner AD (2016) Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol 22(8):2403–2414. https://doi.org/10.3748/wjg.v22.i8.2403
Davidson M, Okines AF, Starling N (2015) Current and future therapies for advanced gastric cancer. Clin Colorectal Cancer 14(4):239–250. https://doi.org/10.1016/j.clcc.2015.05.013
Kitajima Y, Miyazaki K (2013) The critical impact of HIF-1a on gastric cancer biology. Cancers (Basel) 5(1):15–26. https://doi.org/10.3390/cancers5010015
Rohwer N, Cramer T (2010) HIFs as central regulators of gastric cancer pathogenesis. Cancer Biol Ther 10(4):383–385. https://doi.org/10.4161/cbt.10.4.12850
Kouroupi M, Sivridis E, Papazoglou D, Koukourakis MI, Giatromanolaki A (2018) Hypoxia inducible factor expression and angiogenesis—analysis in the pituitary gland and patterns of death. Vivo 32(1):185–190. https://doi.org/10.21873/invivo.11223
Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY (2005) Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol 35(4):207–213. https://doi.org/10.1093/jjco/hyi067
Huang SP, Wu MS, Shun CT, Wang HP, Hsieh CY, Kuo ML, Lin JT (2005) Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J Biomed Sci 12(1):229–241. https://doi.org/10.1007/s11373-004-8177-5
Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, Jia B, Wei B, Wei L, Dai G, Jiao S (2014) HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS ONE 9(3):e90678. https://doi.org/10.1371/journal.pone.0090678
Isobe T, Aoyagi K, Koufuji K, Shirouzu K, Kawahara A, Taira T, Kage M (2013) Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1alpha) expression in gastric cancer. Int J Clin Oncol 18(2):293–304. https://doi.org/10.1007/s10147-012-0378-8
Lu X, Kang Y (2010) Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16(24):5928–5935. https://doi.org/10.1158/1078-0432.CCR-10-1360
Zhang WJ, Chen C, Zhou ZH, Gao ST, Tee TJ, Yang LQ, Xu YY, Pang TH, Xu XY, Sun Q, Feng M, Wang H, Lu CL, Wu GZ, Wu S, Guan WX, Xu GF (2017) Hypoxia-inducible factor-1 alpha correlates with tumor-associated macrophages infiltration, influences survival of gastric cancer patients. J Cancer 8(10):1818–1825. https://doi.org/10.7150/jca.19057
Zhang WJ, Zhou ZH, Guo M, Yang LQ, Xu YY, Pang TH, Gao ST, Xu XY, Sun Q, Feng M, Wang H, Lu CL, Wu GZ, Guan WX, Xu GF (2017) High infiltration of polarized CD163(+) tumor-associated macrophages correlates with aberrant expressions of CSCs markers, and predicts prognosis in patients with recurrent gastric cancer. J Cancer 8(3):363–370. https://doi.org/10.7150/jca.16730
Raiha MR, Puolakkainen PA (2018) Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: a review. Chronic Dis Transl Med 4(3):156–163. https://doi.org/10.1016/j.cdtm.2018.07.001
Murdoch C, Lewis CE (2005) Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer 117(5):701–708. https://doi.org/10.1002/ijc.21422
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61. https://doi.org/10.1016/j.immuni.2014.06.010
Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH (2013) Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res 73(8):2480–2492. https://doi.org/10.1158/0008-5472.CAN-12-3542
Mantovani A, Allavena P (2015) The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 212(4):435–445. https://doi.org/10.1084/jem.20150295
Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, Zhang M, Nan F, Li J, Liu J, Liu J, Jia W, Qiu Y, Song B, Han JJ, Rui L, Duan SZ, Liu Y (2017) The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 18(5):519–529. https://doi.org/10.1038/ni.3709
He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, Liu Z, Yao Z, Wu Q, Liao W, Zhang S, Liu Y, Xiang Y, Liu J, Shi M (2019) MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene 38(23):4637–4654. https://doi.org/10.1038/s41388-019-0747-0
Tanno T, Lim Y, Wang Q, Chesi M, Bergsagel PL, Matthews G, Johnstone RW, Ghosh N, Borrello I, Huff CA, Matsui W (2014) Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood 123(5):725–733. https://doi.org/10.1182/blood-2013-08-524025
Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, Thamboo TP, Chiong E, Zolezzi F, Yang H, Van Ginderachter JA, Poidinger M, Wong AS, Biswas SK (2014) Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity 41(5):815–829. https://doi.org/10.1016/j.immuni.2014.09.014
Shao M, Shan B, Liu Y, Deng Y, Yan C, Wu Y, Mao T, Qiu Y, Zhou Y, Jiang S, Jia W, Li J, Li J, Rui L, Yang L, Liu Y (2014) Hepatic IRE1alpha regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARalpha axis signalling. Nat Commun 5:3528. https://doi.org/10.1038/ncomms4528
Wu Y, Shan B, Dai J, Xia Z, Cai J, Chen T, Lv S, Feng Y, Zheng L, Wang Y, Liu J, Fang J, Xie D, Rui L, Liu J, Liu Y (2018) Dual role for inositol-requiring enzyme 1alpha in promoting the development of hepatocellular carcinoma during diet-induced obesity in mice. Hepatology 68(2):533–546. https://doi.org/10.1002/hep.29871
Luo X, Ryu KW, Kim DS, Nandu T, Medina CJ, Gupte R, Gibson BA, Soccio RE, Yu Y, Gupta RK, Kraus WL (2017) PARP-1 controls the adipogenic transcriptional program by PARylating C/EBPbeta and modulating its transcriptional activity. Mol Cell 65(2):260–271. https://doi.org/10.1016/j.molcel.2016.11.015
Miura K, Kinouchi M, Ishida K, Fujibuchi W, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, Sasaki I (2010) 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers (Basel) 2(3):1717–1730. https://doi.org/10.3390/cancers2031717
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3(3):187–197. https://doi.org/10.1016/j.cmet.2006.01.012
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE (2004) HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 23(9):1949–1956. https://doi.org/10.1038/sj.emboj.7600196
Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR (2011) High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117(23):e207–e217. https://doi.org/10.1182/blood-2010-10-314427
Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J (2019) Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 8(7):1596004. https://doi.org/10.1080/2162402X.2019.1596004
Mimeault M, Batra SK (2010) Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol 224(3):626–635. https://doi.org/10.1002/jcp.22196
Meier JC, Haendler B, Seidel H, Groth P, Adams R, Ziegelbauer K, Kreft B, Beckmann G, Sommer A, Kopitz C (2015) Knockdown of platinum-induced growth differentiation factor 15 abrogates p27-mediated tumor growth delay in the chemoresistant ovarian cancer model A2780cis. Cancer Med 4(2):253–267. https://doi.org/10.1002/cam4.354
Lu L, Ma GQ, Liu XD, Sun RR, Wang Q, Liu M, Zhang PY (2017) Correlation between GDF15, MMP7 and gastric cancer and its prognosis. Eur Rev Med Pharmacol Sci 21(3):535–541
Zhai Y, Zhang J, Wang H, Lu W, Liu S, Yu Y, Weng W, Ding Z, Zhu Q, Shi J (2016) Growth differentiation factor 15 contributes to cancer-associated fibroblasts-mediated chemo-protection of AML cells. J Exp Clin Cancer Res 35(1):147. https://doi.org/10.1186/s13046-016-0405-0
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20(1):51–56. https://doi.org/10.1016/j.gde.2009.10.009
Majmundar AJ, Wong WJ, Simon MC (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40(2):294–309. https://doi.org/10.1016/j.molcel.2010.09.022
Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL (2014) Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci USA 111(50):E5429–E5438. https://doi.org/10.1073/pnas.1421438111
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D (2008) Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99(1):121–128. https://doi.org/10.1111/j.1349-7006.2007.00643.x
Liu L, Ning X, Sun L, Shi Y, Han S, Guo C, Chen Y, Sun S, Yin F, Wu K, Fan D (2007) Involvement of MGr1-Ag/37LRP in the vincristine-induced HIF-1 expression in gastric cancer cells. Mol Cell Biochem 303(1–2):151–160. https://doi.org/10.1007/s11010-007-9467-9
Cottone L, Capobianco A, Gualteroni C, Perrotta C, Bianchi ME, Rovere-Querini P, Manfredi AA (2015) 5-Fluorouracil causes leukocytes attraction in the peritoneal cavity by activating autophagy and HMGB1 release in colon carcinoma cells. Int J Cancer 136(6):1381–1389. https://doi.org/10.1002/ijc.29125
Coussens LM, Zitvogel L, Palucka AK (2013) Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339(6117):286–291. https://doi.org/10.1126/science.1232227
Rojas A, Delgado-Lopez F, Perez-Castro R, Gonzalez I, Romero J, Rojas I, Araya P, Anazco C, Morales E, Llanos J (2016) HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumour Biol 37(3):3321–3329. https://doi.org/10.1007/s13277-015-3940-y
Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN (2018) The MIC-1/GDF15-GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab 28(3):353–368. https://doi.org/10.1016/j.cmet.2018.07.018
Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN (1999) MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol 65(1):2–5. https://doi.org/10.1002/jlb.65.1.2
Emmerson PJ, Duffin KL, Chintharlapalli S, Wu X (2018) GDF15 and growth control. Front Physiol 9:1712. https://doi.org/10.3389/fphys.2018.01712
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant Nos. 81702965, 31842033) and by the Shanghai Committee of Science and Technology, China (Grant Nos. 17411951400, 16DZ1930600).
Author information
Authors and Affiliations
Contributions
SY, TL and FL conceived and designed the studies. SY and QL conducted most of the experiments and analyzed the data. YY and YC assisted to do some of the cell experiments. WL helped to conduct some animal experiments. SY and FL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, S., Li, Q., Yu, Y. et al. Activated HIF1α of tumor cells promotes chemoresistance development via recruiting GDF15-producing tumor-associated macrophages in gastric cancer. Cancer Immunol Immunother 69, 1973–1987 (2020). https://doi.org/10.1007/s00262-020-02598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02598-5