Abstract
Background
Hypoxia is a common feature of rapidly growing solid tumors. Therefore, cellular adaptation to hypoxia and altered glucose metabolism are fundamental to the biology of cancer cells. Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor for more than 60 genes recognized to control the delivery of oxygen and nutrients through the induction of angiogenesis and glycolysis under hypoxic conditions. Therefore, inhibition of the expression of HIF-1α can be expected to be potentially tumor-specific molecular target-based therapy. In this study, we evaluated the significance of HIF-1α expression in relationship to clinicopathological factors, prognosis, vascular endothelial growth factor (VEGF) expression, and microvessel density (MVD).
Methods
Paraffin-embedded tumor specimens from 128 patients who underwent gastrectomy at Kurume University from 2004 to 2005 were used to assess the clinical significance of HIF-1α expression. We used the ABC method to perform an immunohistochemical analysis of the HIF-1α and VEGF expression.
Results
Eighty-four (65.6%) of gastric cancer specimens were positive for HIF-1α expression. Multivariate analysis showed that histology, depth of invasion, VEGF expression, and MVD were significantly associated with HIF-1α expression. On relapse-free and overall survival curves, the HIF-1α-negative group was significantly higher than the HIF-1α-positive group. Moreover, HIF-1α(+)/VEGF(+) patients had the worst prognosis. HIF-1α expression was identified as a significant predictor of relapse-free survival and overall survival by multivariate Cox’s proportional hazard analyses.
Conclusion
Overexpression of HIF-1α was found to be an indicator of poor prognosis for patients with gastric cancer and was significantly correlated with histology, depth of invasion, VEGF, and MVD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of cancer-related death in Japan [1]. Curative resection remains the only treatment associated with improvement in 5-year survival rates, but prognosis depends on the extent of lymph node (LN) metastasis and dissemination [2]. Therefore, a better understanding of the molecular mechanisms governing local invasion and systemic spread of gastric cancer is needed to design and evaluate new therapeutic strategies for this fatal disease [3].
Hypoxia is a common feature of rapidly growing solid tumors, because oxygen is only able to diffuse 100–180 μm from the blood capillaries to cells [4–10]. When tumors grow larger, oxygen and nutrition must be delivered by newly generated vessels. Therefore, cellular adaptation to hypoxia and altered glucose metabolism are fundamental to the biology of cancer cells. Angiogenesis is essential for tumor growth and metastasis. Tumor neovascularization depends on the production of specific angiogenic factors. Vascular endothelial growth factor (VEGF) is one of the major factors that contribute to angiogenesis and metastasis in numerous tumor types, and VEGF overexpression has been associated with tumor progression and poor prognosis [7, 8, 11–13].
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor for more than 60 genes recognized to control the delivery of oxygen and nutrients through the induction of angiogenesis and glycolysis under hypoxic conditions [2, 14–17]. HIF-1α activates the transcription of VEGF, and the expression of glucose transporters (GLUT-1), glycolytic enzymes, and growth factors, which may promote tumor cell survival under hypoxic conditions [17–19].
HIF-1α plays a critical role in angiogenesis during vascular development. HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits; HIF-1α is the oxygen-regulated subunit that determines HIF-1 activity [7]. Under normoxic conditions, HIF-1α is unstable. The instability is regulated, in part, by binding to the von Hippel–Lindau tumor suppressor protein. This binding occurs after hydroxylation of the two HIF-1α proline residues by HIF-prolyl hydroxylases. The von Hippel–Lindau protein is one of the components of the multiprotein ubiquitin-E3-ligase complex. HIF-1α is degraded by the ubiquitin-dependent proteasome pathway [20]. However, under hypoxic conditions, proline hydroxylation is inhibited, allowing HIF-1α to become stable. The stabilized HIF-1α dimerizes with HIF-1β, translocates from the cytoplasm into the nucleus, and binds to hypoxia-responsive elements (HRE) within the nucleus. Its target genes then promote cell proliferation and viability, angiogenesis, and also metabolic adaptations to hypoxia [6, 21–24].
HIF-1α is a primary determinant of HIF activity. HIF-1α overexpression has been studied in several cancers, such as brain, bladder, breast, lung, esophagus, colon, ovary, pancreas, kidney, and prostate cancer [15, 25–31]. These results revealed that HIF-1α is related to the prognosis in these cancers. Therefore, inhibition in the expression of HIF-1α will be expected to be a tumor-specific molecular target-based therapy. So far, few studies have investigated the correlation between HIF-1α and gastric cancer. In this study, immunohistochemical analysis was used to investigate HIF-1α protein and VEGF protein expression. We hypothesized that HIF-1α expression would be correlated with clinicopathological factors, VEGF expression, and microvessel density (MVD) expressed as the mean count of CD34-immunostained vessels, and that this correlation would predict recurrence and overall survival.
Materials and methods
Patients
Samples of gastric cancer were taken from the resected stomach of 128 patients who underwent gastrectomy for gastric carcinoma at the Kurume University Hospital between 2004 and 2005, excluding mucosal cancer, multiple primary cancer, multiple gastric cancer, remnant cancer, and remnant cancer after endoscopic submucosal dissection (ESD). All patients were diagnosed histologically according to the General Rules for Japanese Classification of Gastric Carcinoma of the Japanese Gastric Cancer Association (14th edition) [32]. None of the patients had received any preoperative treatment. Samples were obtained from the central zone of the cancer lesion and preserved by formalin fixation, embedded in paraffin, and stained with hematoxylin and eosin for histological examination. The paraffin blocks were stored until required for immunohistochemistry of HIF-1α and VEGF.
Immunohistochemistry
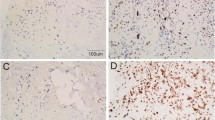
Paraffin-embedded tissues were subjected to immunohistochemical analysis performed by the avidin–biotin–peroxidase complex method (Vectastain ABC Kit; Vector, Burlingame, CA, USA). For immunohistochemistry of HIF-1α and VEGF, paraffin sections were deparaffinized in xylene and rehydrated through graded ethanol solutions. For HIF-1α antigen retrieval, sections were then irradiated by a domestic microwave oven at 99ºC in 10 mM citrate buffer (pH 9.0) for 30 min, and cooled to room temperature. After microwave irradiation, the slides were washed with phosphate-buffered saline (PBS), treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase, and then incubated with the primary antibody in a humidified chamber at 4ºC overnight. As the primary antibody, the rabbit polyclonal antibody H206 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for HIF-1α, diluted at 1:200, and the rabbit polyclonal antibody A-20 (Santa Cruz Biotechnology) for VEGF, diluted at 1:200, were used. Sections were washed three times with PBS, then incubated with biotinylated horse anti-mouse/anti-rabbit immunoglobulin G antibody for 30 min, washed again three times with PBS, and then incubated with avidin-biotinylated peroxidase complex for 30 min. After three additional washings with PBS, staining was developed by incubating the sections in 3-amino-9-ethylcarbazole (Vector) for 10 min. The sections were then counterstained with hematoxylin and mounted. For evaluation of HIF-1α expression, five fields were selected randomly and a total of more than 1,000 tumor cells were counted microscopically under high magnification (400×). The HIF-1α expression through nuclear staining of positive cells was predominant at the invading edge of the tumor margin and at the periphery of necrotic regions within tumors. The HIF-1α expression was defined as positive if nuclear staining was observed in ≥5% of the tumor cells. Concomitant cytoplasmic staining was not counted because HIF-1α in the nucleus determines the functional activity of the HIF-1α complex (Fig. 1a, b) [3]. In regard to overall survival curve, the HIF-1α expression was classified as one of four categories, depending on the percentage of tumor cells stained: – (0–5%), 1+ (5–10%), 2+ (10–15%), 3+ (≥15%). The VEGF expression was defined as positive if cytoplasmic staining was observed in ≥10% of the tumor cells (Fig. 1c, d). The ChemMate Envision method was used for CD34. Endogenous peroxidase activity was inhibited by incubating slides in 3% H2O2 for 5 min. CD34 antigen retrieval was performed by treating with proteinase K for 5 min. Each slide was incubated for 30 min with the antibody at room temperature (Novocastra, Newcastle, UK for CD34, diluted at 1:200). For staining detection, the ChemMate Envision method was used with DAB as chromogen (Fig. 1e). We extracted the digital data of expression using the following image analysis system. CD34-stained specimens were examined to identify the areas with high density. Expression analysis was performed to measure the five expression areas of MVD in all cases, using ‘Win ROOF’ (version 5.7; Mitani, Osaka, Japan) computer software. The digitized data of the expression area were measured and averaged. MVD was classified as either <1,200 or ≥1,200/μm2.
Immunoreactivity for hypoxia-inducible factor-1 alpha (HIF-1α) is mainly identified as positive staining in the nucleus of cancer cells (a, b); for vascular endothelial growth factor (VEGF), immunoreactivity is mainly identified as supranuclear staining or diffuse staining in the cytoplasm of cancer cells (c, d); and for CD34, immunoreactivity is recognized in the endothelium of microvessels (e). a, c, e ×100; b, d ×200
Statistical analysis
Differences in expression rate and association with clinical characteristics were compared by Fisher’s exact test or the chi-square test. Significant factors were extracted for further analysis, carried out using multivariate analysis with a logistic regression method. Moreover, Pearson’s correlation coefficient was used to examine the correlation of positive rate between HIF-1α expression and VEGF expression. The relapse free-survival (the time of surgical resection to the time of recurrence) and the overall survival (the time of surgical resection to patient death) rates were calculated using the Kaplan–Meier method. Univariate analysis of factors thought to influence the relapse-free survival and overall survival was carried out using the log-rank test. The Cox proportional hazards model was used in the multivariate analysis of the factors that were determined to be significant for relapse-free survival and overall survival by univariate analysis. The statistical analyses were performed using a statistical analysis computer program (JUMP 8; SAS Institute, Cary, NC, USA). For all analyses, statistical significance was defined as P < 0.05.
Results
Patients
A total of 128 patients were included in this study; 91 patients were men, and 37 were women. Mean age was 67.3 years, with age ranging from 39 to 85 years. Curative resections were performed in 100 patients (78.1%), and noncurative resection was performed in the other 28 (21.9%). On histological differentiation, 53 cases were differentiated type (41.4%) and 76 cases were undifferentiated type (58.6%). Concerning tumor size, 68 (53.1%) were 6 cm or larger; 62 patients (48.4%) had LN metastasis, and 15 patients (11.7%) had peritoneal metastasis. The postoperative stages of patients were I, II, III, and IV in 48, 24, 34, and 22 patients, respectively.
Clinicopathological significance of HIF-1α protein expression
Eighty-four (65.6%) of gastric cancer specimens were positive for HIF-1α expression; 68 (53.1%) specimens were positive for VEGF expression. Univariate analysis showed that tumor size, macroscopic type, histological type, depth of invasion, LN metastasis, distant metastasis, venous invasion, lymphatic invasion, infiltration (INF), VEGF expression, and MVD were significantly correlated with HIF-1α expression (P = 0.017, P = 0.021, P < 0.0016, P = 0.043, P = 0.006, P = 0.006, P = 0.015, P = 0.022, P = 0.0004, P = 0.0001, and P = 0.0001, respectively). However, there was no significant correlation between the expression of HIF-1α, age, sex, H, P factor, and cancer stroma. As for histological types, HIF-1α expression in signet-ring cell carcinoma or in mucinous adenocarcinoma was significantly higher than that in the other histological types (Table 1). Multivariate analysis was performed for 11 parameters [tumor size, macroscopic type, histological type, depth of invasion, LN metastasis, venous invasion, lymphatic invasion, distant metastasis, infiltration (INF), VEGF expression, and MVD] that had been found to be significant by univariate analysis, using the logistic regression method (Table 2). Multivariate analysis showed that histology, depth of invasion, VEGF expression, and MVD were significantly associated with HIF-1α expression (P = 0.006, P = 0.020, P = 0.0004, and P = 0.014, respectively). However, the correlation of positive rate between HIF-1α expression and VEGF expression was not so strong by Pearson’s correlation coefficient (r = 0.507, P < 0.0001) (Fig. 2).
Relapse-free survival and overall survival curves
The median follow-up duration was 44.9 months (range, 0–60 months) after surgery. The relapse-free survival curve in the HIF-1α-negative group was significantly higher than that in the HIF-1α-positive group in 100 patients who underwent a curative resection (R0) [P = 0.021; hazard ratio (HR), 7.685 (95% confidence interval (CI), 1.471–140.980)] (Fig. 3a). There was no significant correlation between VEGF expression and relapse-free survival (P = 0.381) (Fig. 3b). During the follow-up period, 32 patients died of gastric cancer; the 5-year-overall survival rate was 97.7% in the HIF-1α-negative group and 62.7% in the HIF-1α-positive group. On the overall survival curve, the HIF-1α-negative group was significantly higher than the HIF-1α-positive group [P < 0.0001; HR, 19.480 (95% CI, 4.184–346.672)] (Fig. 3c), and the VEGF negative group was significantly higher than the VEGF positive group [P = 0.034, HR 2.194 (95% CI 1.066–4.846)] (Fig. 3d). In patients with stage III, The survival curve in the HIF-1α-negative group was significantly higher than that in the HIF-1α-positive group (P = 0.0319), but there were no significant difference in the other stages (Fig. 4). The 5-year-overall survival rate according to the percentage of positive tumor staining was 55.6% in (3+), 59.5% in (2+), 69.1% in (1+), and (–) in 97.7%. The prognosis became worse according to the rate of HIF-1α expression (P = 0.0004) (Fig. 5). When stratified for HIF-1α-negative and HIF-1α-positive patients in the VEGF-negative and VEGF-positive subgroups, a statistical difference was observed among the groups. The HIF-1α-negative and VEGF-negative patients had the most favorable prognosis, whereas the HIF-1α-positive and VEGF-positive patients had the worst prognosis (P = 0.0002) (Fig. 6). On overall survival, HIF-1α, VEGF, MVD, tumor size, macroscopic type, histological type, depth of invasion, LN metastasis, venous invasion, lymphatic invasion, liver metastasis, peritoneal metastasis, distant metastasis, cancer stroma, and INF (P < 0.0001, P = 0.034, P = 0.004, P < 0.0001, P < 0.0001, P = 0.025, P < 0.0001, P < 0.0001, P < 0.0001, P < 0.0001, P = 0.033, P < 0.0001, P < 0.0001, P = 0.026, and P = 0.0005, respectively), and on relapse-free survival, HIF-1α, macroscopic type, depth of invasion, LN metastasis, venous invasion, lymphatic invasion, and cancer stroma (P = 0.021, P = 0.009, P = 0.001, P = 0.006, P = 0.002, P = 0.001, and P = 0.030, respectively) were indicators for poor prognosis according to the log-rank test. Multivariate Cox’s proportional hazard analyses of clinicopathological factors that appeared significant in the univariate analyses revealed that HIF-1α was an independent prognostic factor on overall survival and relapse-free survival (Tables 3, 4). HIF-1α expression was identified as a significant predictor of relapse-free survival [P = 0.011; HR, 9.723 (95% CI, 1.568–197.633)] and overall survival [P = 0.016; HR, 7.366 (95% CI, 1.368–137.169)].
Relapse-free survival and overall survival curves in HIF-1α and VEGF. a Relapse-free survival curve in the HIF-1α-negative group was significantly higher than that in the HIF-1α-positive group [P = 0.021; hazard ratio (HR), 7.685 (95% confidence interval (CI), 1.471–140.980)]. b There was no significant correlation between VEGF expression and relapse-free survival (P = 0.381). c On the overall survival curve, the HIF-1α-negative group was significantly higher than the HIF-1α-positive group [P < 0.0001; HR, 19.480 (95% CI, 4.184–346.672)]. d VEGF-negative group was significantly higher than VEGF-positive group [P = 0.034; HR, 2.194 (95% CI, 1.066–4.846)]
Discussion
Under hypoxic condition, angiogenesis is essential for growth and metastasis of solid tumors, because oxygen is only able to diffuse 100–180 μm from the blood capillaries to cells. When tumors grow larger, oxygen and nutrition should be delivered by newly generated vessels. Therefore, cellular adaptation to hypoxia and altered glucose metabolism are fundamental to the biology of cancer cells [5–9, 11–13, 21–24, 28, 33]. For this reason, inhibition in angiogenesis is emerging as a promising strategy for cancer treatment [3]. Tumor angiogenesis and neovascularization require VEGF expression. Binding of HIF-1α to the VEGF promoter is a major pathway resulting in the induction of VEGF expression under hypoxic conditions [34]. VEGF, as well as functioning as a growth factor, is able to function as a vascular permeability factor. Increased permeability of blood vessels facilitates the extravasation of proteins and formation of ascites [35–37]. In previous reports, the expression level of VEGF has been found to be directly associated with the production of ascites and carcinomatosis [37, 38]. Aoyagi et al. [39] reported that VEGF was correlated with peritoneal metastasis from gastric cancer, and that VEGF was a useful indicator of peritoneal recurrence. Moreover, Imaizumi et al. [40] reported that bevacizumab, which is a humanized monoclonal antibody against VEGF, suppressed peritoneal dissemination from gastric cancer using peritoneal metastasis model. These studies provide clear evidence that VEGF is an essential element in the development of peritoneal metastasis from gastric cancer. Some hypoxia-independent mechanisms of HIF-1 activation in tumor cells have also been reported, such as genetic alterations in tumor suppressor genes (p53, VHL, and PTEN) and oncogenes (SRC, HER2, and H-RAS) [41–44]. Activation of certain growth factor receptors (insulin-like growth factor I receptor) has also been shown to increase expression of HIF-1α. Results from recent studies demonstrated that HIF-1α may also regulate the invasiveness of colon cancer cells by altering the expression of genes encoding intermediate filaments (vimentin, keratins), extracellular matrix components (fibronectin), and proteases (matrix metalloproteinase 2 and the urokinase plasminogen activator receptor) [11]. Most studies have shown that HIF-1α overexpression has been detected in several human cancers, such as brain, bladder, breast, lung, esophagus, colon, ovary, pancreas, kidney, and prostate cancer [15, 25–31]. Furthermore, HIF-1α overexpression has been reported to be significantly correlated with highly aggressive disease, resistance to radiation therapy and chemotherapy, and poor prognosis in some cancer types such as oligodendroglioma, breast, ovarian, and oropharyngeal cancer [27, 28, 30, 45–50]. However, few studies have investigated the correlation between HIF-1α and gastric cancer.
In this study, we have investigated the relationship between HIF-1α, VEGF, clinicopathological significance, and patient prognosis in gastric cancer. Our results showed that HIF-1α expression significantly correlated with a malignant behavior category, including increased expression of tumor size, macroscopic type, histological type, depth of invasion, LN metastasis, distant metastasis, venous invasion, INF, VEGF expression, and MVD. However, there were no significant correlations between the HIF-1α expression, H, P, factor, lymphatic invasion and cancer stroma. The multivariate analysis showed that the histology, depth of invasion, VEGF expression, and MVD were significantly associated with the HIF-1α expression. However, the correlation of the positive rate between HIF-1α expression and VEGF expression was not so strong by Pearson’s correlation coefficient, because HIF-1α is a transcription factor of more than 60 genes and HIF-1α induces not only VEGF but also other many factors that affect tumor progression and prognosis. As for histological types, HIF-1α expression in the undifferentiated types was significantly higher than that in the differentiated types. Hypoxic tumors are aggressive and exhibit stem cell-like characteristics. Recent genomics studies have further revealed that poorly differentiated human tumors display a gene expression signature similar to that found in normal embryonic stem cells or lineage-committed progenitor cells [51]. Some clinical studies have shown significant associations between HIF-1α expression and patient outcome in human solid tumors [28, 49, 50]. Our data also demonstrated that relapse-free survival and overall survival curves in the HIF-1α-negative group were significantly higher than those in the HIF-1α-positive group. Moreover, we classified HIF-1α expression as four groups, and a statistical difference was observed among the groups. The prognosis was worse according to the rate of HIF-1α expression. We also found that there was a significant difference among groups stratified to HIF-1α/VEGF expression (P = 0.0002). The patients with HIF-1α(+)/VEGF(+) tumors had the worst prognosis. Concerning the heterogeneity of the expression of the HIF-1α and VEGF within each tissue, other pathways upstream can induce VEGF expression independent of HIF-1α signaling. For example, the oncogene ras upregulates VEGF expression and downregulates endogenous angiogenic inhibitors such as thrombospondin-1 (Tsp-1). Conversely, activation of the tumor suppressor genes p53, PTEN, and Smad4 increases Tsp-1 expression and inhibits the angiogenesis. p53 has been reported to inhibit angiogenesis through the regulation of other unidentified inhibitors [52]. In multivariate analysis, HIF-1α was an independent prognostic factor for relapse-free survival and overall survival. These findings suggested that HIF-1α plays an important role in tumor growth and progression of gastric cancer.
In conclusion, we have demonstrated in this study that HIF-1α expression was correlated with clinicopathological findings. HIF-1α expression was found to be an indicator of poor prognosis for disease recurrence or progression in patients with gastric cancer. In addition, immunoreactivity of combination of HIF-1α and VEGF was a useful marker of the prognosis of gastric cancer.
References
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2:533–543
Liao D, Johnson RS (2007) Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 26:281–290
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Gatenby RA, Kessler HB, Rosenblum JS et al (1988) Oxygen distribution in squamous cell carcinoma metastasis and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 14:531–538
Takahashi R, Tanaka S, Hiyama T et al (2003) Hypoxia-inducible factor 1 alpha expression and angiogenesis in gastrointestinal stromal tumor of stomach. Oncol Rep 10:797–802
Zhong H, Agani F, Baccala AA et al (1998) Increased expression of hypoxia-inducible factor 1 alpha in rat and human prostate cancer. Cancer Res 58:5280–5284
Chen WT, Huang CJ, Wu MT et al (2005) Hypoxia-inducible factor 1 alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol 35:207–213
Mizokami K, Kakeji Y, Oda S et al (2006) Clinicopathological significance of hypoxia-inducible factor 1 alpha overexpression in gastric carcinomas. J Surg Oncol 94:149–154
Yoshikawa T, Tsuburaya A, Miyagi Y et al (2006) Up-regulation of hypoxia-inducible factor 1 alpha and VEGF mRNAs in peritoneal dissemination of patients with gastric cancer. Anticancer Res 63:3849–3854
Sumiyoshi Y, Kakeji Y, Egashira A et al (2006) Overexpression of hypoxia-inducible factor 1 alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res 12:5112–5117
Stoeltzing O, McCarty MF, Wey JS et al (2004) Role of hypoxia-inducible factor 1 alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst 96:946–956
Fukuda R, Kelly B, Semenza GL (2003) Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res 63:2330–2334
Koshikawa N, Iyozumi A, Gassmann M (2003) Constitutive upregulation of hypoxia-inducible factor 1 alpha mRNA occurring in highly metastatic lung carcinoma cells leads to vascular endothelial growth factor overexpression upon hypoxic exposure. Oncogene 22:6717–6724
Semenza GL (2001) Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7:345–350
Saramaki OR, Savinainen KJ, Nupponen NN et al (2001) Amplification of hypoxia-inducible factor 1 alpha gene in prostate cancer. Cancer Genet Cytogenet 128:31–34
Ravi R, Mookerjee B, Bhujwalla ZM et al (2000) Regulation of tumor angiogenesis by p53 induced degradation of hypoxia-inducible factor 1α. Genes Dev 14:34–44
Kung AL, Wang S, Klco JM et al (2000) Suppression of tumor growth through disruption of hypoxia inducible transcription. Nat Med 6:1335–1340
Akakura N, Kobayashi M, Horiuchi I et al (2001) Constitutive expression of hypoxia-inducible factor-1 alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res 61:6548–6554
Dalgard CL, Lu H, Mohyedin A et al (2004) Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J 380:419–424
Huang LE, Gu J, Schau M (1998) Regulation of hypoxia-inducible factor 1 alpha is mediated by O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95:7987–7992
Talks KL, Turley H, Gatter KC et al (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157:411–421
Chun YS, Kim MS, Park JW (2002) Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci 17:581–588
Shi YH, Fang WG (2004) Hypoxia-inducible factor-1 in tumor angiogenesis. World J Gastroenterol 10:1082–1087
Stroka DM, Burkhardt T, Desbaillets I et al (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–2453
Zagzag D, Zhong H, Scalzitti JM et al (2000) Expression of hypoxia-inducible factor 1 alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer (Phila) 88:2606–2618
Theodoropoulos VE, Lazaris A, Sofras F et al (2004) Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol 46:200–208
Bos R, Zhong H, Hanrahan CF et al (2001) Levels of hypoxia-inducible factor 1 alpha during breast carcinogenesis. J Natl Cancer Inst 93:309–314
Kuwa T, Kitada Y, Tanaka S et al (2003) Expression of hypoxia-inducible factor 1 alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer 105:176–181
Wiesener MS, Munchenhagen PM, Berger I et al (2001) Constitutive activation of hypoxia-inducible factor-1 alpha in clear cell renal carcinomas. Cancer Res 61:5215–5222
Birner P, Schindl M, Obermair A et al (2001) Expression of hypoxia-inducible factor 1 alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 7:1661–1668
Buchler P, Reber HA, Buchler M et al (2003) Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas 26:56–64
Japanese Gastric Cancer Association (2010) Japanese classification of gastric carcinoma, 14th edn. Kanehara, Tokyo
Livingston DM, Shivdasani R (2001) Toward mechanism-based cancer care. JAMA 285:588–593
Oskoski R Jr (2007) Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 62:179–213
Senger DR, Galli SJ, Dvorak AM et al (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Dvorak HF, Brown LF, Detmar M et al (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Nagy JA, Masse EM, Herzberg KT et al (1995) Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res 55:360–368
Boocock CA, Charnock-Jones DS, Sharkey AM et al (1995) Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst 87:506–516
Aoyagi K, Kouhuji K, Takeda J et al (2005) VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer 8:155–163
Imaizumi T, Aoyagi K, Shirouzu K et al (2010) Suppressive effect of bevacizumab on peritoneal dissemination from gastric cancer in a peritoneal metastasis model. Surg Today 40:851–857
Chen C, Pore N, Behrooz A et al (2001) Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 276:9519–9525
Jiang BH, Agani F, Passaniti A et al (1997) V-SRC induces expression of hypoxia-inducible factor 1(HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res 57:5328–5335
Laughner E, Taghavi P, Chiles K et al (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21:3995–4004
Mazure NM, Chen EY, Laderoute KR et al (1997) Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90:3322–3331
Aebersold DM, Burri P, Beer KT et al (2001) Expression of hypoxia-inducible factor 1 alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 61:2911–2916
Matuyama T, Nakanishi K, Hayashi T et al (2005) Expression of hypoxia-inducible factor 1α in esophageal squamous cell carcinoma. Cancer Sci 96:176–182
Koukourakis MI, Giatromanolaki A, Skarlatos J et al (2001) Hypoxia-inducible factor (HIF-1α and HIF-2α) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res 61:1830–1832
Beasley NJP, Leek R, Alam M et al (2002) Hypoxia-inducible factor HIF-1α and HIF-2α in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res 62:2493–2497
Griffiths EA, Pritchard SA, Valentine HR et al (2007) Hypoxia-inducible factor 1 alpha expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastroesophageal adenocarcinomas. Br J Cancer 96:95–103
Krishnamachary B, Berg-Dixon S, Kelly B et al (2003) Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 63:1138–1143
Yuri K, Qun L, Daniel Z et al (2009) Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res 69:9271–9280
Wen WM, Alex AA (2009) Novel agents on the horizon for cancer therapy. CA Cancer 59:111–137
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Isobe, T., Aoyagi, K., Koufuji, K. et al. Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1α) expression in gastric cancer. Int J Clin Oncol 18, 293–304 (2013). https://doi.org/10.1007/s10147-012-0378-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0378-8