Abstract
We investigated inflammatory markers such as the neutrophil-to-lymphocyte ratio (NLR) that may predict the response to anti-PD-1 (programmed cell death protein 1) antibody therapy. Data from 54 patients with non-small cell lung cancer (NSCLC) treated with anti-PD-1 antibodies were retrospectively analyzed. The NLR was assessed at baseline and 6 weeks after the start of treatment (post-treatment). Eighteen of 54 patients (33.3%) had objective responses to treatment. Older age, absence of brain metastasis, low post-treatment NLR (< 5), and immune-related adverse events were significantly associated with response. Patients with a high post-treatment NLR (≥ 5) had significantly shorter progression-free survival (PFS) than those with a low post-treatment NLR (median, 1.3 vs. 6.1 months, p < 0.001). Multivariate analysis demonstrated that high post-treatment NLR [hazard ratio (HR) 15.1, 95% confidence interval (CI) 1.5–50.1, p < 0.001], liver metastasis (HR 4.9, 95% CI 1.9–12.4, p = 0.001), and brain metastasis (HR 3.2, 95% CI 1.3–8.2, p = 0.013) were independent prognostic factors of shorter PFS. Overall survival (OS) was significantly different in patients with high and low post-treatment NLRs (median, 2.1 vs. 14.0 months, p < 0.001). A high post-treatment NLR remained an independent prognostic factor for OS in multivariate analysis (HR 3.9, 95% CI 1.6–9.2, p = 0.003). The NLR at 6 weeks after treatment initiation was a prognostic marker in patients with advanced NSCLC treated with anti-PD-1 antibody. Further studies are warranted to evaluate the role of the 6-week NLR as a predictor in anti-PD-1 antibody treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since cancer-related inflammation affects disease progression and survival in many types of cancer [2], several inflammation- and immune-based prognostic scores, such as the neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII), have been developed to predict survival. The NLR has been most extensively investigated in solid tumors and has become a robust prognostic factor in non-small cell lung cancer (NSCLC), as well as in many other cancers [3]. Additionally, the SII has been proven to be a powerful prognostic factor of outcomes in hepatocellular carcinoma and colorectal cancer (CRC) [4,5,6].
Recently, the targeting of immune checkpoint signaling to restore cancer cell-directed immune responses has become a confirmed therapeutic strategy for several types of tumors. Programmed cell death receptor 1 (PD-1), which is found on the surfaces of immune cells in the tumor microenvironment, interacts with the programmed cell death ligand 1 (PD-L1) on tumor cells, enabling these tumor cells to escape host immune surveillance mechanisms [7, 8]. Therapeutic antibodies to PD-1 have shown promising activity in lung cancer [9,10,11,12]. Since anti-PD-1 antibody therapy is expensive and has the risk of developing rare, yet serious, immune-related adverse events (irAEs) [13], it is important to find predictive markers of the therapeutic response to identify patients who would maximally benefit from such therapy. Currently, the only clinically validated predictive marker of treatment with immune checkpoint inhibitors that targets PD-1/PD-L1 is the expression of PD-L1 on tumor cells or tumor-infiltrated immune cells [14].
Recent studies have investigated the predictive role of markers of systemic inflammation in immune checkpoint inhibitor treatment. In melanoma, the presence of a low baseline NLR or a low NLR at an early time-point of treatment with ipilimumab, a monoclonal antibody directed to cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), was shown to be associated with better survival [15, 16]. Similarly, an early increase in lymphocyte count after treatment with ipilimumab has been shown to be associated with better disease control and survival [17, 18].
These findings suggest that the NLR might be a surrogate marker of anti-tumor immunity, and may also be related to the efficacy of anti-PD-1 antibody treatment. However, little is known about the correlation between NLRs or other immune-based prognostic scores and the treatment outcomes of patients treated with anti-PD-1 antibody. Bagley et al. have recently shown that a high pretreatment NLR is associated with worse progression-free survival (PFS) and overall survival (OS) in patients with NSCLC treated with nivolumab [19]. We aimed to investigate clinical factors including immune-based prognostic scores that may help to predict the responses to anti-PD-1 antibody treatment in patients with advanced NSCLC.
Patients and methods
Study population
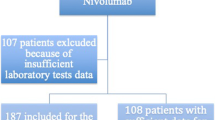
We conducted a retrospective analysis of consecutive patients with NSCLC who had undergone anti-PD-1 antibody (nivolumab or pembrolizumab) treatment at the Seoul National University Hospital (SNUH) and the Seoul National University Bundang Hospital (SNUBH) between October 2013 and April 2016. The inclusion criteria for patients were as follows: (a) pathologically confirmed NSCLC; (b) initial stage IIIB or IV, or recurrence after curative surgery; and (c) administration of nivolumab at 3 mg/kg every 2 weeks or pembrolizumab at 2 mg/kg or 10 mg/kg every 3 weeks as palliative therapy. All but three patients were administered anti-PD-1 antibody through participation in a prospective clinical trial (NCT01295827, NCT01905657, and NCT02175017). Complete blood cell counts were performed pretreatment and before each drug administration. Total white blood cell counts, absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), and platelet counts were analyzed before the beginning of treatment and at 6 weeks after the start of treatment. The NLR was defined as the ratio of ANC to ALC, and categorized using a threshold value of 5 [20]. Platelet-to-lymphocyte ratio (PLR) was defined as the ratio of platelet counts to ALC, and a PLR ≥ 169 was considered to signify elevated levels. The SII was calculated as the platelet count multiplied by the NLR, and an SII ≥ 730 was considered elevated [4].
Chest and abdominopelvic computed tomography scans were performed every 8–12 weeks according to the study protocol, and additionally as needed to assess disease progression. All responses were defined according to the revised RECIST guideline (version 1.1) [21]. Pseudoprogression was defined as an increase in the size of the target lesions or the appearance of a new lesion, followed by subsequent tumor shrinkage. PFS was calculated as the interval from the date of initiation of anti-PD-1 antibody treatment to the date of either disease progression or death. OS was calculated as the duration between the date of initiation of anti-PD-1 antibody treatment and the date of death from any cause. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events version 4.0. In cases with samples available for immunohistochemistry (IHC) analysis, PD-L1 expression was analyzed using PD-L1 IHC 22C3 pharmDx (DAKO, Glostrup, Denmark), with a cut-off tumor proportion score ≥ 50%. The Institutional Review Boards at the SNUH and the SNUBH approved this study (IRB No. J-1607-085-776 and B-1606/349-110), and the study was conducted in compliance with the Declaration of Helsinki.
Statistical analysis
The baseline characteristics of patients and the clinicopathological findings according to their responses to anti-PD-1 antibody treatment were evaluated using the Pearson Chi-square or Fisher’s exact test for categorical variables, and the Mann–Whitney U test for continuous variables. PFS and OS were calculated and compared using the Kaplan–Meier method and the log-rank test. The prognostic values of each variable were evaluated with univariate Cox proportional-hazard regression (PHR) analyses. Multivariate analysis for PFS and OS were performed using the variables that were significant on univariate analysis. All tests were two-sided, and a p value < 0.05 was used to indicate a statistically significant difference. All data were analyzed using SPSS Version 22.0 on data collected through September 2016.
Results
Characteristics of patients
The clinical characteristics of the 54 patients are shown in Table 1. Forty-two (77.8%) of these patients were men. Fifteen (27.8%) patients were non-smokers. Most patients had adenocarcinoma (n = 31); 17 had squamous cell carcinoma, 2 had adenosquamous cell carcinoma, 2 had NSCLC not otherwise specified, 1 had pleomorphic carcinoma, and 1 had large cell neuroendocrine carcinoma. Five patients had epidermal growth factor receptor (EGFR) activating mutations, one patient had a KRAS mutation (Q61H), and one patient had an anaplastic lymphoma kinase (ALK) rearrangement. Metastases to the lungs, bones, central nervous system (CNS), pleura, liver, and adrenal glands were found in 31 (57.4%), 15 (27.8%), 9 (16.7%), 20 (37.0%), 10 (18.5%), and 7 (13.0%) patients, respectively. Thirty-one patients received nivolumab and 23 patients received pembrolizumab. All patients were treated with single agent immunotherapy.
The association of inflammatory markers and outcomes of anti-PD-1 antibody treatment
Eighteen (33.3%) out of 54 patients had clinical objective responses to anti-PD-1 antibody treatment; all patients who exhibited responses showed partial response (PR). The objective response rate (ORR) was significantly associated with the immune-based prognostic scores at 6 weeks after the initiation of treatment. None of the patients with a 6-week post-treatment NLR ≥ 5 achieved PR, whereas 18 (41.9%) of 43 patients, including 2 patients with tumor flares, with a post-treatment NLR < 5 achieved PR (p = 0.011). The ORR was lower in patients with an elevated SII at week 6 than in those with a lower SII in the same week (17.9% vs. 52.0%, p = 0.009). Other variables, including older age, absence of brain metastasis, and the presence of irAEs, were significantly associated with clinical response; gender, smoking history, line of treatment, and baseline NLR, PLR, and SII were not predictive of response. Association between clinicopathological characteristics and treatment responses in patients are shown in Table 1. There was no correction undertaken for multiple comparisons.
The median follow-up period was 26.2 months (range, 6.8–36.2 months), the median PFS and OS were 4.7 months (95% CI, 3.2–6.2) and 12.2 months (95% CI, 9.1–15.3), respectively, and the 1-year PFS and OS were 26.4 and 48.8%, respectively. Figure 1 illustrates PFS according to NLR, PLR, and SII at baseline and week 6 after the start of treatment. Patients with a 6-week post-treatment NLR ≥ 5 had significantly shorter PFS after anti-PD-1 antibody treatment than did those with a post-NLR < 5 (median, 1.3 vs. 6.1 months, p < 0.001). A post-treatment SII ≥ 730 at 6 weeks was also associated with a shorter PFS compared to SII < 730 at 6 weeks (median PFS, 2.8 vs. 8.1 months, p = 0.033).
Progression-free survival (PFS) according to immune-based prognostic scores. PFS according to the neutrophil-to-lymphocyte ratio (NLR) at baseline (a) and week 6 (b); PFS according to the platelet-to-lymphocyte ratio (PLR) at baseline (c) and week 6 (d); PFS according to the systemic immune-inflammation index (SII) at baseline (e) and week 6 (f)
The median OS of patients with post-NLRs ≥ 5 and < 5 were 2.1 and 14.0 months, respectively (p < 0.001; Fig. 1b). Patients with a 6-week post-treatment SII ≥ 730 also had shorter OS than did those with a 6-week SII < 730 (median OS, 7.1 vs. 24.6 months, p = 0.004) (Fig. 2). Baseline NLR, PLR, and SII values were not associated with PFS or OS.
Overall survival (OS) according to immune-based prognostic scores. OS according to the neutrophil-to-lymphocyte ratio (NLR) at baseline (a) and week 6 (b); OS according to the platelet-to-lymphocyte ratio (PLR) at baseline (c) and week 6 (d); OS according to the systemic immune-inflammation index (SII) at baseline (e) and week 6 (f)
Ratio between pre- and post-treatment NLR and outcomes
Reduction in NLR after anti-PD-1 antibody treatment was associated with a higher objective response rate and a significantly improved PFS. Patients with a ratio between pre- and post-treatment NLR of 1 or more had a higher response rate (43.2 vs. 22.2%, p = 0.066) and longer PFS (6.2 vs. 3.0 months, p = 0.035) compared to those with a ratio less than 1 (Supplementary Table 1 and Supplementary Fig. 1).
Univariate and multivariate analysis for PFS and OS
We performed univariate and multivariate analyses to identify the prognostic importance of clinical characteristics and immune-based prognostic scores. In the univariate Cox PHR analyses for PFS, no significant differences were found with respect to patient age, sex, histology, smoking status, performance status, PD-L1 expression, and baseline NLR, PLR, and SII. PD-L1 expression using a different cut-off (5%) was also not associated with PFS (HR 0.69; 95% CI 0.27–1.77; p = 0.437). However, PFS was shorter in patients with CNS (p = 0.003) or liver metastases at diagnosis (p = 0.001), a post-treatment NLR ≥ 5 (p < 0.001), and a post-treatment SII ≥ 730 (p = 0.038). Variables found to be significantly prognostic in the univariate analyses were introduced into a Cox PHR analysis. Multivariable analysis demonstrated that a high post-treatment NLR at 6 weeks [hazard ratio (HR) 15.09, 95% confidence interval (CI) 4.55–50.06, p < 0.001], the presence of liver metastasis (HR 4.92, 95% CI 1.95–12.45, p = 0.001), and CNS metastasis (HR 3.23, 95% CI 1.28–8.16, p = 0.013) were independent prognostic factors for shorter PFS (Table 2).
In the univariate analyses for OS, the presence of CNS (p = 0.022) and liver metastases (p = 0.003), a post-treatment NLR ≥ 5 (p < 0.001), and a post-treatment SII ≥ 730 (p = 0.006) were associated with shorter OS. PD-L1 expression was not associated with OS, irrespective of the cut-off used (5% cut-off, HR 0.71, 95% CI 0.28–1.84, p = 0.485; 50% cut-off, HR 0.46, 95% CI 0.13–1.57, p = 0.215). In multivariate Cox regression analysis, a post-treatment NLR ≥ 5 at week 6 was an independently associated with shorter OS in anti-PD-1 antibody treatment (HR 3.82, 95% CI 1.59–9.17, p = 0.003), along with liver metastasis (HR 3.40, 95% CI 1.44–8.02, p = 0.005) (Table 3). High post-treatment NLR was still independently associated with short PFS and OS in a multivariable analysis model which included additional variables (smoking, histology, and PD-L1 expression) (Supplementary Table 2).
Association of immune-based prognostic scores with PD-L1 expression
Since PD-L1 expression has been suggested as a predictive marker of the treatment response to the anti-PD-1 antibody, we assessed the association between immune-based prognostic scores and PD-L1 expression. Of 54 patients, 36 (66.7%) were evaluable for PD-L1 IHC analysis. Only 7 of 36 patients (19.4%) were positive for PD-L1. Baseline levels of the NLR, PLR, and SII were not associated with PD-L1 expression in tumors. Positive PD-L1 expression was more frequent in patients with lower post-treatment NLRs (23.1 vs. 10%, p = 0.645), PLRs (29.2 vs. 0%, p = 0.070), and SIIs (23.1 vs. 17.4%, p = 0.686) at week 6, but this was not statistically significant (Supplementary Table 3). We also evaluated the association between immune-based prognostic scores and PD-L1 expression using a different cut-off (5%), and there was no statistically significant association between these variables.
Clinical outcomes according to PD-L1 expression and post-treatment NLR
Thirty-six cases were evaluable for both PD-L1 IHC analysis and post-treatment NLR. In this cohort, the response rate was higher in patients with expression of PD-L1 and a low post-treatment NLR (83%, 5 out of 6 patients) compared to those without expression of PD-L1 and a low post-treatment NLR (40%, 8 out of 20 patients). None of the patients with a high post-treatment NLR showed objective response to treatment, regardless of PD-L1 expression (PD-L1 positive, n = 1; PD-L1 negative, n = 9). Supplementary Fig. 2 shows PFS according to both PD-L1 expression and post-treatment NLR. PFS was significantly different according to PD-L1 expression and the post-treatment NLR (median PFS not reached in patients with PD-L1 expression and a low post-treatment NLR vs. 8.9 months in patients without PD-L1 expression and a low post-treatment NLR vs. 1.0 months in a patient with PD-L1 expression and a high post-treatment NLR vs. 1.4 months in patients without PD-L1 expression and a high post-treatment NLR; p < 0.001).
Discussion
In this study, we demonstrated that the post-treatment NLR at week 6 was significantly associated with PFS after anti-PD-1 antibody treatment in patients with advanced NSCLC. In addition, a high post-NLR was also an independent prognostic factor for OS. These findings demonstrated that the post-treatment NLR at week 6 was a prognostic marker for advanced NSCLC treated with anti-PD-1 antibody, and a potential predictive marker of response.
Immune-based prognostic scores, such as the NLR and SII, have been developed and used for many types of cancers. A recent systematic review confirmed that a high NLR was associated with shorter OS in many cancers [3], and, in phase 1 clinical studies, the NLR was an independent prognostic factor for OS [22]. The NLR and SII were predictive of response to both chemotherapy and targeted agents. A low NLR was associated with improved clinical benefits in patients with CRC who were treated with combination chemotherapy and in patients with laryngeal carcinoma treated with chemoradiotherapy [23, 24]. Early reduction in the NLR was a surrogate marker of longer survival in patients with NSCLC who received conventional chemotherapy and gefitinib [25]. In terms of targeted therapy, a low NLR (≤ 3) or decrease in post-treatment NLR at week 6 were associated with better PFS and OS in patients with metastatic renal cell carcinoma (RCC) treated with different targeted agents, including sunitinib and sorafenib [26, 27], and the NLR and SII were predictive markers for patients with metastatic CRC who received chemotherapy plus bevacizumab [6].
Due to the promising efficacies and clinical utilities of immune checkpoint inhibitors, several studies have investigated the role of immune-based scores in prognostication and prediction of response in immune checkpoint inhibitor treatment. Most of the studies focused on patients with advanced melanoma who received ipilimumab [15,16,17,18]. Baseline NLR values < 5 were associated with better PFS and OS [16], and the post-treatment NLR at week 7 or change in lymphocyte count after ipilimumab treatment were associated with PFS [15, 17, 18]. These studies support our finding that the NLR can be used as a predictor for response to immunotherapy.
Ceaseless efforts have been made to explore biomarkers that can predict responses to immune checkpoint inhibition. Among the predictive markers, PD-L1 expression in tumor cells or immune cells seems the most promising. However, PD-L1 expression has been defined differently in many studies using different antibodies and cutoffs [14], and even patients with low or absent PD-L1 expression can achieve robust responses, rendering it difficult to confirm PD-L1 as an exclusionary predictive biomarker [28]. Other biomarkers, such as PD-L1 expression in immune cells, CD8+ T cells, gene alterations in CTLA4, IFNγ inducible genes such as IDO1 and CXCL9, and the tumor mutational load, have been studied; however, most of these biomarkers are not readily available in routine clinical practice [14]. Therefore, the pursuit of a method of optimal patient selection continues. Bagley et al. have recently shown that a high pretreatment NLR was an independent predictive factor for PFS and OS in nivolumab-treated NSCLC patients [19]. Unlike the study by Bagley et al., our data showed that the post-treatment NLR was associated with response rate and survival in patients with NSCLC treated with anti-PD-1 antibody. To the best of our knowledge, our study is the first to evaluate the prognostic role of multiple post-treatment, immune-based prognostic scores in NSCLC, and our study suggests that NLR could be a potential predictor of response. Further studies are needed to evaluate the predictive role of NLR in this setting.
The calculation of the NLR is straightforward, readily available, and reproducible at almost all institutions, with no additional expenditure. Since baseline NLR was not associated with survival or response to the anti-PD-1 antibody, advance patient selection remains difficult. However, our results show that the anticancer immune response is associated with NLR in the early phase of the treatment, and our data can help in clinical decision-making in circumstances such as discriminating tumor flares (or pseudoprogressions) from true progressions and facing moderate-to-severe irAEs.
The biologic basis of this result is not thoroughly understood. The expression of the Fas ligand, which was associated with poor prognosis in patients with RCC, showed a positive association with the NLR [29]. Patients with high NLRs showed lower percentages of CD8+ T cells and lower densities of tumor-infiltrating CD3+ T cells than patients with low NLRs [30]. In our study, high post-treatment NLR, PLR, and SII at week 6 were negatively correlated with PD-L1 expression, although statistical significance was not reached. Because of the small sample size and the fact that only 67% of patients had tumor tissue available for IHC analysis, an association between peripheral blood immune markers and tumor PD-L1 expression could have been masked. Nevertheless, many studies, including ours, suggest that the NLR in the peripheral blood could be a surrogate marker of anti-tumor immunity in patients receiving immunotherapy.
Another compelling aspect of our study is that the presence of liver metastasis was associated with the outcome of anti-PD-1 antibody treatment. The liver is an immunological organ, and has a large population of macrophages (resident Kupffer cells [KCs]), natural killer cells, and natural killer T cells, and yet maintains its immune tolerance to non-pathogenic antigens [31, 32]. Many cells that are resident in the liver, such as the liver sinusoidal endothelial cells, KCs, and dendritic cells, have crucial roles in reducing the immune response and maintaining an immune-suppressive status with the low abundance of major histocompatibility complexes and the lack of costimulatory molecules [33,34,35]. The immune tolerance maintained in the liver might explain the poor response to anti-PD-1 antibody treatment in patients with liver metastases. The presence of liver metastasis can help guide the selection of patients before anti-PD-1 antibody treatment, since this information would be available at the time of treatment initiation.
There are some limitations in our study. First, this was a retrospective analysis of two prospective clinical trials, involving two centers in Korea. The generalizability of this study is limited, since all patients were treated in the clinical trials. Second, the response rate of 33% in our study patients is relatively high compared to the response rate previously reported with anti-PD-1 antibody treatment in NSCLC. This is possibly related to the small number of study population and the retrospective nature of our study that may have led to selection bias. Indeed, the relatively low percentages of response to anti-PD-1 antibody treatment and its substantial cost, and rare, but severe, immunologic AEs encourage us to search for more readily available biomarkers that could aid in identifying patients who are likely to respond to anti-PD-1 antibody treatment, thereby avoiding its toxicities in patients not expected to respond. Therefore, these limitations do not diminish the importance of our study, and the use of the post-treatment NLR at week 6 as an early marker of response that should be evaluated in future studies.
In conclusion, the NLR value at 6 weeks after initiation of treatment is a prognostic marker for patients with advanced NSCLC treated with anti-PD-1 antibody, and a potential predictive marker of response. Other clinical factors, including the site of metastasis, are also predictive of anti-PD-1 antibody efficacy. Further studies are warranted to evaluate the role of the 6-week NLR as a predictor in anti-PD-1 antibody treatment.
Abbreviations
- AE:
-
Adverse event
- ALC:
-
Absolute lymphocyte counts
- ALK:
-
Anaplastic lymphoma kinase
- ANC:
-
Absolute neutrophil counts
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- CRC:
-
Colorectal cancer
- EGFR:
-
Epidermal growth factor receptor
- HR:
-
Hazard ratio
- IRAE:
-
Immune-related adverse events
- KC:
-
Kupffer cells
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- PHR:
-
Proportional-hazard regression
- PFS:
-
Progression-free survival
- PLR:
-
Platelet-to-lymphocyte ratio
- SII:
-
Systemic immune-inflammation index
- SNUBH:
-
Seoul National University Bundang Hospital
- SNUH:
-
Seoul National University Hospital
References
Suh KJ, Kim SH, Kim YJ et al (2017) P3.02c-061 Neutrophil/lymphocyte ratio predicts the efficacy of anti-PD-1 antibody in patients with advanced lung cancer. J Thorac Oncol 12:S1312–S1313. https://doi.org/10.1016/j.jtho.2016.11.1856 (poster)
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Templeton AJ, McNamara MG, Šeruga B et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106:dju124. https://doi.org/10.1093/jnci/dju124
Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, Zhang X, Wang W-M, Qiu S-J, Zhou J, Fan J (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20:6212–6222. https://doi.org/10.1158/1078-0432.CCR-14-0442
Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, Zhang W, Chen S, Lu L, Chen X (2015) Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget 6:43090–43098. https://doi.org/10.18632/oncotarget.5719
Passardi A, Scarpi E, Cavanna L, Dall’Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Brandes AA, Tenti E, Vespignani R, Frassineti GL et al (2016) Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 7:33210–33219. https://doi.org/10.18632/oncotarget.8901
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R (2011) Interaction of tumor cells with the microenvironment. Cell Commun Signal 9:18. https://doi.org/10.1186/1478-811X-9-18
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn M-J, Felip E et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. https://doi.org/10.1056/NEJMoa1501824
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, Majem M, Fidler MJ, de Castro G et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, Kähler KC, Loquai C, Reinmuth N, Steins M, Zimmer L, Sendl A, Gutzmer R (2016) Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 45:7–18. https://doi.org/10.1016/j.ctrv.2016.02.003
Shien K, Papadimitrakopoulou VA, Wistuba II (2016) Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer 99:79–87. https://doi.org/10.1016/j.lungcan.2016.06.016
Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, Biagioli M, Altomonte M, Maio M (2013) Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 62:1021–1028. https://doi.org/10.1007/s00262-013-1418-6
Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GCA, De Galitiis F, Marchetti P, Amato G, Lazzeri A, Pala L, Cocorocchio E et al (2015) Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 112:1904–1910. https://doi.org/10.1038/bjc.2015.180
Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E, Sandomenico F, Petrillo A, Botti G et al (2014) Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 63:675–683. https://doi.org/10.1007/s00262-014-1545-8
Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AMM, Routier E, Robert C (2013) Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 24:1697–1703. https://doi.org/10.1093/annonc/mdt027
Bagley SJ, Kothari S, Aggarwal C et al (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013
Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88:218–230. https://doi.org/10.1016/j.critrevonc.2013.03.010
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Kumar R, Geuna E, Michalarea V, Guardascione M, Naumann U, Lorente D, Kaye SB, de Bono JS (2015) The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br J Cancer 112:1157–1165. https://doi.org/10.1038/bjc.2015.67
Chua W, Charles KA, Baracos VE, Clarke SJ (2011) Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104:1288–1295. https://doi.org/10.1038/bjc.2011.100
Zeng Y-C, Chi F, Xing R, Xue M, Wu L-N, Tang M-Y, Wu R (2016) Pre-treatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol 46:126–131. https://doi.org/10.1093/jjco/hyv175
Lee Y, Kim SH, Han J-Y, Kim HT, Yun T, Lee JS (2012) Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol 138:2009–2016. https://doi.org/10.1007/s00432-012-1281-4
Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, Carducci MA (2012) The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer 48:202–208. https://doi.org/10.1016/j.ejca.2011.09.001
Templeton AJ, Knox JJ, Lin X, Simantov R, Xie W, Lawrence N, Broom R, Fay AP, Rini B, Donskov F, Bjarnason GA, Smoragiewicz M, Kollmannsberger C et al (2016) Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol 70:358–364. https://doi.org/10.1016/j.eururo.2016.02.033
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847–856. https://doi.org/10.1158/1535-7163.MCT-14-0983
Sejima T, Iwamoto H, Morizane S, Hinata N, Yao A, Isoyama T, Saito M, Takenaka A (2013) The significant immunological characteristics of peripheral blood neutrophil-to-lymphocyte ratio and Fas ligand expression incidence in nephrectomized tumor in late recurrence from renal cell carcinoma. Urol Oncol 31:1343–1349. https://doi.org/10.1016/j.urolonc.2011.09.008
Lin G, Liu Y, Li S et al (2016) Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget 7:50963–50971. https://doi.org/10.18632/oncotarget.7680
Jenne CN, Kubes P (2013) Immune surveillance by the liver. Nat Immunol 14:996–1006. https://doi.org/10.1038/ni.2691
Crispe IN (2009) The liver as a lymphoid organ. Annu Rev Immunol 27:147–163. https://doi.org/10.1146/annurev.immunol.021908.132629
Wahl C, Bochtler P, Chen L, Schirmbeck R, Reimann J (2008) B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology 135:980–988. https://doi.org/10.1053/j.gastro.2008.05.076
You Q, Cheng L, Kedl RM, Ju C (2008) Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 48(3):978–990. https://doi.org/10.1002/hep.22395
Bomble M, Tacke F, Rink L, Kovalenko E, Weiskirchen R (2010) Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem Biophys Res Commun 396:342–347. https://doi.org/10.1016/j.bbrc.2010.04.094
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suh, K.J., Kim, S.H., Kim, Y.J. et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 67, 459–470 (2018). https://doi.org/10.1007/s00262-017-2092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2092-x