Abstract
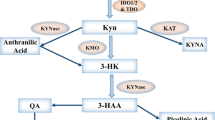
IDO2 is a newly discovered enzyme with 43 % similarity to classical IDO (IDO1) protein and shares the same critical catalytic residues. IDO1 catalyzes the initial and rate-limiting step in the degradation of tryptophan and is a key enzyme in mediating tumor immune tolerance via arrest of T cell proliferation. The role of IDO2 in human T cell immunity remains controversial. Here, we demonstrate that similar to IDO1, IDO2 also degrades tryptophan into kynurenine and is inhibited more efficiently by Levo-1-methyl tryptophan (L-1MT), an IDO1 competitive inhibitor, than by dextro-methyl tryptophan (D-1MT). Although IDO2 enzyme activity is weaker than IDO1, it is less sensitive to 1-MT inhibition than IDO1. Moreover, our results indicate that human CD4+ and CD8+ T cell proliferation was inhibited by IDO2, but both L-1MT and D-1MT could not reverse IDO2-mediated arrest of cell proliferation, even at high concentrations. These data indicate that IDO2 is an inhibitory mechanism in human T cell proliferation and support efforts to develop more effective IDO1 and IDO2 inhibitors in order to overcome IDO-mediated immune tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first step of tryptophan degradation is catalyzed by the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO1), which plays a key role in immune counter-regulation in a range of clinically significant syndromes such as chronic infections, autoimmunity, cancer, and allograft resistance to destructive host immunity [1]. IDO1 suppresses immune response possibly through tryptophan depletion and via the generation of pro-apoptotic metabolites [2, 3]. It has been shown that IDO1+ human antigen-presenting cells reduce tryptophan, suppress T cell activation, and promote tolerance in the tumor microenvironment [4], and tumor draining lymph nodes [5]. Tumor expression of IDO1 has been shown to correlate with poor prognosis in several human malignancies including endometrial [6], colon [7], and epithelial ovarian carcinomas (EOC) [8]. In a previous study, we demonstrated that EOC patients with higher frequencies of intraepithelial CD8+ T cells demonstrated improved survival compared with patients with lower frequencies [9]. These observations have prompted our interest in the use of IDO-inhibitor drugs to overcome IDO-mediated arrest of T cell proliferation and potentially enhance the efficacy of vaccine-induced immune responses in human ovarian cancer.
Previous studies showed that the L stereoisomer of 1-MT was a more potent inhibitor of IDO1, while D stereoisomer was shown to have superior antitumor activity and to be more effective in inhibiting IDO1-expressing tolerogenic DCs in preclinical models [10]. Recently, we have shown that L-1MT is more efficient than D-1MT in inhibiting enzyme activities of IDO1 and reversing IDO-1-mediated arrest of T cell proliferation [11]. A potential explanation for the discrepant biochemical and antitumor effects was provided by the discovery of the IDO2 gene that appears to be preferentially inhibited by D-1MT [12]. Human IDO2 residing immediately downstream of the classic IDO1 gene on 8p12 has been discovered, along with the IDO2 ortholog in the mouse [12]. There is about 43 % similarity between IDO2 and IDO1 in humans, and the same level of similarity between murine IDO2 and IDO1 [13]. Critical catalytic residues between IDO1 and IDO2 are identical and functionally conserved, and IDO2 functions like IDO1 biochemically in tryptophan catabolism [14]. Expression of IDO2 has been found in human pancreatic cancer and cell lines [15]. However, the biological role of IDO2 in T cell immunity remains unclear.
In order to study biological role of IDO2 in cellular immunity, we assessed susceptibility of human lymphocytes to IDO2-expressing cells and examined the ability of different isomers of 1-MT in inhibiting IDO2 enzyme activity. Our data indicate that L-1MT is more efficient than D-1MT in inhibiting both IDO1 and IDO2 enzyme activities. Like IDO1, IDO2 also suppresses T cell proliferation. Although human IDO2 activity can be inhibited by D-, L-, and racemic (D/L) 1MT, none of these 1-MTs could efficiently reverse T cell suppression mediated by IDO2. The data are relevant to understanding IDO2 biological functions as well as in selecting IDO inhibitors for clinical trials.
Materials and methods
Normal donors and cell lines
Blood samples were obtained from normal healthy volunteers. All specimens were collected under an approved protocol from the Institutional Review Board (IRB) at Roswell Park Cancer Institute (RPCI), Buffalo, NY. Human embryonic kidney cell line 293 and IDO1 (INDO)-transfected 293 cell line (293IDO1) were the gifts from Dr. Benoît J. Van den Eynde [3] and cultured in RPMI 1640 media supplemented with 10 % fetal bovine serum, 2 mM l-glutamine (Gibco/Invitrogen, Carlsbad, CA), 100 U/ml penicillin/streptomycin (Gibco/Invitrogen), and 0.05 mM nonessential amino acids (Gibco/Invitrogen) as complete medium (CM). Human and mouse IDO2 (INDOL1)-transfected 293 cells were provided by Dr. Richard Metz [12] and maintained in CM supplemented with 5 ng/ml blasticidin and 100 μg/ml Zeocin and treated with doxycycline (Dox) to induce transgene expression per Invitrogen’s procedure. All cells were cultured in regular RPMI 1640 media.
Measurement of kynurenine production
Kynurenine (Kyn) production in cells was analyzed essentially as described [16]. Briefly, 200 μl of the test sample was mixed in a 96-well plate with 100 μl 30 % TCA. After centrifuging, supernatants (100 μl) were transferred to a new dish, mixed with 100 μl Ehrlich’s reagent (2 % p-dimethylaminobenzaldehyde w/v in glacial acetic acid), and incubated 10 min at room temperature. Absorbance at 490 nm was determined on a plate reader and the data collected and analyzed using Excel software (Microsoft). Samples were analyzed in triplicate.
Assessment of cellular proliferation and suppression
CD4+ and CD8+ cell proliferation was measured by CFSE dilution after appropriate gating. Magnetic bead separation technology (Invitrogen Dynal AS, Oslo, Norway) was used to isolate CD8+ and CD4+ T cell subsets. Subsequently, 1 × 107/ml cells separated from the aforementioned protocol were independently labeled with 5 μM CFSE (Invitrogen) according to the manufacturer’s instructions. And 5 × 104 cells were separately co-cultured with 1 × 104 of 293, 293IDO1, or 293IDO2 cells and activated with plate-bound anti-CD3 (UCHT1 clone, 5 μg/ml) and soluble anti-CD28 (clone 28.1, 1 μg/ml, both from BD Biosciences) mAbs in 96-well U-bottom plates (Nalgene Nunc, Rochester, NY). The ability of IDO2 to suppress the growth of responder T cells was assessed by FACS after co-culturing for 3–6 days. In some experiments, T cells were stimulated with 9 μg/ml PHA (HA15, Murex Diagnostics, Dartford, UK). Activated T cells cultured alone without any 293 cells were used as positive control. Nonactivated T cells were used as negative control.
Antibodies and fluorescence-activated cell sorting analysis
Anti-CD3-APC H7, anti-CD4-APC, and anti-CD8-PE-Cy7 were purchased from Becton–Dickinson Pharmingen. LIVE/DEAD Fixable Dead Cell Stain Kits were purchased from Invitrogen. Surface staining was performed for 15–20 min at 4 °C in fluorescence-activated cell sorting (FACS) tubes containing total cells harvested from aforementioned co-cultures resuspended in 100 μl staining buffer [PBS with 1 % FCS and 0.05 % NaN3 (Sigma)]. LIVE/DEAD samples were stained according to manufacturer’s instructions. Stained samples were acquired on an LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ) using FACSDiVa software, and listmode data were analyzed with WinList (Verity Software House, Topsham, MN). Dead cells were removed from analyses by gating on events that were considered negative for the LIVE/DEAD dye. With each sample, an isotype-matched negative control was used to determine the positive and negative cell populations.
Statistical analysis
Comparison between paired or unpaired groups was performed using the appropriate Student’s t test. A p value <0.05 was defined as statistically significant.
Results
IDO2 has enzyme activity that degrades tryptophan and generates kynurenine
Human and mouse IDO2 genes under the control of Dox-activated promoters were transfected into human embryonic kidney cells (293 cells), respectively [12]. These cells express IDO2 in the presence of Dox and grow in a similar manner as parental 293 cells in the absence of Dox. The expression levels of IDO1 and IDO2 proteins are similar as detected by Western blot [12]. To study tryptophan (Trp) metabolism mediated by IDO2 enzyme, these cells were cultured in complete medium supplemented with various amount of Trp and 20 ng/ml of Dox. On day 5, the supernatant was collected, and concentrations of Kyn were measured by Ehrlich reagent. As shown in Fig. 1a, the concentrations of Kyn increased from both IDO2 cells treated with Dox as Trp concentrations increased, indicating that both human and mouse IDO2 were able to generate Kyn by degrading Trp. There was more Kyn produced by Dox-treated cells than by cells without Dox. Furthermore, the amount of Kyn in supernatants derived from mouse IDO2 cells treated with Dox was greater than those from human IDO2, indicating that mouse IDO2 (MuIDO2) has a stronger enzyme activity than human IDO2, considering that these cells express same amount of IDO2 protein in our system. Next, we cultured MuIDO2 cells treated with Dox and HuIDO1 cells for 5 days supplemented with various amount of Trp. Kyn concentration analysis from cultured supernatants revealed that HuIDO1 cells produced more amount of Kyn than MuIDO2 cells. These data indicated that human IDO1 derived from a human IDO1-transfected 293 cells has the strongest enzyme activity when compared with both human and mouse IDO2 (Fig. 1b).
Human and mouse IDO2 degrades Trp into Kyn. a Human and mouse IDO2 gene–transfected 293 cells were cultured in complete media supplemented with various amount of Trp and 20 ng/ml of Dox for 5 days, and concentrations of Kyn derived from each culture supernatant were measured. b IDO1 enzyme activity is stronger than IDO2. Concentrations of Kyn were compared between MuIDO2 cells and HuIDO1-transfected 293 cells from supernatant on day 5. Kyn production from 293 parental cells and 293IDO2 cells without Dox was also presented. Tests were repeated at least three times with similar results
L-1MT is more efficient in suppressing IDO2 activity
Next, we wanted to determine whether IDO2 can be suppressed by IDO inhibitors and if this is the case, which isomer is more efficient in inhibiting IDO2 enzyme activity. 293IDO2 cells were cultured in the absence or presence of 20 ng/ml of Dox with or without different isomers of 1 mM 1-MT. On day 5, supernatant from each culture was harvested, and Kyn concentrations were measured. 293IDO2 cells produced very little Kyn when they were cultured without Dox treatment, which was independent of the presence of different 1-MTs, indicating that 1-MTs alone had no effect on 293IDO2 cells. Consistent with Fig. 1, 293IDO2 cells produced higher amounts of Kyn when treated with Dox (p = 0.0005 compared with no Dox treatment) (Fig. 2a). Surprisingly, contrary to previous report [12], IDO2 activity was not inhibited by D-1MT (p = 0.65). On the contrary, L-1MT but not D-1MT partially suppressed IDO2 activity (p = 0.03). To further investigate IDO2 sensitivity to D- or L-1MT, serial-diluted amounts of D-1MT and L-1MT were incubated with 293IDO2 cells treated with Dox (Fig. 2b). 293IDO2 cells cultured with serial-diluted D-1MT and L-1MT without Dox treatment serving as negative controls generated negligible amounts of Kyn. After Dox treatment, noticeable amounts of Kyn were produced from these 293IDO2 cells in the absence of 1-MTs. However, IDO2 activity began to decrease when cells were treated with as low as 250 μM of L-1MT and continued to decrease as concentration of L-1MT increased. On the other hand, Kyn concentration decreased very slowly when cells were cultured in the presence of various amounts of D-1MT. We confirmed these observations by studying kinetics of Kyn production. IDO2 cells were cultured with different 1-MTs, supernatants were collected at different time points, and concentrations of Kyn were measured (Fig. 2c). IDO2 cells without Dox treatment served as negative control. Kyn production from Dox-treated IDO2 cells appeared by day 3 and continued to increase with time. It was clearly shown that at each of the time points, L-1MT was more efficient than D-1MT in inhibiting IDO2 activity. Collectively, our data indicated that IDO2 activity was inhibited more efficiently by L-1MT than by D-1MT.
L-1MT is more efficient than D-1MT in inhibiting IDO2 activity. a Kyn concentrations derived from supernatant of mouse IDO2 cells cultured with different 1 mM of 1-MTs were measured. b Mouse IDO2 cells were cultured with various amounts of different 1-MTs for 4 days. c Mouse IDO2 cells were cultured in 1 mM of different 1-MTs for different days. IDO2 cells without Dox treatment were used as negative control. Tests were repeated at least three times with similar results
Both IDO1 and IDO2 are suppressed more efficiently by L-1MT than by D-1MT
Recently, we have reported that IDO1 is inhibited more efficiently by L-1MT than by D-1MT [11]. It is important to compare efficiencies of different 1-MTs on different IDO enzymes. 293IDO1 cells and 293IDO2 cells treated with Dox were cultured with various amounts of different 1-MTs for 5 days. Analysis of Kyn concentrations revealed superior enzyme activity of IDO1 to IDO2 (Fig. 3a). Consistent with our previous report [11], L-1MT was more efficient than D-1MT in suppressing IDO1 activity. Since IDO2 enzyme activity was much weaker than IDO1, we plotted percentage inhibition of Kyn production after treatment with 1-MTs. As shown in Fig. 3b, inhibitions of Kyn production were in the following order: 293IDO1 with L-1MT > 293IDO2 with L-1MT > 293IDO2 with D-1MT > 293IDO1 with D-1MT. The data indicated that L-1MT is more efficient than D-1MT in inhibiting both IDO1 and IDO2 activities. Although IDO2 is more efficiently inhibited by L-1MT than D-1MT, there is only about 30 % highest inhibition in IDO2 at high concentrations of L-1MT, while IDO1 was inhibited by about 50 % by the same amount of L-1MT, suggesting that 1-MT is not very efficient in inhibiting IDO2 enzyme activity.
Both IDO1 and IDO2 were suppressed more efficiently by L-1MT than by D-1MT. Kyn concentrations (a) and %inhibition of Kyn by 1-MT which was calculated as percentage of Kyn difference after 1-MT treatment divided by Kyn without 1-MT treatment with no background subtraction. b Derived from 293IDO1 and mouse 293IDO2 cells treated with Dox were shown. Tests were repeated at least three times with similar results. The reading from media alone was used as negative control (−)
IDO2 inhibits proliferation of CD4+ and CD8+ T cells
In order to determine the effect of IDO2 expression on T cell proliferation, CD4+ and CD8+ T cells were purified from normal donor PBMC by magnetic beads; 5 × 104 CFSE labeled CD4+ or CD8+ T cells were co-cultured with 1 × 104 of 293IDO2 cells treated or not with 20 ng/ml Dox. Plate-bound anti-CD3 and soluble CD28 were used to stimulate T cells. After 4 days, T cell proliferation was measured by CFSE dilution after gating by FACS. As shown in Fig. 4a, a few CD4+ T cells proliferated without anti-CD3/CD28 stimulation. However, anti-CD3/CD28 stimulation resulted in CD4+ T cell proliferation when cultured with or without 293 cells in the presence or absence of Dox, suggesting that co-culturing with 293 cells or treatment with Dox does not affect T cell proliferation. In contrast, less amounts of T cells divided when co-cultured with 293IDO2 cells treated with Dox. T cell growth was also inhibited by 293IDO1 cells. Unlike the IDO2 experiments, IDO1-mediated T cell suppression was independent of Dox treatment. The data indicate that in our in vitro system, human CD4+ (Fig. 4a) and CD8+ (Fig. 4b) T cell proliferation was inhibited by IDO2-expressing cells. We could not reverse T cell suppression mediated by IDO2 by either L- or D-1MT by co-culturing T cells with IDO2-expressing cells in the presence of different 1-MTs (Fig. 4b). In contrast, consistent with our previous report [11], T cell suppression by IDO1 activity can be reversed by L-1MT but not by D-1MT (Fig. 4b). Analysis of Kyn production from these supernatants revealed that IDO2 was induced from 293IDO2 cells after Dox treatment (Fig. 4c), which further confirmed our T cell proliferation assay results. In these supernatants, we also found that L- and DL-1MTs were more efficient in inhibiting IDO2 activity than D-1MT. IDO1 was inhibited by L- and DL-1MT in the presence or absence of Dox. The higher efficiency of L-1MT in inhibiting IDO1 activity is consistent with the results indicating that L- but not D-1MT can reverse IDO1-mediated T cell suppression.
IDO2-suppressed CD4+ and CD8+ T cells proliferation. Isolated CD4+ (a) or CD8+ (b)T cells stimulated with anti-CD3/CD28 were co-cultured with MuIDO2 293 cells in the absence or presence of Dox for 4 days, and T cells cultured alone activated (+) or not (−) were used as controls. T cell proliferation (a, b) and Kyn production (c) were studied. d CD4 cells stimulated by PHA were co-cultured with different 293 cells supplemented with various amounts of Trp for 4 days, and T cell proliferation was determined by CFSE dilution. T cells cultured alone with or without PHA were served as positive (+) or negative (−) controls, respectively. Experiments were repeated three times with similar results
IDO1 suppresses T cell proliferation through Trp starvation and Kyn production. In order to determine whether this is also related to IDO2-mediated T cell suppression, we co-cultured CD4 T cells with 293IDO2 cells supplemented with various amount of Trp in complete media. As shown in Fig. 4d, CD4 T cell proliferation was not suppressed upon co-culture with 293, 293 Dox, 293IDO2, when compared to CD4 T cells cultured without any 293 cells. However, T cell growth was slightly inhibited when co-cultured with 293IDO2 cells treated with Dox (IDO2 Dox). This suppression was not reversed by increasing the amounts of Trp in culture. On the contrary, although CD4 T cell proliferation was suppressed by IDO1 cells, the suppression can be reversed as supplemented by Trp.
Discussion
We have previously reported that L-1MT is more efficient than D-1MT in inhibiting IDO1 enzyme activity [11]. Here, we demonstrate that IDO2, a protein sharing similar critical catalytic residues as IDO1, also produced Kyn by degrading Trp and was also inhibited more efficiently by L-1MT than by D-1MT. This observation is consistent with recent studies [17, 18] but is contrary to a previous report, in which IDO2 was found to be suppressed by D-1MT while L-1MT was inefficient [12]. While D- and L-1MTs were tested from 0 to 100 μmol/l in the previous report, we tested a wider range (0–1,000 μmol/l) in the current study. Since IDO2 is less sensitive to both 1-MTs than IDO1, higher concentrations of 1-MTs are likely needed to show inhibition of Kyn production. Indeed, we could only observe suppression of Kyn production by L-1MT in 293IDO2 cultures at >125 μmol/l concentration. In addition, we found that IDO2 was more efficiently suppressed by L-1MT than by D-1MT at different time points.
In previous studies, while the L stereoisomer of 1-MT was a more potent inhibitor of IDO1, the D stereoisomer was shown to have superior antitumor activity and to be more effective in inhibiting IDO1-expressing tolerogenic DCs in preclinical models [10]. The discrepant biochemical and antitumor effects were attributed to the IDO2 gene that appears to be preferentially inhibited by D-1MT [12]. Our data demonstrate that both IDO1 and IDO2 are more efficiently inhibited by L-1MT than by D-1MT, indicating that the superior antitumor effects of D-1MT in preclinical models may not be applicable to humans.
Studies on IDO2 indicated that IDO2-expressing cells such as tumor cells and DCs also express IDO1 [19, 20]. Therefore, we studied the function of IDO2 in IDO2-transfected eukaryotic cells to exclude IDO1 interference. In addition, IDO2’s enzyme activity is very low in constitutively or in IFN-γ-induced IDO2-expressing cells, making functional study of IDO2 in physiological conditions almost impossible [12, 14]. Analysis of classic IDO1 and novel IDO2 proteins expressed as transgenes in bacteria or eukaryotic cells suggested that IDO2 has only 3–5 % of the enzymatic activity of IDO1 [12, 14, 21], and biochemical study also found that the K(m) of human IDO2 for l-tryptophan is much higher than that of IDO1 [22]. In the present study, we demonstrated IDO2-dependent Trp degradation and Kyn production using an in vitro system. This model allowed us to obtain reproducible results by culturing IDO2-expressing cells with different isomers of 1-MT. Although our artificial culture model does not elucidate which cell types in the physiologic system are the biologically relevant sites of expression for IDO2, it provides an accessible model for studying the efficiencies of IDO2 inhibitors.
Lob et al. studied human IDO2 expression and function in DCs [19] and tumor cells [23]. They found that these cells expressed both IDO1 and IDO2 detected by RT-PCR. After blocking IDO1 expression by siRNA, Kyn production was dramatically decreased, which indicated that almost all the IDO activity from these cells derived from IDO1 but not from IDO2. These studies demonstrated that human IDO2 has little or no functional activity and may not be critical for human tumor immunity. However, in these same studies, there was noticeable amount of Kyn produced even after IDO1 siRNA treatment. Although siRNA blocking is usually not 100 % efficient, this raises the possibility that IDO2 enzyme activity was contributing to Kyn production. Indeed, a recent report described expression of IDO2 in human pancreatic cancer and cell lines [15]. Although this report did not address the impact of IDO2 expression on cancer patients’ survival, it is clear from these studies and our data that human IDO2 has functional activity and may play a role in human tumor immunity. IDO2 might be a useful target for anticancer immunotherapeutic strategies, which has been demonstrated by the existence of IDO2-specific cytotoxic T cells in both normal and cancer patients [24].
IDO1 plays an essential inhibitory role in the immune system by suppressing T cell function. Here, we also demonstrate that IDO2 suppresses proliferation of human CD4+ and CD8+ T cells, which was concomitant with tryptophan degradation and kynurenine generation. We did not detect any IDO2-mediated T cell death (data not shown), suggesting that IDO2 suppresses human T cell proliferation by mechanism(s) other than apoptotic cell death. Although we observed that L-1MT inhibits IDO2 activity more efficiently than D-1MT, both L- and D-1MT could not reverse IDO2-mediated T cell suppression. Since the level of Kyn from functional 293IDO2 cells in the presence of high concentrations of L-1MT was still higher than nonfunctional 293IDO2 cells, we propose that a threshold of kyunerine suppression is required for reversing IDO2-mediated arrest of T cell proliferation, and neither L- nor D-1MT could reach this threshold. On the other hand, L-1MT is efficient in inhibiting IDO1 enzyme activity as demonstrated by almost complete inhibition of Kyn generation, and this effect was accompanied by reversal of IDO1-mediated arrest of T cell proliferation. Taken together with our previous study [11], L-1MT is able to reverse IDO1-mediated T cell suppression, whereas it is unable to reverse IDO2-mediated arrest of T cell proliferation, even at high concentrations.
Since IDO1 activity suppresses T cell proliferation by depletion of Trp and generation of Kyn derivatives, we went on to study the effect of additional amount of Trp on IDO2-mediated T cell suppression. Unlike IDO1, IDO2 suppression on T cell growth could not be reversed by increasing Trp concentration. Therefore, although IDO2 degrades Trp into Kyn which is inhibited by L-1MT, IDO2-mediated T cell suppression could not be reversed by either L-1MT or extra Trp. This suggests that the mechanism of IDO2-mediated T cell suppression differs from IDO1-mediated T cell suppression and warrants further investigation.
References
Mellor AL, Munn DH (2008) Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev 8(1):74–80
Grohmann U, Fallarino F, Puccetti P (2003) Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 24(5):242–248
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9(10):1269–1274
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4(10):762–774
Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL (2004) Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 114(2):280–290
Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F (2006) Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 95(11):1555–1561
Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A (2006) Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 12(4):1144–1151
Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M (2005) Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 11(16):6030–6039
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Nat Acad Sci USA 102(51):18538–18543
Munn DH (2006) Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol 18(2):220–225
Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD Jr, Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, Beck A, Liao J, Cheney R, Moysich K, Lele S, Shrikant P, Old LJ, Odunsi K (2009) Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res 69(13):5498–5504
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC (2007) Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res 67(15):7082–7087
Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH (2009) Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol 41(3):467–471
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH (2007) Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396(1):203–213
Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, Brody JR (2009) Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg 208(5):781–787; discussion 787–789
Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V (2006) 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol 177(4):2061–2071
Yuasa HJ, Ball HJ, Austin CJ, Hunt NH (2010) 1-L-methyltryptophan is a more effective inhibitor of vertebrate IDO2 enzymes than 1-D-methyltryptophan. Comp Biochem Physiol 157(1):10–15
Austin CJ, Mailu BM, Maghzal GJ, Sanchez-Perez A, Rahlfs S, Zocher K, Yuasa HJ, Arthur JW, Becker K, Stocker R, Hunt NH, Ball HJ (2010) Biochemical characteristics and inhibitor selectivity of mouse indoleamine 2,3-dioxygenase-2. Amino acids 39(2):565–578
Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P (2008) Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111(4):2152–2154
Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P (2009) Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev 9(6):445–452
Yuasa HJ, Ball HJ, Ho YF, Austin CJ, Whittington CM, Belov K, Maghzal GJ, Jermiin LS, Hunt NH (2009) Characterization and evolution of vertebrate indoleamine 2, 3-dioxygenases IDOs from monotremes and marsupials. Comp Biochem Physiol 153(2):137–144
Meininger D, Zalameda L, Liu Y, Stepan LP, Borges L, McCarter JD, Sutherland CL (2011) Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta 1814(12):1947–1954
Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P (2009) IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58(1):153–157
Sorensen RB, Kollgaard T, Andersen RS, van den Berg JH, Svane IM, Straten P, Andersen MH (2011) Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res 71(6):2038–2044
Acknowledgments
We thank Dr. Benoît J. Van den Eynde for providing us with 293IDO1 cells and Dr. Richard Metz for 293IDO2 cells. This work was supported by a grant from the Cancer Research Institute Ovarian Cancer Working Group Grant (K.O.) Anna-Marie Kellen Clinical Investigator Award of the CRI (to K.O.), the Ovarian Cancer Research Fund (K.O. and P.S.), and a Roswell Park Cancer Institute Alliance Foundation grant.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, F., Liao, J., Villella, J. et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother 61, 2013–2020 (2012). https://doi.org/10.1007/s00262-012-1265-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1265-x