Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) is a multifunctional transcription factor that regulates adipogenesis, immunity and inflammation. Our laboratory previously demonstrated that PPARγ ligands induce apoptosis in malignant B cells. While malignant B lineage cells such as B cell lymphoma express PPARγ, its physiological function remains unknown. Herein, we demonstrate that silencing PPARγ expression by RNAi in human Burkitt’s type B lymphoma cells increased basal and mitogen-induced proliferation and survival, which was accompanied by enhanced NF-κB activity and increased expression of Bcl-2. These cells also had increased survival upon exposure to PPARγ ligands and exhibited a less differentiated phenotype. In contrast, PPARγ overexpression in B lymphoma cells inhibited cell growth and decreased their proliferative response to mitogenic stimuli. These cells were also more sensitive to PPARγ-ligand induced growth arrest and displayed a more differentiated phenotype. Collectively, these findings support a regulatory role for PPARγ in the proliferation, survival and differentiation of malignant B cells. These findings further suggest the potential of PPARγ as a therapeutic target for B cell malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burkitt’s lymphoma (BL) is an aggressive non-Hodgkin B cell lymphoma usually diagnosed in children and young adults. In the United States and Western Europe, it constitutes about 1–2% of all adult lymphomas and 30–50% of pediatric lymphomas [14, 28]. BL has also been associated with Epstein–Barr virus (EBV) latent infection, which results in a lymphoproliferative phenotype and increased resistance to apoptosis [21]. Intensive chemotherapeutic regimens have greatly increase prognosis, but can have significant toxicity, including treatment-related deaths [14]. New courses of therapy are aimed at minimizing toxicity without compromising outcome, and include monoclonal antibody (Rituximab) and steroid therapies. Recent molecular evidence of the role of transcription factors in BL is yielding promise as therapeutic targets [41].
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of transcription factors that regulate lipid metabolism and adipose differentiation [6]. There are three known PPAR isoforms: PPARα, PPARβ/δ and PPARγ. The human PPARγ gene is located on chromosome 3, band 3p25 [3]. This gene gives rise to three mRNA isoforms (PPARγ1, γ2 and γ3) through alternate promoter usage and splicing [18]. Both PPARγ1 and PPARγ3 mRNA translate into PPARγ1 protein and PPARγ2 mRNA gives rise the PPARγ2 isoform that contains 28 extra amino acids [18]. All PPAR isoforms heterodimerize with members of the retinoid X receptor (RXR) subfamily of nuclear hormone receptors. These complexes then bind to the peroxisome proliferator response element (PPRE) in the promoter regions of target genes. PPARγ is activated by natural ligands such as 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2) and by certain polyunsaturated fatty acids. PPARγ can also be activated by synthetic ligands such as the thiazolidinediones (TZDs) class of anti-diabetic drugs. PPARγ also has anti-proliferative, anti-inflammatory and pro-differentiating properties in immune cells [20]. Importantly, PPARγ regulates B lymphocyte function. B lymphocytes from PPARγ-haploinsufficient mice exhibit increased proliferation and survival [54]. Our laboratory (and others) have demonstrated that both normal and malignant B lymphocytes express PPARγ and that exposure to certain PPARγ ligands inhibits B cell proliferation and induces apoptosis [42, 43, 49]. Some studies have shown that PPARγ ligands induce differentiation of malignant cells [11, 39]. Moreover, PPARγ expression increases during differentiation of monocytes to macrophages [40]. In hematological malignancies, PPARγ ligands can induce monocytic differentiation in myeloid leukemia cells and help sensitize malignant cells to the pro-differentiation effects of all trans-retinoic acid [16, 24, 34, 35, 57]. These studies support the concept that PPARγ is an important transcription factor in B cells and serves as a pro-differentiation factor for malignant cells.
The ability of PPARγ to alter B cell proliferation and apoptosis may relate to its ability to repress other transcription factors, such as nuclear factor kappa-B (NF-κB) [20]. NF-κB controls B cell proliferation and survival [19, 30] and is constitutively active in several human cancers, including B cell lymphomas [44, 51]. Moreover, EBV activates NF-κB in the process of B cell transformation to malignancy [27]. Straus et al. [56] reported that the natural PPARγ ligand 15d-PGJ2 inhibits multiple steps in the NF-κB signaling pathway. PPARγ may also regulate NF-κB by obstructing its transcription [9]. A recent report by Pascual et al. demonstrated that PPARγ-ligand-dependent sumoylation of PPARγ leads to the recruitment of PPARγ to the repressor complexes on the promoter regions of genes regulated by NF-κB, ultimately suppressing NF-κB driven gene expression [45].
Thus, PPARγ may control B cell lymphoma proliferation and survival through alterations in NF-κB activity. We hypothesized that the level of PPARγ plays an important role in B lymphoma cell survival. We speculated that high levels of PPARγ would inhibit proliferation/survival and induce differentiation, while low PPARγ levels would enhance B lymphoma survival and enhance their undifferentiated phenotype. Herein, we investigated the effects of PPARγ expression on B cell lymphoma proliferation, survival and differentiation.
Materials and methods
Reagents and antibodies
Ciglitazone was purchased from Biomol (Plymouth Meeting, PA). CDDO was synthesized by Dr. T. Honda and kindly provided by Dr. Michael Sporn (Dartmouth College, Hanover, NH) [25]. [3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] MTT, DMSO and anti-Flag M2 monoclonal antibody peroxidase conjugate were from Sigma (St Louis, MO). The anti-PAX-5 was purchased from Millipore (Billerica, MA). The anti-BLIMP-1 was purchased from Novus Biologicals (Littleton, CO). The rabbit anti-human PPARγ antibody was purchased from Biomol. Anti-Bcl-2 (sc-7382) and anti-p65 (sc-372) antibodies were purchased from Santa Cruz (Santa Cruz, CA). Total actin (CP-01) antibody was from Oncogene (Cambridge, MA).
Construction of lentiviral vectors
The lentiviral vector encoding the short hairpin RNA (shRNA) against PPARγ transcripts (nucleotides 1095-1113) was constructed using the oligonucleotide sequence 5′GTTTGAGTTTGCTGTGAAG3′, as described by Katayama et al. [31]. The two complementary oligonucleotides were cloned downstream of the human RNA polymerase III U6 promoter and then subcloned into the FG12 lentiviral vector (gift of Dr. David Baltimore), as described earlier [48] (see also Fig. 1a).
Construction of a lentiviral vector for delivering human PPARγ siRNA. a Schematic diagram of the siRNA-expressing lentiviral vector. The short hairpin form of siRNA is expressed under the control of a human U6-RNA Pol III promoter (Pol III). The vector contains a GFP marker under the UbiC promoter for tracking transduced cells b HEK 293 cells were mock transfected (lane 1) or were co-transfected with a FLAG-tagged-PPARγ-WT vector (Flag-PPARγ) and with either an empty DNA vector, (pcDNA3.1, lane 2), increasing DNA concentrations of empty parental FG12 vector (lanes 3, 4) or increasing DNA concentrations of the FG12 vector expressing siRNA against PPARγ (lane 5, 6). Numbers represent microgram amounts of plasmid DNA. All transfections included equivalent concentrations of DNA, which were normalized with the empty DNA vector, pcDNA3.1. c Ramos cells were infected at an MOI of 5 and the GFP-positive cells were sorted by FACS. After 5 days, cells were analyzed by flow cytometry to determine the purity. LV-control and LV-PPARγ-siRNA infected cells showed >95% GFP-positive cells. d Reduction of PPARγ protein levels in Ramos cells transduced with LV-PPARγ-siRNA (siRNA). Total actin was used as a loading control
The LV-PPARγ1-WT and the LV-empty vector were produced as described earlier [17].
Lentiviral vector production
Human embryonic kidney 293FT cells (Invitrogen, Carlsbad, CA) were grown to 50–70% confluency in Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum in T-175 flasks. Subsequently, the VSVG pseudotyped HIV vector was generated by co-transfecting with 5 μg of envelope vector (pCMV-VSVG), 14 μg of transfer vector and 14 μg of packaging vector pCMV-Δ89.2, using Lipofectamine LTX (Invitrogen, Carlsbad, CA). Cells were split into two T-175 flasks 6 h post-transfection. Supernatants were collected 48 and 72 h post-transfection. Virus was harvested by ultracentrifugation at 50,000×g for 2 h at 4°C using a Beckman SW 28 rotor. The concentrated virus stocks were titered on 293FT cells based on GFP expression.
Cells and culture conditions
Ramos (EBV negative) and Raji (EBV positive) B lymphoma cells were cultured in RPMI 1640 tissue culture medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 5 × 10−5 M β-mercaptoethanol (Eastman Kodak, Rochester, NY), 10 mM HEPES (US Biochemical Corp., Cleveland, OH), 2 mM l-glutamine (Life Technologies), 50 μg/ml gentamicin (Life Technologies). Human embryonic 293FT cells were purchased from ATCC (Manassas, VA) and were grown in Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum.
Lentiviral infections and cell sorting
Ramos and Raji B lymphoma cells were plated at a density of 1 × 106 cells/well in a 12 well plate and infected with the different lentiviral vectors at MOIs of 1–5 in the presence of 6 μg/ml of polybrene. Twenty-four hours post-infection, the growth media was replaced. Lentiviral transduced cells were identified on the basis of the GFP expression. GFP-positive cells were sorted by flow cytometry using a FACSAria (BD Bioscience, San Jose, CA).
Immunofluorescence
Ramos B lymphoma cells infected with the lentiviral constructs were incubated with mouse anti-human CD19-APC (BD Biosciences), anti-human CD38-PE (BD Biosciences), anti-human CD20-PE (BD Biosciences) or with anti-human CD40-biotin (Axxora/Ancell, Bayport, MN) in cold PBS with sodium azide (0.02%) and BSA (0.3%) for 20 min at 20°C. For CD40 surface staining, cells were washed and incubated with secondary APC-conjugated streptavidin (Caltag, Burlingame, CA).
Electrophoretic mobility shift assay (EMSA) for NF-κB
Gel shift assay of nuclear extracts from uninfected, LV-control and LV-PPARγ-siRNA infected Ramos cells was performed as described [49].
PPARγ activity assay
Nuclear extracts from LV-Empty infected or LV-PPARγ-WT infected Ramos cells were collected using a nuclear extract kit (Active Motif, Carlsbad CA). To determine PPARγ activity, a TransAM PPARγ activity assay kit was used (Active Motif, Carlsbad CA) as described [1].
Viability and proliferation assays
Ramos B lymphoma cells transduced with LV-PPARγ-siRNA and LV-control-GFP (1 × 105 cells per well) were plated in a 96-well flat bottom microtiter plate. MTT was performed to assess cell viability. The tetrazolium salt MTT is taken up by viable cells and reduced to a formazan residue by functional mitochondria of living cells [4]. Cells were treated with increasing concentrations of the PPARγ ligand CDDO and 10 μl per well of a 5 mg/ml of MTT (in 1× PBS) was added for the last 4 h of incubation. After incubation, the plate was centrifuged, the media removed and DMSO was added to each well to dissolve the precipitate. The plate was read at 510 nm on a Benchmark microplate reader (BioRad, Hercules, CA).
For the proliferation assay, cells were cultured as described above, and were left untreated or were treated with CD40L [29], 10 μg/ml of rabbit anti-human F(ab′)2 anti-IgM Ab (Jackson ImmunoResearch Laboratories) and 1/1,000 dilution of Pansorbin (Staphylococcus aureus Cowen I strain; Sigma–Aldrich) or different combinations of these mitogens; 1 μCi/well of 3H-thymidine was added for the last 18 h of culture. The cells were harvested onto a 96-well filter plate and the 3H-thymidine incorporation was detected as counts per minute (cpm) using a Topcount Luminometer (PerkinElmer, Boston, MA).
Cell cycle analysis
Cell cycle analysis of human BL cells was performed using the APC Bromodeoxyuridine (BrdU) Flow kit (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions. In the set of experiments using ciglitazone, cells were treated with ciglitazone (5 μM) for 48 h. Cells were then pulsed for 30 min with 10 nM BrdU and stained the APC-BrdU flow kit.
Western blots
Whole cell extracts were collected using ELB buffer [50 mM HEPES (pH 7), 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, 50 μM ZnCl2, supplemented with 0.1 mM PMSF, 1 mM DTT and a mixture of protease and phosphatase inhibitors] and total protein was quantified using bicinchoninic acid protein assay (BCA assay kit) (Pierce, Rockford, IL). A total of 25 μg protein was electrophoresed on 8–16% Precise™ protein gels (Pierce, Rockford, IL) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millopore, Billerica, MA). The membranes were analyzed for immunoreactivity with the indicated primary antibody, washed and then incubated with an appropriate horse radish peroxidase-conjugated secondary antibody. The membranes were visualized by chemiluminescence using an ECL kit (Pierce, Rockford, IL).
Statistical analysis
Results are expressed as the mean ± standard deviation. Two-tailed Student t test was performed and P < 0.05 were considered significant. All experiments were repeated at least three times.
Results
Construction of a lentiviral-based vector for delivery of PPARγ siRNA
Our laboratory previously demonstrated that both normal and malignant B cells express PPARγ and that certain PPARγ ligands induce apoptosis [42, 43, 49, 50]. To determine the physiological role of PPARγ expression in certain human B cell lymphomas, we used a lentivirus-mediated shRNA expression system to knockdown PPARγ expression in Burkitt’s B lymphoma cells. To construct the siRNA expression cassette, a target sequence of siRNA for PPARγ was selected to knockdown both PPARγ1 and PPARγ2 [31]. The 19 bp PPARγ target sequence was cloned downstream of the human RNA polymerase III U6 promoter and then subcloned into the FG12 lentiviral vector, which also expresses GFP under the Ubiquitin C (UbiC) promoter (Fig. 1a) [48]. First, to determine whether the siRNA sequence effectively targets human PPARγ mRNA, the DNA plasmid vector containing the siRNA sequence was tested in human embryonic kidney (HEK) 293 cells. HEK 293 cells were mock transfected (Fig. 1b, pcDNA 3.1, lane 1) or were co-transfected with a FLAG-tagged PPARγ-wild type vector (Fig. 1b, Flag-PPARγ, lanes 2–6) and with an empty DNA vector (Fig. 1b, pcDNA 3.1, lane 2), or increasing DNA concentrations of the parental empty vector (FG12-empty, lanes 3 and 4), or increasing concentrations of the PPARγ siRNA expression vector (FG12-siRNA-PPARγ, lanes 5 and 6). Exogenous PPARγ expression was tested using an anti-FLAG antibody and endogenous PPARγ expression was tested using an anti-PPARγ antibody. Both exogenous and endogenous PPARγ protein levels were dramatically reduced in HEK293 cells that co-expressed the PPARγ-Flag vector and the FG12-siRNA-PPARγ vector (Fig. 1b, compare lane 2 with lanes 5 and 6), but not in cells co-transfected with PPARγ-Flag and the FG12-empty vector (Fig. 1b, compare lane 2 with 3 and 4). This indicates that the siRNA sequence against PPARγ was successful at knocking down PPARγ expression. Next, the pseudotyped lentiviral vectors were generated as described in Materials and methods and designated LV-control and LV-PPARγ-siRNA. Both vectors also express GFP. Ramos B lymphoma cells were infected at an MOI of 5. Approximately 30% of the Ramos B cells were transduced as determined by GFP expression (Fig. 1c, left panel). These GFP- positive cells were sorted and 5 days post-sorting, the cells were analyzed by flow cytometry to determine the purity. More than 98% of the cells were GFP-positive (Fig. 1c, right panel). Western blot analysis confirmed that LV-PPARγ-siRNA infected Ramos B cells have dramatically reduced levels of PPARγ protein (Fig. 1d).
Reduction of PPARγ expression in Ramos B lymphoma cells results in enhanced proliferation and reduced sensitivity to PPARγ agonist-induced cell death
We first investigated whether PPARγ plays a role in B lymphoma proliferation. Proliferation was measured by 3H-thymidine incorporation. Control (LV-Control) and PPARγ-knockdown (LV-PPARγ-siRNA) Ramos cells were left untreated or were stimulated with Pansorbin (fixed S. aureus), human CD40L, anti-IgM, CD40L + anti-IgM or CD40L + Pansorbin for 24 h. Basal proliferation (Fig. 2a, untreated) was significantly increased following lentiviral delivery of PPARγ-siRNA. There was also a significant increase in the proliferative response to mitogenic stimuli in PPARγ-knockdown cells in comparison to control Ramos cells (Fig. 2a). This indicates that the presence of PPARγ dampens B lymphoma cell proliferation.
Reduction of PPARγ expression in Ramos B lymphoma cells results in enhanced proliferation and reduced sensitivity to PPARγ ligand induced cell death. a LV-control and LV-PPARγ-siRNA transduced Ramos B lymphoma cells were untreated or treated with 1:1,000 Pansorbin (fixed S. aureus), human CD40L, 10 μg/ml anti-IgM, a combination of CD40L + anti-IgM, and CD40L + Pansorbin for 24 h. Proliferation was measured by [3H] thymidine incorporation. b LV-Control and LV-PPARγ-siRNA infected Ramos cells were exposed to increasing concentrations of the PPARγ ligand CDDO. Viability was measured by MTT assay. PPARγ-knockdown Ramos cells have increased survival in comparison to control cells when exposed to the PPARγ agonist CDDO. (*P < 0.05), ns not significant
We next evaluated whether PPARγ-knockdown Ramos B cells would have increased survival upon exposure to the synthetic PPARγ ligand, CDDO (Fig. 2b). Control and PPARγ-knockdown Ramos cells were exposed to increasing concentrations of CDDO for 24hr and viability measured by MTT. PPARγ-knockdown Ramos cells have increased survival when exposed to lethal doses of PPARγ-ligand CDDO. This increase in survival was also confirmed using 7-AAD staining (data not shown). PPARγ-knockdown cells also had better survival following serum depravation compared to control cells (data not shown). Taken together, these results indicate that PPARγ regulates cell proliferation and survival in B lymphoma cells.
PPARγ knockdown B lymphoma cells display a less differentiated phenotype
To explore the effect of PPARγ on B cell differentiation and activation, we examined the effect of PPARγ knockdown on several important B cell markers. During B cell differentiation, CD38 expression increases while CD19 and CD20 levels are downregulated [2, 7]. Therefore, we compared the levels of CD20, CD19 and CD38 of control Ramos cells versus PPARγ-knockdown Ramos cells by flow cytometry. The surface expression of CD38 in PPARγ-siRNA Ramos cells was slightly lower than PPARγ-expressing Ramos cells (LV-control) (Fig. 3a, panel 3). Conversely, surface expression of CD20 and CD19 in PPARγ-siRNA Ramos cells was increased (Fig. 3a, panels 1 and 2). These results suggest that PPARγ participates in B cell differentiation.
PPARγ-knockdown Ramos human B lymphoma cells have a less differentiated phenotype. a Ramos B lymphoma cells stably transduced with LV-control or LV-PPARγ-siRNA were analyzed for the expression of CD20, CD19 and CD38. Cells that express PPARγ-siRNA showed increased levels of CD20 and CD19 and decreased levels of CD38. MFI’s are shown in the histogram. This experiment is representative of three separate experiments. b LV-control and LV-PPARγ-siRNA infected cells were lysed and expression of PAX-5 and BLIMP-1 were analyzed by western blot as indicated. Total actin was used to normalize protein loading
We also investigated the effects of PPARγ knockdown on the expression of two transcription factors crucial for B cell differentiation: Paired box protein 5 (PAX-5) and B lymphocyte-induced maturation protein 1 (BLIMP-1)[33]. During B cell differentiation PAX-5 levels are downregulated and/or inactivated, while BLIMP-1 is upregulated [33]. We observed a marked reduction in BLIMP-1 expression in PPARγ-knockdown cells compared to control cells, while the levels of PAX-5 remain relatively unchanged (Fig. 3b). Together, these results, in conjunction with the changes seen in B cell differentiation surface markers (Fig. 3a), suggest that the reduction of BLIMP-1 expression is the result of transcriptional regulation by PPARγ and correlates with a less differentiated phenotype.
PPARγ knockdown Ramos B lymphoma cells have enhanced NF-κB activity and express higher levels of the NF-κB-dependent pro-survival gene Bcl-2
NF-κB is an important transcription factor for B cell proliferation and survival [22]. Because previous studies have demonstrated the direct effects of PPARγ on NF-κB [32, 46], we investigated whether PPARγ expression affected NF-κB. First, the expression of the NF-κB subunit p65 was investigated in PPARγ-knockdown Ramos cells versus uninfected and control Ramos cells. Nuclear and cytoplasmic extracts were collected from uninfected, control and siRNA-PPARγ transduced Ramos cells and western blot analysis for the NF-κB p65 subunit was performed. An accumulation of p65 occurred in the nucleus (≈threefold induction compared to controls), with a concomitant decrease in the cytoplasm (≈fivefold reduction compared to controls), of PPARγ-knockdown Ramos cells in comparison to uninfected and control cells (Fig. 4a). Next, an EMSA was performed on nuclear extracts (described in "Materials and methods") from uninfected, LV-control and LV-PPARγ-siRNA infected Ramos cells to measure NF-κB DNA binding activity. Figure 4b shows that PPARγ-knockdown Ramos cells have increased NF-κB DNA binding activity in comparison to uninfected and control cells. Bcl-2 is an anti-apoptotic protein that has an important role in normal and malignant B cell proliferation and survival [52]. Bcl-2 is also a key target gene of NF-κB. Therefore, we investigated the levels of Bcl-2 in PPARγ-siRNA Ramos cells, uninfected and control Ramos cells. PPARγ-knockdown Ramos cells had ≈tenfold higher Bcl-2 levels compared to uninfected or control Ramos cells (Fig. 4c). These findings support the idea that PPARγ regulates NF-κB activation.
PPARγ knockdown Ramos B lymphoma cells have enhanced NF-κB activity and express higher levels of the NF-κB-dependent pro-survival gene Bcl-2. a Nuclear and cytoplasmic extracts from uninfected, LV-control and LV-PPARγ-siRNA transduced Ramos cells were collected and NF-κB p65 levels analyzed by western blot. Total actin was used as a loading control. Graph shows representative densitometry. b Nuclear extracts were incubated with a radiolabeled DNA binding sequence for NF-κB and a gel shift assay was performed. c Bcl-2 protein expression was analyzed by western blot in whole cell lysates from uninfected, LV-control and LV-PPARγ-siRNA transduced Ramos B cells. Graph shows representative densitometry
Design of a lentiviral-based vector for PPARγ1 overexpression in human B lymphoma cells
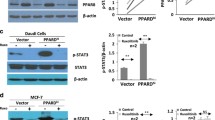
To further determine the physiological role of PPARγ in human B cell lymphoma, overexpression studies were performed using a lentiviral vector we constructed for PPARγ gene delivery. The lentiviral vector contains a Flag-tagged PPARγ1 cDNA under the control of the CMV promoter and Copecod GFP (CopGFP) under the EF1-α promoter. As a control, the backbone lentiviral vector that only expresses CopGFP was used (Fig. 5a). Ramos cells were infected at an MOI of 5 and GFP-positive Ramos cells were sorted by flow cytometry. Interestingly, we observed a decrease in GFP expression over time after sorting. We hypothesized that selective pressure was responsible for decreasing the levels of both PPARγ and GFP (Fig. 5b). For these reasons, cells were only cultured for a maximum of 1 month after sorting, at which time we evaluated the effects of PPARγ overexpression. The presence of exogenous PPARγ expression was determined by western blot using an anti-FLAG antibody. We found that cells stably transduced with LV-PPARγ, but not LV-empty nor uninfected cells expressed the Flag-tagged PPARγ protein (Fig. 5c). To test whether the overexpressed PPARγ was capable of binding DNA, an ELISA-based assay to quantify the binding of PPARγ to its promoter response element was used. Cells overexpressing PPARγ had a twofold increase in PPARγ activity compared to LV-empty infected cells (Fig. 5d). These results demonstrate that the increased levels of PPARγ were able to bind DNA and activate transcription.
Design of a lentiviral vector for PPARγ overexpression in human B cell lymphoma. a Schematic diagram of the PPARγ1-expressing lentiviral vector. The Flag-PPARγ1 cDNA is expressed under the control of a CMV promoter. The vector also contains an elongated factor 1α (EF-1α) promoter driving the GFP marker gene for tracking transduced cells. 5′LTR, HIV-1 5′LTR; Δ3′LTR, HIV-1 self-inactivating 3′LTR; WPRE woodchuck hepatitis B virus RNA regulatory element. b Ramos B lymphoma cells were transduced with LV-empty and LV-PPARγ (MOI = 5). At 48 h post-transduction, cells were analyzed for GFP expression using flow cytometry. GFP-positive cells were then sorted on the basis of GFP expression. GFP expression was monitored over time (one day and one week after sorting) to determine purity and the stability of gene expression c Exogenous PPARγ1 expression was evaluated by western blotting using an anti-FLAG antibody. After sorting, the FLAG-PPARγ protein was detected in the cells infected with LV-PPARγ, but not in the LV-empty infected cells. β-Tubulin levels were assayed to normalize protein loading. d Nuclear extracts from Ramos cells stably transduced with either LV-empty or LV-PPARγ were collected and transactivation of PPARγ was analyzed by ELISA using TransAm® technology (*P < 0.05)
Ramos B lymphoma cells transduced with LV-PPARγ have a decreased proliferative response
We next determined the effects of PPARγ overexpression on B lymphoma cell proliferation using 3H-thymidine incorporation. Both basal proliferation, as well as stimulated proliferative responses was tested. Ramos B lymphoma cells stably transduced with LV-empty or LV-PPARγ were untreated or were stimulated with CD40L, anti-IgM, Pansorbin and combination of CD40L + anti-IgM or CD40L + Pansorbin for 24 h. LV-empty expressing cells were able to respond to stimuli, as seen by an increase in 3H-thymidine incorporation (Fig. 6a). However, LV-PPARγ transduced cells poorly responded to mitogenic stimuli. The robust proliferative response to CD40L (with or without anti-IgM or Pansorbin) was dramatically reduced following PPARγ overexpression (Fig. 6a). Since PPARγ overexpression inhibited B lymphoma cell proliferation, we next assessed cell cycle kinetics using BrdU and 7-AAD double staining. LV-PPARγ transduced cells had a decrease in the percentage of cells in the S-phase of the cell cycle when compared to those of LV-empty infected control cells (47% in LV-empty cells vs. 39% in LV-PPARγ cells) (Fig. 6b). These results confirm that PPARγ overexpression inhibited basal cell growth of B lymphoma cells. To determine whether PPARγ overexpression had the same effect on another BL cell line, EBV+ Raji B lymphoma cells were infected at an MOI of 5. GFP-positive cells were then sorted and tested for basal proliferation using BrdU and 7-AAD staining. LV-PPARγ overexpressing Raji B lymphoma cells also showed a decrease in the percentage of cells in S-phase, while the fraction of cells with G0/G1 and G2/M DNA content was increased in comparison to LV-empty cells (Fig. 6c). We next investigated whether these cells were more sensitive to PPARγ ligand-mediated growth inhibition. To test this, we treated LV-empty and LV-PPARγ Raji cells with a sublethal dose of the PPARγ ligand, Ciglitazone (5 μM). Ciglitazone treatment had a minimal effect on proliferation of LV-empty infected cells (compare LV-empty and LV-empty + Cig). However, ciglitazone treatment resulted in further growth inhibition when PPARγ was overexpressed (LV-PPARγ), as seen by an even lower number of cells in the S-phase of the cycle (Fig. 6c, compare LV-PPARγ and LV-PPARγ + Cig). These findings indicate that PPARγ negatively regulates cell proliferation by decreasing the number of cells entering S-phase and further enhanced their susceptibility to PPARγ-ligand induced cell cycle arrest.
Ramos B lymphoma cells transduced with LV-PPARγ have decreased basal and stimulatory proliferative responses. a Ramos B lymphoma cells stably transfected with LV-Empty and LV-PPARγ were left untreated or were treated with human CD40L, 10 μg/ml anti-IgM, a combination of CD40L plus anti-IgM, 1:1,000 Pansorbin (fixed S. aureus), and CD40L plus Pansorbin for 24 h. Proliferation was measure by [3H] thymidine incorporation. (*P < 0.05), (**P < 0.001) b Cell cycle analysis: Ramos cells transduced with LV-empty and LV-PPARγ were incubated for 30 min with BrdU and labelled with anti-BrdU and 7-AAD (gated on GFP-positive cells). Cells that overexpressed PPARγ showed a reduction in the percentage of cells entering the S-phase of the cycle and a G2/M cell cycle arrest. This profile is representative of three separate experiments. c Raji B lymphoma cells were infected with either LV-empty or LV-PPARγ at an MOI of 5 and sorted by GFP expression. Cells were left untreated or were treated with 5 μM ciglitazone (+ Cig) for 48 h. Cells were pulsed with BrdU for 30 min and intracellularly stained with an anti-BrdU antibody. Cells that were infected with LV-PPARγ had a slight reduction in BrdU incorpotation in comparison to LV-empty infected cells. When cells were treated with ciglitazone, a dramatic reduction in BrdU incorporation was observed in the cells that overexpress PPARγ (LV-PPARγ), but not in the LV-empty infected cells. These results are representative of three separate experiments
PPARγ-overexpressing B lymphoma cells display a more differentiated phenotype
Since reduced PPARγ expression in B lymphoma cells resulted in a less differentiated phenotype (Fig. 3), we next explored whether PPARγ overexpression had an effect on B cell differentiation markers (CD20, CD19 and CD38) and activation marker (CD40) expression (Fig. 7a). Surface expression of CD38 was not changed and CD19 expression was slightly reduced by PPARγ overexpression. Additionally, surface expression of CD20 and CD40 were dramatically reduced in PPARγ-overexpressing Ramos cells (Fig. 7a). These results suggest a role for PPARγ in B cell differentiation and activation.
Ramos B lymphoma cells overexpressing PPARγ showed a more differentiated phenotype. a Ramos B lymphoma cells stably transduced with LV-Empty or LV-PPARγ were analyzed for the expression of CD20, CD19, CD38 and CD40. Cells that overexpress PPARγ showed a slight decrease on CD19 expression and a dramatic decrease on CD20 and CD40, but no changes in CD38 expression. b LV-empty and LV-PPARγ infected cells were lysed and expression of PAX-5 and BLIMP-1 were analyzed by western blot as indicated. Total actin was used to normalize protein loading
To confirm that PPARγ regulates differentiation of Ramos cells, we next examined the effects of PPARγ overexpression on PAX-5 and BLIMP-1. BLIMP-1 expression was upregulated in LV-PPARγ-infected cells in comparison to LV-empty infected cells. In contrast, the levels of PAX-5 were downregulated in these cells (Fig. 7b). Therefore, we propose that PPARγ promotes differentiation of germinal center (GC) B cell-derived cells toward plasma cells.
Discussion
Burkitt’s lymphoma is an aggressive form of non-Hodgkin lymphoma, and it is the most common childhood cancer in Central Africa [14]. Although the survival rates have increased, new innovative therapies are needed. PPARγ and PPARγ ligands have emerged as a new molecular target to treat cancer. Data from our laboratory (and others) have demonstrated that ligand-initiated activation of PPARγ inhibits growth and induces apoptosis in B cell malignancies [34, 42, 43, 47, 49, 50]. Further, PPARγ expression correlates with patient prognosis in certain cancers [53, 58]. In the present study, we demonstrated that alterations of PPARγ expression levels in the GC B cell-derived cells Ramos and Raji affect cell proliferation, survival and differentiation. Interestingly, a decrease in PPARγ expression was associated with an increase in B lymphoma cell proliferation and survival, both basally and after mitogenic stimuli (Fig. 2a). Moreover, PPARγ-knockdown malignant B cells survive better when exposed to increasing doses of CDDO in comparison to PPARγ-expressing cells (Fig. 2b). These data, in conjunction with our recently published results [42, 43, 50], further confirm that part of the cytotoxic effects of PPARγ ligands are mediated directly through PPARγ.
Nuclear Factor-kappa B is an important transcription factor for B cell development and survival. Activation of the NF-κB pathway induces expression of anti-apoptotic proteins, such as the Bcl-2 family members [22]. The anti-inflammatory effects of PPARγ have been linked to the inhibition of NF-κB [46]. Studies performed in PPARγ haploinsufficient mice indicate that PPARγ is important in controlling B cell proliferation and survival. These mice exhibit enhanced B cell proliferation after LPS stimulation and increased NF-κB activity in comparison to their wild type counterparts [54]. Here, we showed that PPARγ knockdown human B lymphoma cells have increased NF-κB activity and increased levels of the anti-apoptotic protein Bcl-2, which supports the mouse studies. PPARγ has also been proposed to have pro-differentiating properties [5, 16, 34]. During normal B cell differentiation, the levels of CD20 and CD19 are downregulated, whereas the levels of CD38 are upregulated. We observed that upon PPARγ downregulation, there was a reduction of CD38 surface expression and an increase of CD20 and CD19 expression, indicative of a less differentiated phenotype. These findings demonstrate that the expression of PPARγ, as well as its activity, is necessary to control B cell lymphoma proliferation, survival and differentiation. Although we did not observed changes in CD38 expression upon PPARγ overexpression, we observed a slight decrease in CD19 surface expression and a considerable decrease in CD20 expression on PPARγ-overexpressing B lymphoma cells, which correlates with a more differentiated phenotype. Therefore, we speculate that PPARγ overexpression would improve the outcome of patients with B cell lymphoma, since a more differentiated phenotype correlates with a better prognosis [15].
Studies performed in thyroid carcinoma cells demonstrated that PPARγ overexpression resulted in an induction of cell cycle arrest and cell death [37]. In concordance with these studies, when we overexpressed PPARγ in BL cells, and recently in multiple myeloma [17], we found that PPARγ overexpression inhibited both basal and stimulated proliferation in B lymphoma cells. Interestingly, we observed a decrease in GFP expression in lymphoma cells over time. These results might explain a direct effect of PPARγ overexpression on GFP transcription, or a deleterious effect of PPARγ that confers growth disadvantage to PPARγ overexpressing cells. We hypothesize that over time, selective pressure was responsible for decreasing the levels of both PPARγ and GFP. In addition, we observed a decrease of CD40 expression in cells overexpressing PPARγ. Previous studies have shown that CD40 ligation can rescue BL from B cell receptor crosslinking-induced cell death [26]. Therefore, we speculate that reduced levels of surface expression of CD40 would render the lymphoma cells less responsive to CD40 ligand stimulation and subsequently contribute to their reduced cell growth and survival.
We propose that PPARγ regulates B lymphoma cell differentiation. PAX-5 is inactivated by an unknown stimulus and is one of the first steps needed for plasma cell differentiation, while BLIMP-1 is upregulated. BLIMP-1 functions as an important regulator of plasma cell differentiation by inhibiting proliferation through inhibition of c-myc, an important factor for cell proliferation [36, 55]. BLIMP-1 also represses PAX-5, which is crucial during B cell differentiation [55]. In addition to the changes on surface expression of B cell differentiation markers (Fig. 3a and Fig. 7a), there was also a reduction of BLIMP-1 in the PPARγ knockdown cells and an upregulation with PPARγ overexpression. While the expression of PAX-5 was relatively unchanged upon PPARγ knockdown, the levels of PAX-5 were downregulated in cells overexpressing PPARγ. Collectively, these findings suggest that PPARγ may regulate B cell differentiation via direct or indirect regulation of key transcription factors, such as BLIMP-1 and PAX-5 and may contribute, at least in part, to the inhibitory effects of PPARγ on proliferation and cell cycle progression in B cell lymphomas.
Collectively, our findings support PPARγ as a potential new target to control malignant B lymphoma proliferation, survival and differentiation. Therapeutic efforts to alter PPARγ levels in malignant B cells may demonstrate synergistic cytotoxicity when used in conjugation with PPARγ ligands. One mechanism to selectively target malignant B cells in vivo would be to use lentiviral vectors that have a B cell lymphoma-specific promoter, thereby allowing therapeutic gene expression only in malignant B cells. A new generation of lentiviral vectors known as self-inactivating vectors (SIN) allows restriction or silencing of gene expression regulated by the viral promoter [10]. This feature makes possible the use of an internal promoter (e.g. B cell lymphoma-specific) to drive the gene of interest. In fact, this method has recently been used to introduce therapeutic genes in mammalian cells [8, 12, 13, 38]. For example, in multiple myeloma, the use of a minimal immunoglobulin promoter as well as the kappa light chain intronic and 3′ enhancers to drive the gene of interest was able to selectively transduce myeloma cells [12]. Therefore, using a cell or tissue-specific promoter and/or enhancer element could target a specific cell population. One possible candidate would be the promoter or enhancer regions of c-myc, which is highly expressed in BL [23].
References
Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP (2004) Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood 104:1361–1368
Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ (1995) Generation of memory B cells and plasma cells in vitro. Science 268:720–722
Beamer BA, Negri C, Yen CJ, Gavrilova O, Rumberger JM, Durcan MJ, Yarnall DP, Hawkins AL, Griffin CA, Burns DK, Roth J, Reitman M, Shuldiner AR (1997) Chromosomal localization and partial genomic structure of the human peroxisome proliferator activated receptor-gamma (hPPAR gamma) gene. Biochem Biophys Res Commun 233:756–759
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11:127–152
Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143
Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137:354–366
Campana D, Suzuki T, Todisco E, Kitanaka A (2000) CD38 in hematopoiesis. Chem Immunol 75:169–188
Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L (2002) Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood 99:399–408
Daynes RA, Jones DC (2002) Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2:748–759
Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D, Aebischer P (2000) Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson’s disease. Hum Gene Ther 11:179–190
Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S (1999) Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A 96:3951–3956
Dingli D, Diaz RM, Bergert ER, O’Connor MK, Morris JC, Russell SJ (2003) Genetically targeted radiotherapy for multiple myeloma. Blood 102:489–496
Dresch C, Edelmann SL, Marconi P, Brocker T (2008) Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J Immunol 181:4495–4506
Evens AM, Gordon LI (2002) Burkitt’s and Burkitt-like lymphoma. Curr Treat Options Oncol 3:291–305
Ferry JA (2006) Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist 11:375–383
Fujimura S, Suzumiya J, Nakamura K, Ono J (1998) Effects of troglitazone on the growth and differentiation of hematopoietic cell lines. Int J Oncol 13:1263–1267
Garcia-Bates TM, BA, Phipps RP (2008) Peroxisome proliferator activated receptor gamma (PPARγ) overexpression suppresses growth and induces apoptosis in human multiple myeloma cells. Clin cancer res
Garcia-Bates TM, Lehmann GM, Simpson-Haidaris PJ, Bernstein SH, Sime PJ, Phipps RP (2008) Role of peroxisome proliferator-activated receptor gamma and its ligands in the treatment of hematological malignancies. PPAR Res 2008:834612
Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109(Supp):S81–S96
Glass CK, Ogawa S (2006) Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6:44–55
Gregory CD, Dive C, Henderson S, Smith CA, Williams GT, Gordon J, Rickinson AB (1991) Activation of Epstein–Barr virus latent genes protects human B cells from death by apoptosis. Nature 349:612–614
Gugasyan R, Grumont R, Grossmann M, Nakamura Y, Pohl T, Nesic D, Gerondakis S (2000) Rel/NF-kappaB transcription factors: key mediators of B cell activation. Immunol Rev 176:134–140
Hermeking H (2003) The MYC oncogene as a cancer drug target. Curr Cancer Drug Targets 3:163–175
Hirase N, Yanase T, Mu Y, Muta K, Umemura T, Takayanagi R, Nawata H (1999) Thiazolidinedione induces apoptosis and monocytic differentiation in the promyelocytic leukemia cell line HL60. Oncology 57(Suppl 2):17–26
Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB (1998) Design and synthesis of 2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 8:2711–2714
Hristov KK, Knox KA, Mitev VI (2005) Regulation of tyrosine phosphorylation during the CD40-mediated rescue of Ramos-BL B cells from BCR-triggered apoptosis. Int J Mol Med 16:937–941
Izumi KM, Kieff ED (1997) The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci USA 94:12592–12597
Jaffe ES, Diebold J, Harris NL, Muller-Hermelink HK, Flandrin G, Vardiman JW (1999) Burkitt’s lymphoma: a single disease with multiple variants. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Blood 93:1124
Jumper MD, Nishioka Y, Davis LS, Lipsky PE, Meek K (1995) Regulation of human B cell function by recombinant CD40 ligand and other TNF-related ligands. J Immunol 155:2369–2378
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663
Katayama K, Wada K, Miyoshi H, Ohashi K, Tachibana M, Furuki R, Mizuguchi H, Hayakawa T, Nakajima A, Kadowaki T, Tsutsumi Y, Nakagawa S, Kamisaki Y, Mayumi T (2004) RNA interfering approach for clarifying the PPARgamma pathway using lentiviral vector expressing short hairpin RNA. FEBS Lett 560:178–182
Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S (2004) Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 5:104–112
Klein U, Dalla-Favera R (2008) Germinal centres: role in B cell physiology and malignancy. Nat Rev Immunol 8:22–33
Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, Schober WD, Wang RY, Chism D, Kornblau SM, Younes A, Collins SJ, Koeffler HP, Andreeff M (2004) Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther 3:1249–1262
Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M (2002) Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood 99:326–335
Lin Y, Wong K, Calame K (1997) Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276:596–599
Martelli ML, Iuliano R, Le Pera I, Sama I, Monaco C, Cammarota S, Kroll T, Chiariotti L, Santoro M, Fusco A (2002) Inhibitory effects of peroxisome poliferator-activated receptor gamma on thyroid carcinoma cell growth. J Clin Endocrinol Metab 87:4728–4735
May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, Sadelain M (2000) Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 406:82–86
Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM (1998) Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell 1:465–470
Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93:229–240
O’Neil J, Look AT (2007) Mechanisms of transcription factor deregulation in lymphoid cell transformation. Oncogene 26:6838–6849
Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP (2000) Peroxisome proliferator activator receptor-gamma agonists and 15-deoxy-Delta(12, 14)(12, 14)-PGJ(2) induce apoptosis in normal and malignant B-lineage cells. J Immunol 165:6941–6948
Padilla J, Leung E, Phipps RP (2002) Human B lymphocytes and B lymphomas express PPAR-gamma and are killed by PPAR-gamma agonists. Clin Immunol 103:22–33
Panwalkar A, Verstovsek S, Giles F (2004) Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies. Cancer 100:1578–1589
Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437:759–763
Pascual G, Glass CK (2006) Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab 17:321–327
Piva R, Gianferretti P, Ciucci A, Taulli R, Belardo G, Santoro MG (2005) 15-Deoxy-delta 12, 14-prostaglandin J2 induces apoptosis in human malignant B cells: an effect associated with inhibition of NF-kappa B activity and down-regulation of antiapoptotic proteins. Blood 105:1750–1758
Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 100:183–188
Ray DM, Akbiyik F, Bernstein SH, Phipps RP (2005) CD40 engagement prevents peroxisome proliferator-activated receptor gamma agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-kappaB-dependent mechanism. J Immunol 174:4060–4069
Ray DM, Akbiyik F, Phipps RP (2006) The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-Delta12, 14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol 177:5068–5076
Rayet B, Gelinas C (1999) Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938–6947
Reed JC (2008) Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood 111:3322–3330
Sasaki H, Tanahashi M, Yukiue H, Moiriyama S, Kobayashi Y, Nakashima Y, Kaji M, Kiriyama M, Fukai I, Yamakawa Y, Fujii Y (2002) Decreased perioxisome proliferator-activated receptor gamma gene expression was correlated with poor prognosis in patients with lung cancer. Lung Cancer 36:71–76
Setoguchi K, Misaki Y, Terauchi Y, Yamauchi T, Kawahata K, Kadowaki T, Yamamoto K (2001) Peroxisome proliferator-activated receptor-gamma haploinsufficiency enhances B cell proliferative responses and exacerbates experimentally induced arthritis. J Clin Invest 108:1667–1675
Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17:51–62
Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK (2000) 15-deoxy-delta 12, 14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA 97:4844–4849
Tabe Y, Konopleva M, Kondo Y, Contractor R, Tsao T, Konoplev S, Shi Y, Ling X, Watt JC, Tsutsumi-Ishii Y, Ohsaka A, Nagaoka I, Issa JP, Kogan SC, Andreeff M (2007) PPARgamma-active triterpenoid CDDO enhances ATRA-induced differentiation in APL. Cancer Biol Ther 6:1967–1977
Theocharis S, Kanelli H, Politi E, Margeli A, Karkandaris C, Philippides T, Koutselinis A (2002) Expression of peroxisome proliferator activated receptor-gamma in non-small cell lung carcinoma: correlation with histological type and grade. Lung Cancer 36:249–255
Acknowledgments
This study was supported by DE11390, ES01247, a Hematology Training Grant NHLBI- T32HL007152, a Leukemia and Lymphoma Society Translational Research Award and a Lymphoma Research Foundation Award. Carolyn J. Baglole was supported by a Parker B. Francis Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Bates, T.M., Peslak, S.A., Baglole, C.J. et al. Peroxisome proliferator-activated receptor gamma overexpression and knockdown: impact on human B cell lymphoma proliferation and survival. Cancer Immunol Immunother 58, 1071–1083 (2009). https://doi.org/10.1007/s00262-008-0625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0625-z