Abstract
Intravenously-applied bacteria tend to accumulate in tumors and can sporadically lead to tumor regression. Systemic administration of attenuated Salmonella typhimurium is safe and has shown no significant adverse effects in humans. The purpose of this study was to test the hypothesis that engineering S. typhimurium to express a chemokine, CCL21, would increase anti-tumor activity. We engineered an attenuated strain of S. typhimurium to produce the chemokine CCL21. Attenuated S. typhimurium expressing CCL21 significantly inhibited the growth of primary tumors and pulmonary metastases in preclinical models of multi-drug-resistant murine carcinomas, while control bacteria did not. Histological analysis of tumors showed marked inflammatory cell infiltrates in mice treated with CCL21-expressing but not control bacteria. Levels of cytokines and chemokines known to be induced by CCL21 [e.g., interferon-γ (INFγ), CXCL9, and CXCL10] were significantly elevated in tumors of mice treated with CCL21-expressing but not control S. typhimurium. The anti-tumor activity was found to be dependent on CD4- and CD8-expressing cells, based on antibody-mediated in vivo immuno-depletion experiments. Anti-tumor activity was achieved without evidence of toxicity. In summary, chemokine-expressing, attenuated bacteria may provide a novel approach to cancer immunotherapy for effective and well-tolerated in vivo delivery of immunomodulatory proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies that have used bacteria to treat tumors have been documented as far back as 1893 [1], but the significant toxicity associated with this strategy precluded widespread use [1–3] and has thus far been limited to bacillus Calmette-Guerin for the treatment of superficial bladder carcinomas [4, 5]. Recently, interest in this strategy of employing bacteria to target tumor cells has received heightened interest, particularly with regard to the use of attenuated strains of Salmonella typhimurium. S. typhimurium are facultative anaerobic bacteria that are able to accumulate within tumors in vivo to concentrations of up to 109 colony forming units (CFU) per gram of tumor tissue [6, 7]. This tumor accumulation may be explained by a variety of mechanisms, such as increased access to nutrients provided by rapidly growing tumor and necrotic cells, adaptation of S. typhimurium to growth in hypoxic areas, lack of bactericidal activity of macrophages and neutrophils due to reduced oxygen within tumor areas, suppression of the immune system in tumor regions due to secretion of cytokines such as TGF-β, and absence of circulating antibodies and complement factors due to irregular vasculature and positive pressure within tumors.
Recently, a lipid A-negative strain of S. typhimurium has been used in phase I clinical trials [8, 9]. Although S. typhimurium was found to be safe at doses up to 109 CFU/m2 body surface area in these studies, and the bacteria did indeed accumulate within tumor tissues, no significant anti-tumor activity was observed. Because attenuated S. typhimurium display an excellent safety profile [7, 10] and are able to synthesize functional cytokines at high levels [11], we have employed these facultative anaerobic bacteria to express proteins with anti-tumor or immunomodulatory activities, testing them in mouse tumor models [12].
In this study, we engineered an attenuated strain of S. typhimurium to stably express the cytokine CCL21. CCL21, also known as 6Ckine or secondary lymphoid tissue chemokine, is a member of the chemokine family of small secreted proteins that controls the migration of lymphocytes [13], dendritic cells [14], T [15] and natural killer (NK) cells [16]. The activities of CCL21 suggest that it may induce the colocalization of naïve T cells and antigen-stimulated dendritic cells, which may help to overcome tumor-induced immunosuppression, thereby leading to an effective cell-mediated immune response and subsequent tumor elimination. Several studies demonstrated the feasibility of such an approach to use this chemokine to induce tumor rejection [17–19], but were limited to intratumoral injections. In addition, CCL21 appears to have antitumor functions that do not depend on the immune system, but are mediated through the binding of the chemokine receptor CXCR3 [20], including angiostatic properties [21–24]. In this study, we used an attenuated strain of S. typhimurium to deliver the immunostimulatory cytokine CCL21 to tumors and show that CCL21 expression results in inhibition of tumor growth without significant toxicity in murine models.
Materials and methods
Animals, bacterial strains, cell lines, and plasmid vectors
Female mice were purchased at about 6–8 weeks of age from The Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by the Animal Care Committee of the Burnham Institute for Medical Research (#AUF 04-152). Attenuated lipid A-negative S. typhimurium (pur−/msb−) were generated as previously described [6]. The D2F2 cell line was a gift from Dr Wei-Zen Wei (Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA); CT-26 and B16 melanoma cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). cDNAs encoding luciferase (pGL3; Promega Corporation, Madison, WI, USA), enhanced green fluorescent protein (pEGFP; Clontech, Mountain View, CA, USA), or murine CCL21 (InvivoGen, San Diego, CA, USA), were inserted into pGEN206. This vector is a slightly modified form of pGEN222 [25] in which the parA locus has been exchanged with a parM and a parR locus. In addition, a repA locus has been introduced between the origin of replication and the multiple cloning sites.
Immunoblotting
Protein expression of pGEN206-CCL21 was demonstrated by Western blotting of 109 CFU S. typhimurium (expressing nothing or pGEN206-CCL21) and their respective culture supernatants after resuspending in Laemmli sample buffer and running an SDS-PAGE followed by immunoblotting with a polyclonal goat anti-CCL21 antibody (Abcam, Cambridge, MA, USA).
Mouse experiments
Tumor cell infectivity was performed via a standard gentamycin protection assay. For experiments involving primary tumors, BALB/c mice (n = 5) were challenged subcutaneously (SC) with 1.25 × 105 D2F2 breast carcinoma cells and treated as indicated with PBS or 5 × 106 CFU of indicated S. typhimurium per mouse after 14 days. Volumes of SC tumors were measured in two dimensions and calculated as length/2 × width2. Mice were challenged with a lethal dose of 2.5 × 105 CT-26 colon carcinoma cells and treated intravenously (IV) with either PBS or indicated S. typhimurium (5 × 106 CFU S. typhimurium per mouse) after 9, 14 and 19 days. In experiments in which pulmonary metastases were established, BALB/c mice (n = 8) were first injected IV with 5 × 104 D2F2 breast carcinoma cells and then treated IV with PBS or indicated S. typhimurium (5 × 106 CFU S. typhimurium per mouse) after 6, 13, and 20 days. After 50 days, lungs were weighed (mean ± SE) and examined for metastases using the following scale to determine the percentage of lung surface covered by metastases: 0 = 0%, 1 = <20%, 2 = 20–50%, 3 = >50%. For experiments involving antibody-mediated depletion/blocking, C57/Bl6 mice (n = 5) were challenged SC with 1.25 × 105 B16 melanoma cells and treated IV after 7 days with PBS, S. typhimurium, S. typhimurium plus empty vector, or S. typhimurium plus CCL21-bearing vector, including groups of mice that were treated intraperitoneally (IP) with antibodies (500 μg/mouse; [26]) against CD4+ T cells (anti-CD4 clone GK1.5, ATCC; Manassas, VA, USA), CD8+ T cells (anti-CD8 clone 2.43, ATCC; Manassas, VA, USA), NK cells (anti-asialo-GM; Wako Chemical, Dallas, TX, USA), or with antibodies (100 μg/mouse; [27]) against IFNγ (anti-IFNγ clone R4-6A2, ATCC; Manassas, VA, USA), CXCL9 or CXL10 (gifts from Dr Valbuena; [28]) after 5 and 15 days.
Immunohistochemistry and ELISA
Mice were sacrificed with an overdose of IP-administered Avertin anesthetic (0.017 ml/g body weight; Aldrich, Milwaukee, WI, USA) followed by cervical dislocation. For histological analyses, tissues were fixed in paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E). For immunohistochemistry, paraffin sections were stained using an antibody specific for the Ly-6G antigen (BD, Franklin Lakes, NJ, USA). For ELISA, tumors from mice (n = 3 per group) were extracted using a solution consisting of PBS containing 1% NP-40 and protease inhibitors, normalized for total protein content, and analyzed by ELISA for cytokines CXCL9, CXCL10 (R&D Systems, Minneapolis, MN, USA) and IFNγ (eBioscience, San Diego, CA, USA). Results represent amounts of cytokines per gram of protein (mean ± SD).
Statistics
Statistical significance of differential findings between controls and treatment groups was determined by two-tailed Student’s t-test (Figs. 2c, 3) or by ANOVA. The significance of metastases scores was determined by Mann–Whitney U-test. Findings were regarded as significant if P-values were less than 0.05.
Results and discussion
CCL21 protein expression by attenuated S. typhimurium
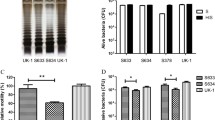
To engineer the attenuated S. typhimurium strain purI−/msbB− to express CCL21, we used the plasmid pGEN206, in which a murine CCL21 cDNA was expressed under the control of an ompC promoter. The engineered bacteria were grown in culture, recovered by centrifugation, and the resulting cell-containing pellet and cleared culture supernatant were tested for CCL21 protein by immunoblotting. The results demonstrated the presence of CCL21 in both cells and supernatant (Fig. 1). The production of CCL21 in vitro in bacterial cultures is >50 pg/μg. Control S. typhimurium (transformed with empty plasmid) and their culture supernatants did not contain CCL21 protein (Fig. 1).
CCL21 expression by S. typhimurium. Attenuated lipid A-negative S. typhimurium (pur−/msb−) were generated as previously described [6]. A cDNA encoding murine CCL21 (InvivoGen, San Diego, CA, USA) was inserted into pGEN206. This vector is a slightly modified form of pGEN222 [25] in which the parA locus has been exchanged with a parM and a parR locus. In addition, a repA locus has been introduced between the origin of replication and the multiple cloning sites. Expression of the 12 kD CCL21 protein was demonstrated by immunoblot analysis of cell lysates (“pellet”) or culture supernatants (“SUP”) from 109 CFU of S. typhimurium containing either empty vector (Sal) or CCL21-bearing plasmid-transformed S. typhimurium (Sal + CCL21). Samples were normalized for total protein content, resuspended in Laemmli sample buffer and subjected to SDS-PAGE followed by immunoblotting with a polyclonal goat anti-CCL21 antibody (Abcam, Cambridge, MA, USA). Results are representative of three independent experiments
S. typhimurium-expressing CCL21 inhibit primary and metastatic tumor growth
Recently, we showed that the attenuated strain of Salmonella used here accumulates in subcutaneous tumors in vivo in mice, using luciferase-expressing bacteria in conjunction with bioluminescence imaging [12]. We tested the anti-tumor activity of CCL21-expressing bacteria in vivo by performing experiments in which mice were challenged SC with CT-26 colon (Fig. 2a) or D2F2 breast carcinoma cells (Fig. 2b) to form SC tumors, or IV with CT-26 cells to form experimental lung metastases (Fig. 2c). Following tumor challenge, mice were treated IV with PBS, empty S. typhimurium or S. typhimurium bearing control or CCL21 vectors. In the case of SC tumors, mice received treatment at a consistent time point after tumors reached a clearly visible size. Marked reduction of subcutaneous tumor growth was observed in mice receiving S. typhimurium expressing CCL21 compared with control groups (Fig. 2a, b). Recovering bacteria from excised tumors and various organs of the mice sacrificed at the termination of the study confirmed accumulation of Salmonella predominantly in tumor tissue: (938 ± 259 million CFU/g tumor tissue compared to 0.10 ± 0.26 million CFU/g liver tissue, 0.16 ± 0.38 million CFU/g spleen tissue, 0.90 ± 1.34 million CFU/g kidney tissue and 0 ± 0 million CFU/g lung tissue) (Supplemental data). In addition to suppressing growth of subcutaneous tumors, a marked reduction in lung metastases was observed after treatment with CCL21-expressing bacteria compared with control mice. This suppression of metastatic growth was assessed by determining the weight of the lungs of sacrificed animals (Fig. 2c) and determining metastases scores for excised lungs (Fig. 2d).
Effect of CCL21-targeting S. typhimurium on tumor growth. Female mice were purchased at about 6–8 weeks of age from The Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by the Animal Care Committee of the Burnham Institute for Medical Research (#AUF 04-152). a BALB/c mice (n = 8) were challenged SC with 1.25 × 105 CT-26 colon carcinoma cells (from ATCC; Manassas, VA, USA) and treated IV with PBS (dark filled diamond), or 5 × 106 CFU of S. typhimurium (dark filled square), S. typhimurium with empty vector (dark filled triangle), or S. typhimurium with CCL21 vector (open circle) after 14 days. Bar graphs indicate average tumor volumes measured in two dimensions by external calipers and calculated as length/2 × width2 (mean ± SE). b Mice were challenged SC with 2.5 × 105 D2F2 breast carcinoma cells and treated IV with 5 × 106 CFU of S. typhimurium (dark filled square), S. typhimurium containing empty vector (dark filled triangle), or S. typhimurium bearing CCL21 vector (open circle) after 9, 14 and 19 days. Bar graphs indicate average tumor volumes of five mice (mean ± SD). c, d Mice (n = 8) were injected IV with 5 × 104 CT26 colon carcinoma cells and then treated IV with PBS, or 5 × 106 CFU of S. typhimurium (Sal), S. typhimurium containing empty vector (Sal + vector), or S. typhimurium bearing the CCL21 plasmid (Sal + CCL21) after 6, 13 and 20 days. After 29 days, lungs were weighed (c mean ± SD; normal lung weight ~0.15 g) and examined for metastases (d), assessing the percentage of lung surface covered by metastases as follows: 0 = 0%, 1 = <20%, 2 = 20–50%, 3 = >50%. Asterisks indicate P < 0.05. Results are representative of four independent experiments
CCL-21-expressing bacteria induce inflammatory cell infiltration into tumors and elevate intratumoral levels of cytokines and chemokines
We examined the intratumoral levels of INFγ, CXCL9, and CXCL10, chemokines and cytokines known to be induced by CCL21 [23, 24]. In mice treated with CCL21-expressing Salmonella, intratumoral levels of INFγ, CXCL9, and CXCL10 were significantly elevated, compared to either PBS- or control Salmonella-treated mice (Fig. 3a). Consistent with elevated levels of cytokines and chemokines, histological analysis of tumors excised from treated mice showed abundant infiltrates of immune (mononuclear) and especially inflammatory (polymorphonuclear) cells in mice treated with CCL21-expressing bacteria, with far less cell infiltration observed in mice receiving PBS or control Salmonella (Supplementary information). Abundant neutrophils (confirmed by Ly-6G immunostaining) and evidence of tumor necrosis suggested that some of the inflammatory infiltrates may be secondary to tumor lysis.
Analysis of mechanism of anti-tumor activity of CCL21-expressing S. typhimurium. a Portion of tumors from sacrificed mice (n = 3) was extracted using a 1% NP-40 solution in PBS, and analyzed by ELISA (eBioscience, San Diego, CA, USA) for the CXCL9 (dark filled square) and CXCL10 (dark filled square) chemokines as well as IFNγ (open square). Data represent ng cytokine per gram of total protein (mean ± SD). b C57/Bl6 mice (n = 5) were challenged SC with 1.25 × 105 B16 melanoma cells (from ATCC; Manassas, VA, USA). After 7 days, mice were injected IV with PBS, or 5 × 106 CFU of S. typhimurium (Sal), S. typhimurium containing empty vector (Sal + vector) or S. typhimurium bearing CCL21 vector (Sal + CCL21), including groups of mice that were treated IP with (500 μg/mouse) antibodies against CD4+ T cells (Sal + CCL21 + anti-CD4; anti-CD4 clone GK1.5, ATCC; Manassas, VA, USA), CD8+ T cells (Sal + CCL21 + anti-CD8; anti-CD8 clone 2.43, ATCC; Manassas, VA, USA), NK cells (Sal + CCL21 + anti-NK; anti-asialo-GM, Wako Chemical, Dallas, TX), or (100 μg/mouse) antibodies against IFNγ (Sal + CCL21 + anti-IFN γ; ATCC; Manassas, VA, USA), CXCL10 (Sal + CCL21 + anti-CXCL10; gift from Dr Valbuena), or CXCL9 (Sal + CCL21 + anti-CXCL9; gift from Dr Valbuena) after 5 and 15 days. Depicted are the tumor weights (mean ± SD) after 22 days. Asterisks indicate P < 0.05 compared to mice treated with PBS, Salmonella, or Salmonella + vector. Results are representative of three independent experiments
Anti-tumor activity of CCL21-expressing bacteria requires CD4 and CD8 cells
We challenged C57/Bl6 mice SC with B16 melanoma cells and various groups of mice were then treated with complement-fixing antibodies to deplete in vivo CD4+ T cells, CD8+ T cells, or NK cells. Furthermore, IFNγ, CXCL9, and CXCL10 were neutralized in mice with antibodies (Fig. 3b). As demonstrated previously for intratumoral injected CCL21 [17], CD4+ T cells and CD8+ T cells (but not NK cells) were indispensable for significant inhibition of tumor growth by CCL21-expressing bacteria (Fig. 3b). Thus, both CD4+ and CD8+ T-cells appear to contribute to the observed antitumor activity.
Attempts to individually neutralize CCL21-induced cytokine/chemokines with antibodies showed that CXCL9 and CXCL10 significantly contributed to inhibition of tumor growth (Fig. 3b). In contrast, antibody neutralization of INFγ did not significantly impair anti-tumor activity.
Though demonstrating potent anti-tumor activity, CCL21-expressing S. typhimurium did not cause greater systemic toxicity than the unmodified attenuated S. typhimurium strain (data not shown). Grossly, the mice did not show decreased activity or grooming abnormalities until several weeks after S. typhimurium injections, after tumor burden increased, suggesting that the tumor (rather than the therapy) contributed to these signs of illness. Histological analysis of multiple organs (data not shown) showed minimal changes that were comparable to the results previously reported for attenuated S. typhimurium control bacteria that did not express the CCL21 chemokine [7, 10].
Concluding remarks
We describe a novel bacteria-based strategy using an attenuated strain of S. typhimurium engineered to produce a chemokine in vivo. The concept was to exploit the intrinsic capability of these bacteria to accumulate specifically within tumors with the intend to produce functional human chemokine locally within the tumor lesions [11]. We chose CCL21 based on reported activities of this chemokine as a contributor to host responses to tumors [17–19]. Using the same mouse cancer models, we have shown previously [12] and here that the attenuated S. typhimurium strain localizes predominantly to tumors in mice, confirming several other studies that have documented tumor targeting by these facultative anaerobes [7, 10].
Our data demonstrate that IV delivery of CCL21-expressing attenuated S. typhimurium induces an inflammatory response within tumors and causes a substantial reduction of tumor burden in mice challenged with multidrug-resistant CT26 colon, D2F2 breast, and B16 melanoma cells [26]. In contrast, control Salmonella showed little or no anti-tumor activity, thus demonstrating the contribution of CCL21 to the therapeutic effect. The mechanisms responsible for the anti-tumor activity of CCL21-expressing Salmonella are probably multifactoral, with the predominant mechanism possibly varying with the tumor involved, anatomical location (e.g., subcutaneous vs. pulmonary), mouse strain, and other variables. In the B16 melanoma model, we showed that the anti-tumor activity of CCL21-expressing bacteria was significantly impaired in mice depleted of CD4+ or CD8+ T cells, which is in accordance with previous reports involving the intratumoral application of CCL21 [17–19]. In contrast, antibodies targeting NK cells did not significantly impact anti-tumor activity. However, it should be recognized that in vivo antibody immunodepletion is never completely effective for eliminating cell populations, and thus a role for NK cells cannot be entirely excluded. In addition, experiments using neutralizing antibodies targeting cytokines or chemokines known to be induced by CCL21 provided evidence that CXCL9 and CXCL10 contribute significantly to the anti-tumor activity. While antibodies neutralizing INFγ did not significantly impair anti-tumor activity of CCL21-expressing Salmonella, we cannot entirely exclude a role for this cytokine because, individually, some cytokines may not be essential but in combination with others, they may make important contributors to the observed anti-tumor activity of CCL21-expressing Salmonella.
Histological examination demonstrated the induction of an inflammatory response within tumors following infection with CCL21-producing bacteria, with far less immune and inflammatory cell infiltration in the tumors of mice treated with control bacteria lacking CCL21. In contrast, inflammation of non-cancerous organs was limited principally to spleen, suggesting preferential targeting of the host response to tumors, though we cannot exclude a broader systemic effect of these chemokine-producing bacteria. The infiltrates in tumors of CCL21-treated mice included mononuclear and polynuclear cells. CCL21 is known to be chemotactic for lymphocytes and dendritic cells (mononuclear cells) but not polymorphonuclear cells. However, chemokines induced by CCL21 (e.g., CXCL9, CXCL10) are chemotactic for neutrophils [29]. Also, it bears noting that evidence of tumor necrosis was found histologically in mice treated with CCL21-expressing Salmonella, suggesting that some of the neutrophil infiltrates could be secondary to tumor lysis. In this regard, CCL21 has been reported to have a direct anti-angiogenic effect that could contribute to tumor hypoxia and cause subsequent necrosis. The anti-tumor activity of CCL21-expressing Salmonella therefore may be multifactoral, providing a distinct advantage over more target-focused therapies for which tumor resistance often emerges, thus making the pleiotrophic actions of CCL21-expressing Salmonella a distinct advantage. Conversely, it should be recognized that the pleiotropic actions may include adverse mechanisms, such as induction of regulatory T cells and myeloid-suppressor cells, which could reduce efficacy of this immunotherapy strategy [30].
Taken together, our preclinical findings demonstrate the feasibility of a novel microbial-based approach for cancer immunotherapy involving engineering S. typhimurium to express the chemokine CCL21. These findings extend our recent analysis of bacteria armed to express the cytokine LIGHT, a TNF-family member [12], and thus demonstrate the potential of using chemokines or cytokines for this application. Future studies involving other types of immunomodulatory proteins should help to reveal the full repertoire of molecules that might be appropriate to consider for cancer immunotherapies using engineered non-virulent S. typhimurium. Ultimately, however, human clinical trials will be needed to test the utility of such approaches and the therapeutic index of specific types of molecules, given the important genomic differences between rodent species and humans with respect to their repertoires of genes encoding cytokines and their receptors [31].
Regardless of the relative merits of various chemokines or cytokines for engineering non-virulent S. typhimurium, a bacteria-based strategy for de novo production of immunomodulatory proteins may have several advantages over other approaches. For example, virus-based cytokine gene delivery is difficult to terminate, given the general lack of antiviral drugs capable of halting viral infection should unacceptable toxicity be encountered. Most of the viruses under clinical investigation also lack biological features that may preferentially target them to tumors. Furthermore, recombinant cytokines are typically expensive to produce, lack tumor-targeting features, and often cause severe systemic inflammatory reactions that would limit clinical use. In contrast, genetically engineered bacteria are inexpensively produced, can selectively target tumors, and are readily eradicated by antibiotic therapy in the event of unacceptable toxicity. Altogether, these advantages, along with the results from this and similar studies, indicate that further preclinical evaluations are warranted of chemokine-producing bacteria as a novel microbial approach to cancer immunotherapy.
References
Coley WB (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res (262):3–11
Coley WB, Hoguet JP (1916) Melanotic cancer: with a report of 91 cases. Ann Surg 64(2):206–241
Coley WB (1906) Late results of the treatment of inoperable sarcoma by the mixed toxins of Erysipelas and Bacillus prodigosus. Am J Med Sci 131:375–430
Herr HW (1997) Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br J Urol 80(5):762–765
Jackson AM, Ivshina AV, Senko O, Kuznetsova A, Sundan A, O’Donnell MA et al (1998) Prognosis of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer by immunological urinary measurements: statistically weighted syndrome analysis. J Urol 159(3):1054–1063
Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J et al (2000) Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 181(6):1996–2002
Rosenberg SA, Spiess PJ, Kleiner DE (2002) Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother 25(3):218–225
Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ et al (2002) Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 20(1):142–152
Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I et al (2004) Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 22(3):313–320
Thamm DH, Kurzman ID, King I, Li Z, Sznol M, Dubielzig RR et al (2005) Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res 11(13):4827–4834
Carrier MJ, Chatfield SN, Dougan G, Nowicka UT, O’Callaghan D, Beesley JE et al (1992) Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol 148(4):1176–1181
Loeffler M, Le’Negrate G, Krajewska M, Reed JC (2007) Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A 104(31):12879–12883
Rollins BJ (1997) Chemokines. Blood 90(3):909–928
Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM (1999) The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol 162(7):3859–3864
Nagira M, Imai T, Yoshida R, Takagi S, Iwasaki M, Baba M et al (1998) A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur J Immunol 28(5):1516–1523
Kim CH, Pelus LM, Appelbaum E, Johanson K, Anzai N, Broxmeyer HE (1999) CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKbeta-11/MIP-3beta/ELC, are chemoattractants for CD56(+)CD16(-) NK cells and late stage lymphoid progenitors. Cell Immunol 193(2):226–235
Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX et al (2000) Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol 164(9):4558–4563
Yang SC, Batra RK, Hillinger S, Reckamp KL, Strieter RM, Dubinett SM et al (2006) Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res 66(6):3205–3213
Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M et al (2004) Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res 10(8):2891–2901
Soto H, Wang W, Strieter M, Copeland NG, Gilbert DJ, Jenkins NA et al (1998) The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci U S A 95(14):8205–8210
Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S et al (1995) Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 182(1):155–162
Sgadari C, Angiolillo AL, Tosato G (1996) Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87(9):3877–3882
Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL (1995) The role of CXC chemokines as regulators of angiogenesis. Shock 4(3):155–160
Wang JM, Deng X, Gong W, Su S (1998) Chemokines and their role in tumor growth and metastasis. J Immunol Methods 220(1–2):1–17
Galen JE, Nair J, Wang JY, Wasserman SS, Tanner MK, Sztein MB et al (1999) Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun 67(12):6424–6433
Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA (2006) Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 116(7):1955–1962
Spitalny GL, Havell EA (1984) Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med 159(5):1560–1565
Valbuena G, Walker DH (2004) Effect of blocking the CXCL9/10-CXCR3 chemokine system in the outcome of endothelial-target rickettsial infections. Am J Trop Med Hyg 71(4):393–399
Shi G, Partida-Sanchez S, Misra RS, Tighe M, Borchers MT, Lee JJ et al (2007) Identification of an alternative G{alpha}q-dependent chemokine receptor signal transduction pathway in dendritic cells and granulocytes. J Exp Med 204(11):2705–2718
Flanagan K, Moroziewicz D, Kwak H, Horig H, Kaufman HL (2004) The lymphoid chemokine CCL21 costimulates naive T cell expansion and Th1 polarization of non-regulatory CD4+ T cells. Cell Immunol 231(1–2):75–84
Reed JC, Doctor KS, Godzik A (2004) The domains of apoptosis: a genomics perspective. Sci STKE 2004(239):RE9
Acknowledgments
We thank M. Hanaii and T. Siegfried for manuscript preparation, M. Cuddy for manuscript review, Dr W. Z. Wei for D2F2 cells, and Dr G. Valbuena for CXCL9 and CXCL10 antibodies. We also acknowledge the generous support of the Austrian Academy of Sciences, APART Fellowship Program (M. Loeffler) and the NIH (CA-69381) (J. C. Reed).
Author information
Authors and Affiliations
Corresponding author
Additional information
Markus Loeffler and John C. Reed should be considered senior authors.
Rights and permissions
About this article
Cite this article
Loeffler, M., Le’Negrate, G., Krajewska, M. et al. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother 58, 769–775 (2009). https://doi.org/10.1007/s00262-008-0555-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0555-9