Abstract
Purpose
Catumaxomab is a trifunctional monoclonal antibody with binding sites directed to human EpCAM and the human T cell antigen CD3 (anti-EpCAM × anti-CD3). Catumaxomab demonstrated efficacy when administered intraperitoneally in patients with EpCAM positive malignant ascites from ovarian cancer in terms of tumor cell killing and reduction of ascites generation. As EpCAM is also overexpressed in NSCLC, the present study was conducted in order to evaluate safety and tolerability of intravenous treatment with catumaxomab.
Patients and methods
UICC stage IB–IV NSCLC patients were eligible, if they had at least one prior therapy. Other inclusion criteria were: age 18–75 years, adequate bone marrow function and AST or gamma-GT ≤ 2.5 ULN. Escalating doses of catumaxomab were administered over 8 h as a continuous intravenous infusion. Dose escalation started at 2 μg and was increased to 7.5 μg. In addition various doses of dexamethasone as premedication were investigated. All patients were pretreated with antihistamines.
Results
Dose limiting toxicity (DLT) was a grade 3 and 4 elevation of ALT, AST and gamma-GT, which was observed in dose level IV (10 mg dexamethasone premedication, 5 μg catumaxomab) and V (40 mg dexamethasone followed by 7.5 μg catumaxomab). Elevated liver enzymes decreased to grade 2 within 3–7 days and were at baseline level within 14 days after infusion. The maximum tolerated dose (MTD) was defined in dose level III (40 mg of dexamethasone followed by 5 μg of catumaxomab).
Conclusions
Five micrograms of catumaxomab with a pre-medication of 40 mg dexamethasone and antihistamines can be recommended as first dose for i.v. therapy consisting of multiple catumaxomab infusions for patients with NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expression of the membrane-embedded glycoprotein epithelial cell adhesion molecule (EpCAM) in vivo is related to an increased epithelial cell proliferation and is negatively correlated with cell differentiation [1]. EpCAM is overexpressed in a variety of epithelial tumor cells like in colorectal cancer, prostate cancer, ovarian cancer and non-small cell lung cancer (NSCLC). Because of the wide expression on tumor cells it has been considered a tumor associated antigen [2, 3] and innovative immunotherapeutic approaches targeting EpCAM are of special interest [4, 5]. EpCAM has a direct impact on cell cycle and proliferation, and the ability to rapidly up-regulate the proto-oncogene c-myc and cyclin A/E. Thus, inhibition of EpCAM expression leads to a strong decrease in proliferation and metabolism in human carcinoma cells [6].
Catumaxomab is a new hybrid-hybridoma derived, trifunctional monoclonal antibody with two different binding specificities directed to EpCAM and the T cell marker CD3 (anti-EpCAM × anti-CD3). While binding to EpCAM-positive tumor cells and T cells, catumaxomab is thought to simultaneously recruit accessory cells via binding of its Fc-region to Fcγ-receptors type I and III resulting in an efficient killing of tumor cells by induction of apoptosis, release of cytokines and perforin-mediated lysis as well as by antibody dependent cellular cytotoxicity (ADCC) (Fig. 1) [7, 8]. A phase I/II clinical trial of intraperitoneal therapy with 5–200 μg of catumaxomab resulted in an effective elimination of tumor cells from malignant ascites in patients with ovarian cancer [9]. In addition five out of nine patients with peritoneal carcinomatosis from various solid tumors had a response to intraperitoneal therapy with catumaxomab in another study [10].
Despite several improvements of NSCLC treatment, the prognosis and outcome of NSCLC patients’ still remain unfavourable with a 5-year overall survival rate of 14–17% [11]. Surgery as well as radio and/or chemotherapy in early tumor stages may lead to short term improvement of the disease but result in high local recurrence rates [12, 13]. Especially for patients with progressive disease only few treatment options exist and these options are mainly palliative [14–16]. Therefore, there is still an urgent medical need for the development of new treatment strategies for NSCLC [17].
Based on the fact that EpCAM is expressed in 86.5% [5] of all NSCLC patients we conducted this phase I dose escalation study in order to investigate safety and tolerability, and to determine the MTD (maximum tolerated dose) of a single intravenous infusion of catumaxomab in NSCLC patients.
Patients and methods
Patient population
Patients with histologically or cytologically confirmed NSCLC, UICC stage IB-IV with at least one prior treatment (surgery, chemotherapy and/or radiotherapy) were enrolled into the study. Inclusion criteria were age between 18 and 75 years, a negative pregnancy test and Karnofsky performance status >60%. Patients with acute or chronic infection, serum haemoglobin <9 g/dl, thrombocytes <80/nl, white blood cell count >13 or <3/nl, aspartate aminotransferase (AST) and/or γ-GT (GGT) >2.5 × upper limit of normal (ULN), estimated creatinine clearance (according to Cockroft and Gault [18]) <30 ml/min, chemo- or radiotherapy within the last 28 days, previous treatment with mouse monoclonal antibodies were also excluded from the study. The study protocol was approved by the local Ethics Committee and all patients gave their written informed consent.
Trial design
The study was conducted to investigate safety and tolerability of increasing doses of 2–20 μg catumaxomab, administered once by a constant-rate 8-h intravenous infusion (with and without dexamethasone premedication) to patients with NSCLC. The MTD was to be identified. The production of the antibody had been published earlier [7, 8].
The trial was designed as a multi-center, uncontrolled, sequential dose-rising tolerability study with each subject being investigated once. Generally, three patients were investigated at each dose level, up to six at the presumed MTD level. Patients were closely monitored for 72 h post-infusion and follow-up visits took place on 7 and 28 days after the infusion. The dose escalation and patients safety surveillance were carefully monitored by a Dose Steering Board (DSB). In cases of unexpected toxicities or a critical adverse event (CAE) the DSB immediately had to judge whether recruitment into a dose level should be terminated beforehand.
Treatment
Thirty minutes before administration of the study medication, premedication was given containing paracetamol (0.5–1 g orally), clemastine (2–4 mg i.v.) and cimetidine (400 mg i.v.) or ranitidine (50 mg i.v.) with or without dexamethasone (40 or 10 mg i.v.). All patients received one single dose of catumaxomab as an 8-h continuous infusion via an intravenous catheter. The various doses given according to the planned dose-escalation schemes are provided in Table 1. The solution for infusion was prepared using the sterile antibody syringe containing 10-μg intact antibody in 0.1 ml 100 mmol sodium citrate buffer and a sterile sodium chloride solution of 0.9%.
Toxicity analysis (safety parameters)
The following safety measurements were carried out in all patients: recording of adverse events (AE), clinical evaluation of the patient during the treatment and follow-up period, physical examination, vital signs, 1-lead ECG continuous monitoring during the first 24 h of treatment, 12-lead ECG, and safety laboratory parameters. Grading of toxicities was performed according to NCI CTC grading version 2.0. Additional medication and the need to discontinue the infusion were to be reported. An AE was considered to be CAE when at least one of the following criteria was fulfilled: (a) NCI CTC grade ≥3 AE which demanded interruption of the infusion and which then could not be relieved within 2 h by conventional measures, (b) delayed infusion reactions with toxicity grade ≥3 within the first 24 h after the start of infusion which then could not be relieved by conventional measures or (c) grade ≥3 laboratory test abnormalities within the first 48 h after the start of infusion with regard to serum electrolytes, ALT, AST, gamma-GT, serum creatinine or evidence of haemolysis. All CAE had to be discussed within the DSB to define the clinical relevance.
The MTD was determined as follows:
-
If none of the three patients at a certain dose level suffered a CAE, which was judged to be clinically relevant by the DSB, the next three patients were treated with the next dose level.
-
If one of the three patients suffered a CAE, additional three patients were investigated at the same dose level, if none of these further patients suffered a CAE, which was clinically relevant according to the DSB discussion the DSB decided whether the next higher dose was to be implemented.
-
If two patients at a given dose suffered a clinically relevant CAE no further patient was treated at that dose or a higher dose. The DSB decided if the previous given dose level should be considered as MTD.
The DSB was allowed to terminate recruitment into a dose level even before three patients had been enrolled, if a CAE was attributed to be caused by a decrease in the dose of the premedication.
HAMA/HARA (human anti-mouse/human anti-rat antibody)-tests
For the determination of HAMA/HARA, samples of patient serum were collected at screening and during follow-up on day 7 and 28 after the catumaxomab infusion. The samples were analyzed with an ELISA (enzyme linked immunosorbent assay) developed by Gallati consisting of four separate ELISAs: human anti-mouse Ig antibody, human anti-mouse IgG2a antibody, human anti-rat Ig antibody and human anti-rat IgG2a antibody. The anti-antibodies of the patients sample were captured by a mixture of rat and mouse Ig immobilized on a micro titer plate, detected by the corresponding peroxidase labelled antibody (mouse Ig, mouse IgG2a, rat Ig, rat IgG2a) and quantified with a colouring enzyme–substrate reaction with TMB (3,3′,5,5′-tetramethylbenzidine). The quantification of HAMA and HARA was conducted by calibration with goat anti-mouse Ig or goat anti-rat Ig, respectively. Measurements of extinction were performed at 450 nm in an ELISA Reader and results were given in Gallati units (representing the concentration of the calibrator goat anti-mouse Ig or goat anti-rat Ig).
Results
Patient characteristics
Between December 2001 and 2003 a total of 21 patients had been treated within the trial. Fifteen patients had been treated according to the study protocol and were evaluable for toxicity analysis and MTD determination. Nine patients were not evaluable, because they received an incorrect dilution of the antibody resulting in a ten times decreased dose of the antibody. Median age of evaluable patients was 57 years (range 48–71). Tumor stages at study entry were heterogeneous with 1, 5, 5 and 4 patients in UICC stage I, II, III and IV (Table 2). Patients with stage I–IIIA had prior resection of the tumor followed by adjuvant catumaxomab therapy within this trial. Patients with stage IIIB and IV had measurable disease. These patients had prior chemotherapy. At the time of disease progression all patients received chemotherapy and some received tyrosine-kinase inhibitors (TKIs) of the epidermal growth factor receptor (EGF-R) also.
In dose levels I, II, III, IV and V a total of 3, 1, 4, 5 and 2 patients were evaluable for toxicity analysis (for details see Table 2). Only one patient had been treated in dose level II (2 μg of catumaxomab without dexamethasone). This patient experienced syncope during infusion of catumaxomab, which resulted in an immediate discontinuation of the infusion. Therefore the criteria for an unexpected CAE were met. The DSB decided to stop further accrual into this dose level. Because one possible explanation for the syncope could be treatment without dexametason premedication, DSB decided to continue accrual into a dose level with a dexametason premedication. In dose level V the first two patients had a dose limiting toxicity (DLT), therefore no third patient had been treated within this dose level.
Toxicities
A total of six (40%) patients experienced a total of 16 treatment-emergent adverse events (TEAEs) of CTC grade ≥3 with a tendency of a higher incidence in the higher dose groups. No patient experienced a treatment-emergent serious adverse event or died during the study. One patient (dose group II) experienced a drug-related TEAE leading to discontinuation of catumaxomab.
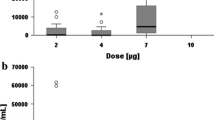
The most frequent TEAEs (8 patients with 20 TEAEs) were of hepatic and hepatobiliary disorders of which 13 events were of CTC grade ≥3. The reported incidence tended to be higher in the higher dose groups and did not correlate with the patient’s performance status. All events were considered to be related to the study drug. In dose level I (2 μg + 40 mg dexamethasone), IV (5 μg + 10 mg dexamethasone), and V (7.5 μg + 40 mg dexamethasone), the liver function parameters, gamma-glutamyltransferase (γGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and alkaline phosphatasis, increased 12–24 h after the start of the infusion reaching a peak at 48 h and returned to screening level at the end of study. Elevated liver enzymes decreased to grade 2 within 3–7 days and were at baseline level within 14 days after infusion. No increases of liver enzymes were observed in dose level II (2 μg + without dexamethasone) and III (5 μg + 40 mg dexamethasone) (Table 3).
Thus the DLT was found to be CTC grade 3 and 4 transient elevation of ALT and AST as well as a CTC grade 3 elevation of γGT, which occurred in dose level IV and V. In dose level IV (5 μg catumaxomab + 10 mg dexamethasone), three out of five patients had CTC grade 3 or 4 elevations of ALT and AST and two patients had a CTC grade 3 elevation of γGT, which had not been observed at the same dose of catumaxomab (5 μg) administered with a higher dose of premedication (40 mg dexamethasone) as in dose level III (Table 3). In dose level V (7.5 μg + 40 mg dexamethasone) one out of two patients had grade 3 elevation of ALT, and all patients had grade 3 elevation of AST and γGT (Table 3). Since the DLT was observed in dose level IV and V, dose level III (5 μg + 40 mg dexamethasone) was defined to be the MTD. However no deterioration of liver synthesis was observed. Since ≥2 patients in dose level IV and V experienced a grade III or IV elevation of liver parameters, it was decided to terminate accrual into dose level IV and V after five and two patients, respectively.
Haematological toxicity was mild. A transient, reversible CTC grade 3 decrease of lymphocyte counts in dose level II, IV and V was observed (Table 4). The decrease of lymphocytes occurred 8 h after start of the infusion of the antibody in three patients. Lymphocyte counts returned to pre-infusion values within 48–72 h after start of the infusion. C-reactive protein (CRP) increased 24 h after start of the infusion in one patient in the highest dose level (7.5 μg + 40 mg dexamethasone) and in one of dose level II (2 μg + without dexamethasone) and returned to pre-treatment values on day 7.
No additional CTC grade 3 or 4 toxicities were observed. Most frequent CTC grade 1 or 2 toxicities were symptoms of the cytokine release syndrome as pyrexia (N = 3), nausea (N = 2), dizziness (N = 3), headache (N = 2) and tachycardia (N = 2) (Table 5).
Human-anti-mouse-antibodies (HAMA) and human-anti-rat-antibodies (HARA)
All patients were screened for pre-existing human-anti-mouse- or rat-antibodies (HAMA or HARA). In one out of fifteen patients pre-existing HARA are detectable in serum at very low concentrations (32 ng/ml), the patient was included into the study per exemption request. No patient had pre-existing HAMA.
Out of 14 evaluable patients, no patient developed HAMA and only the patient with pre-existing HARA had a slightly increased HARA-level on day 7 (90 ng/ml) and 28 (80 ng/ml) after infusion of catumaxomab (Table 6).
Time to progression and survival
Two out of five patients diagnosed with stage IIB died 3 and 11 months after the infusion due to disease progression, three patients are still alive without tumor progression at 29, 30 and 35 months after catumaxomab infusion at dose levels I (two patients) and V (one patient). The stage IIIA patient (N = 1) and all four stage IIIB patients are still alive at 26–37 months after treatment in dose level III and IV, although three of four stage IIIB patients relapsed 2.5, 7 and 9 months after treatment. One out of the four UICC stage IV patients had a tumor progression 3 months after treatment in dose level IV but is still alive after 28 months. The other stage IV patients progressed 1, 3 and 3.5 months after treatment and died after 9, 13 and 15 months.
Discussion
The results of treatment of non-small cell lung cancer remain disappointing despite the great efforts made in the last decade. Even novel therapies with small molecules or monoclonal antibodies only offer a survival benefit of few months for patients with locally advanced and metastatic disease [15, 16]. In the adjuvant setting substantial benefit can be seen with chemotherapy, but still a large number of patients suffer a local or distant relapse [12, 13]. It is certain that there is and will be a great demand on new agents helping to treat the disease.
EpCAM is expressed on the tumor cells in 86.5% of all NSCLC patients and might therefore be an interesting target for new therapeutic interventions [5]. Several immunotherapeutic approaches targeting EpCAM have been developed in recent years. A new immunotherapeutic agent might be the trifunctional antibody catumaxomab with its two different binding specificities directed to EpCAM and the T cell marker CD3 (anti-EpCAM × anti-CD3). Catumaxomab is able to bind to EpCAM-positive tumor cells and T cells and simultaneously recruits Fcγ-receptor type I and III positive accessory cells via binding of its Fc region. This characteristic results in the formation of a so-called tricell complex and induces a cross activation of T cells and accessory cells including co-stimulatory signals [19]. As a consequence, tumor cells are effectively killed by several mechanisms like release of cytokines, perforin-mediated lysis, phagocytosis, ADCC or induction of apoptosis [7, 8, 20]. Especially antibody-mediated phagocytosis of tumor cells by accessory cells might result in the processing and presentation of tumor-associated and tumor-specific antigens on the surface of antigen presenting cells, which might lead to a polyclonal humoral and cellular immune response against known and unknown epitopes derived from tumor associated antigens [21].
As the trifunctional antibody consists of a mouse IgG2a and a rat IgG2b chain there might be induction of human-anti-mouse-antibodies (HAMA) and human-anti-rat-antibodies (HARA) when administered in vivo [22, 23]. Therefore the HAMA/HARA status was determined in all patients before and after treatment with the antibody. After treatment, no patient developed HAMA or HARA within 28 days; only one patient who had pre-existing HARA at a very low concentration had a slightly increased HARA level at the end of trial. This suggests that treatment with catumaxomab using amounts up to 7.5 μg as a single infusion dose does not result in generation of anti-antibodies within 28 days after treatment and seems to be safe. Repeated administration of the antibody can be considered in the future and must be investigated in further clinical trials.
The DLT we found was a transient CTC grade 3 and 4 elevation of the liver enzymes ALT, AST and gamma-GT after administration of dose level IV (5 μg catumaxomab + 10 mg dexamethasone premedication) and V (7.5 μg catumaxomab + 40 mg dexamethasone). The MTD was therefore determined as dose level III (5 μg catumaxomab + 40 mg dexamethasone). There was no clinically relevant change in other parameters of liver function e.g., bilirubin or prothrombin time. Elevated liver enzymes decreased to baseline within 14 days after infusion in every patient. The mechanisms responsible for the liver cell damage observed are unclear. One hypothesis is that the release of cytokines during activation of the immune response might damage hepatocytes resulting in a release of liver enzymes into the serum. Another mechanism might be that the strong expression of EpCAM on epithelial cells of the biliary duct might lead to a damage of cells of the biliary duct resulting in a local release of mediators damaging hepatocytes. Chronic liver damage or even liver failure had never been observed. Elevations of liver enzymes were also found after intraperitoneal infusion of catumaxomab in patients with malignant ascites due to ovarian cancer but to a lower extend. This might be explained by local application route and a lower systemic reaction. Three patients had a transient, CTC grade 3 reversible decrease of lymphocytes starting 8 h and returning to baseline values within 48–72 h after start of infusion. This abnormality did not result in any clinical consequence. In this context, a study by Molema et al. [24] suggests that infused bispecific antibodies induce activation of lymphocytes accompanied by an upregulation of adhesion molecules leading to an interaction of lymphocytes with endothelial cells, followed by a generalized redistribution of lymphocytes into tissues.
Therefore, the transient and reversible decrease of lymphocytes might be interpreted at least partly as an antibody-induced migration of T cells out of the blood stream into the tissue and possibly to the tumor. No other hematological toxicity ≥CTC grade 3 occurred. One of the potential problems of T cell activation via CD3 might be the appearance of a cytokine release syndrome, which is usually reflected by pyrexia, nausea, headache and tachycardia. In this study cytokine release related symptoms appeared after catumaxomab infusion in 12 patients but none were more severe than of CTC grade 2. Overall these side effects were marginal especially when compared to potential side effects induced by chemotherapy and could easily be controlled by symptomatic treatment without further complications.
The effectiveness of catumaxomab has been demonstrated in other studies. In patients with malignant ascites due to ovarian cancer a phase I/II clinical trial of intraperitoneal therapy with the antibody resulted in an effective reduction of ascites fluid and elimination of tumor cells from malignant ascites [9]. In addition five out of nine patients with peritoneal carcinomatosis from various solid tumours showed a response after intraperitoneal therapy with catumaxomab in another study [10].
Remarkably, the survival observed in this study was very favourable, although survival analysis was not the primary endpoint. All four patients with locally advanced disease UICC stage IIIB are still alive at 26 (two patients), 27 and 28 months after catumaxomab treatment. One of the patients without any tumor progression and the other three with a relapse after 2.5, 7 and 9 months. Additionally one patient with metastatic disease UICC stage IV is still alive at 28 months after treatment but having had a relapse at 3 months. Even though this study was not designed to assess the clinical effects of catumaxomab and most of these patients received additional tumor therapies after the catumaxomab treatment these findings are remarkable and might indicate clinical efficacy. However, it is clear that this needs to be confirmed in future randomized trials.
In summary we demonstrate that administration of catumaxomab as a single intravenous infusion of 5 μg with a premedication of 40 mg dexamethasone to patients with NSCLC is feasible. The data obtained in this trial are the basis for a further evaluation of catumaxomab treatment in NSCLC.
References
Munz M, Zeidler R, Gires O (2005) The tumour-associated antigen EpCAM upregulates the fatty acid binding protein E-FABP. Cancer Lett 225:151–157
Herlyn M, Steplewski Z, Herlyn D, Koprowski H (1979) Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci USA 76:1438–1442
Fleuren GJ, Gorter A, Kuppen PJ, Litvinov S, Warnaar SO (1995) Tumor heterogeneity and immunotherapy of cancer. Immunol Rev 145:91–122
Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S (2004) Frequent EpCam protein expression in human carcinomas. Hum Pathol 35:122–128
Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Krononen J, Simon R, Sauter G, Baeuerle PA (2006) Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer 94:128–135
Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O (2004) The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 23:5748–5758
Riesenberg R, Buchner A, Pohla H, Lindhofer H (2001) Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (alpha EpCAM × alpha CD3). J Histochem Cytochem 49:911–917
Zeidler R, Mysliwietz J, Csanady M, Walz A, Ziegler I, Schmitt B, Wollenberg B, Lindhofer H (2000) The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer 83:261–266
Jaeger M, Stroehlein MA, Schoberth A, Burges A, Heiss MM, Lindhofer H (2004) Immunotherapy with the trifunctional antibody removab leads to significant elimination of tumor cells from malignant ascites in ovarian cancer: results of a phase I/II study. J Clin Oncol 23(Suppl):2504
Stroehlein M, Jaeger M, Lindhofer H, Peschel C, Jauch KW, Heiss MM (2004) Intraperitoneal immunotherapy of peritoneal carcinomatosis from solid tumors by trifunctional antibodies. J Clin Oncol 23(Suppl):2532
Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jaminson PM, Wingo PA, Howe HL, Anderson RN, Edwards BK (2004) Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 101:3–27
Winton T, Livingston R, Johnson D et al (2005) Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589–2597
The International Adjuvant Lung Cancer Trial Collaborative Group (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351–360
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DF, ECOG (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Sandler AB, Gray R, Brahmer J et al (2005) Randomized phase II/III trial of paclitaxel (P) plus carboplatin (C) with or without bevacizumab (NSC #704865) in patients with advanced non-squamous non-small cell lung cancer (NSCLC): an Eastern Cooperative Oncology Group (ECOG) Trial-E4599. J Clin Oncol 23:24
Carney DN (2002) Lung cancer—time to move on from chemotherapy. N Engl J Med 346:126–128
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H (1999) Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J Immunol 163:1246–1252
Zeidler R, Mayer A, Gires O, Schmitt B, Mack B, Lindhofer H, Wollenberg B, Walz A (2001) TNFalpha contributes to the antitumor activity of a bispecific, trifunctional antibody. Anticancer Res 21:3499–3503
Ruf P, Lindhofer H (2001) Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood 98:2526–2534
Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JM, Colnaghi MI, Canevari S (1999) Level of anti-mouse-antibody response induced by bi-specific monoclonal antibody OC/TR in ovarian-carcinoma patients is associated with longer survival. Int J Cancer 84:62–68
Azinovic I, Denardo GL, Lamborn KR, Mirick G, Goldstein D, Bradt BM, DeNardo SJ (2006) Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother 55:1451–1458
Molema G, Tervaer JW, Kroesen BJ, Helfrich W, Meijer DK, de Leij LF (2000) CD3 directed bispecific antibodies induce increased lymphocyte-endothelial cell interactions in vitro. Br J Cancer 82:472–479
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sebastian, M., Passlick, B., Friccius-Quecke, H. et al. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM × anti-CD3): a phase I study. Cancer Immunol Immunother 56, 1637–1644 (2007). https://doi.org/10.1007/s00262-007-0310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0310-7