Abstract
Purpose
To evaluate the diagnostic performance of biphasic contrast-enhanced CT in differentiation of lipid-poor adenomas from pheochromocytomas.
Methods
129 patients with 132 lipid-poor adenomas and 93 patients with 97 pheochromocytomas confirmed by pathology were included in this retrospective study. Patients underwent unenhanced abdominal CT scan followed by arterial and venous phase. Quantitative and qualitative imaging features were compared between the two groups using univariate analysis. Risk factors for pheochromocytomas were evaluated by multivariate logistic regression analysis and a diagnostic scoring model was established based on odd ratio (OR) of the risk factors.
Results
Pheochromocytomas were larger and showed cystic degeneration more frequently compared with lipid-poor adenomas (p < 0.01). No significant difference was found in peak enhancement phase between the two groups (p = 0.348). Attenuation values on unenhanced phase (CTU), arterial phase (CTA), and venous phase (CTV) of pheochromocytomas were significantly higher than that of lipid-poor adenomas while enhancement ratio on arterial and venous phase (ERA, ERV) of pheochromocytomas was significantly lower than that of lipid-poor adenomas (all p < 0.05). Multivariate analysis revealed lesion size > 29 mm (OR: 5.74; 95% CI 2.51–13.16; p < 0.001), CTA > 81 HU (OR: 2.54; 95% CI 1.04–6.17; p = 0.04), CTV > 97 HU (OR: 11.19; 95% CI 3.21–38.97; p < 0.001), ERV ≤ 1.5 (OR: 20.23; 95% CI 6.30–64.87; p < 0.001), and the presence of cystic degeneration (OR: 6.22, 95% CI 1.74–22.25; p = 0.005) were risk factors for pheochromocytomas. The diagnostic scoring model yielded an area under the curve (AUC) of 0.911.
Conclusions
Biphasic contrast-enhanced CT showed good diagnostic performance in differentiation of lipid-poor adenomas from pheochromocytomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence rate of adrenal incidentalomas is increasing with wide usage of radiological examination, accounting for 4% of all abdominal CT scan [1]. Among adrenal incidentalomas, 70%-80% are adenomas and the less common masses include adrenocortical carcinomas (8%), pheochromocytomas (7%), metastases (5%), and so on. [2]. Identification of benign leave-out masses from the ones that need further work-up is crucial for clinical management. Most adenomas are nonfunctional and do not need management. Pheochromocytomas are catecholamine-secreting tumors that may cause life-threatening hypertension crisis during surgery and they have the potential of biological aggressiveness and require early intervention [3]. The typical symptoms, for instance, hypertension, headache, sweating, and positive biochemical test of plasma or 24-h urinary metanephrine, indicate the diagnosis of pheochromocytomas. However, up to 25% of patients with pheochromocytomas are asymptomatic [2, 4], and 50% of small pheochromocytomas may show modestly elevated or normal biochemical marker levels [5]. Under the circumstances, imaging plays a vital role in differentiation of adenomas from pheochromocytomas.
Most adenomas are rich in intracellular lipid, leading to low attenuation values on unenhanced CT. Attenuation values less than 10 HU on unenhanced CT is reliable to diagnose lipid-rich adenomas [6]. However, approximately 29% of lipid-poor adenomas show attenuation values greater than 10 HU on unenhanced CT, making them indeterminate to diagnose [6]. In such settings, washout characteristics obtained from delayed phase are warranted as adenomas show rapid washout features compared with pheochromocytomas [7,8,9,10]. However, additional studies found that 33%-50% of pheochromocytomas met washout criteria established for adenomas, leading to misdiagnosis [11,12,13]. Furthermore, delayed scan ranging from 5 to 15 min is not applicable in the work practice [7, 8]. For adrenal masses incidentally found in abdominal dual-phase contrast-enhanced CT, additional delayed scan increases medical cost and radiation dose for patients.
Several studies explored the value of biphasic contrast-enhanced CT in differentiation of adenomas from pheochromocytomas [14,15,16]. They found peak enhancement during arterial phase, higher enhancement level on arterial and venous phase could assist in the identification of pheochromocytomas, especially those that demonstrated adenomas-like washout features. However, these studies solely focused on the individual features and other quantitative and qualitative imaging features were not taken into consideration for the differentiation of adenomas from pheochromocytomas. Moreover, the sample size, especially of pheochromocytomas, was relatively small. Therefore, the purpose of our study was to investigate whether biphasic contrast-enhanced CT could differentiate lipid-poor adenomas from pheochromocytomas when combined with quantitative and qualitative features.
Materials and methods
This retrospective study was performed in Tongde Hospital of Zhejiang Province and Sir Run Run Shaw Hospital of Zhejiang University School of Medicine hospital and was approved by the institutional review board. Informed content was waived due to the retrospective analysis of the study.
Study population
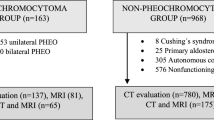
We searched the pathology database during January 2011 and October 2020 with key words of “adrenal adenoma” and “adrenal pheochromocytoma,” respectively. Initially, 465 adrenal adenomas and 126 pheochromocytomas were found. The inclusion criteria of the study were as follows: (1) the presence of preoperative biphasic contrast-enhanced CT imaging, (2) lipid-poor adenomas with mean attenuation values > 10 HU on unenhanced CT [6], and (3) the presence of enhanced region within masses for attenuation values measurement. Lesions with completely cystic degeneration were excluded, (4) lesion size ≥ 10 mm to ensure measurement accuracy and to avoid partial volume averaging artifact. Finally, the study population consisted of 129 patients with 132 lipid-poor adenomas (3 bilateral) and 93 patients with 97 pheochromocytomas (4 bilateral). The flowchart of our study is shown in Fig. 1.
Images protocol
Multiple CT scanners were used due to retrospective analysis of the study, including SOMATOM Sensation 16 and SOMATOM Definition Flash (Siemens Healthcare, Forchheim, Germany) and LightSpeed VCT (GE Healthcare, Milwaukee, WI, USA). All patients underwent unenhanced scan followed by arterial phase (25–30 s) and venous phase (60 s) after infusion of contrast material. A total of 100–120 mL non-ionic iodine contrast material was injected intravenously using a power injector at a rate of 2.5–3.0 mL/s. The CT scan parameters were as follows: tube voltage, 120 kVp; tube current, 250–300 mA; slice thickness, 1.0–3.0 mm.
Imaging analysis
Two abdominal radiologists (Y. An and J. Wang, with 3 and 15 years experience, respectively) blind to pathological results and clinical information independently evaluated all preoperative CT images. Disagreement was settled by consensus. The following imaging data were evaluated: (1) size: longest diameter on axial images; (2) attenuation values measured on unenhanced phase (CTU), arterial phase (CTA), and venous phase (CTV). For attenuation values measurement, a 20–40 mm2 circular region of interest (ROI) was placed on the enhanced part of the lesion. The attenuation values were measured 3 times and the mean of these values was recorded as final results; (3) enhancement ratio, we calculated the enhancement ratio on arterial phase (ERA) and venous phase (ERV) as ERA = (CTA – CTU)/CTU, ERV = (CTV – CTU)/CTU; (4) peak enhancement phase was defined as the phase in which maximum enhancement level was 5 HU greater than another phase after contrast. If the difference of enhancement level was less than 5 HU between arterial and venous phases, the lesion was deemed as equally enhanced [14, 17]; (5) the presence of cystic degeneration, which was defined as low-density region without enhancement; (6) the presence of hemorrhage, which was defined as hyperdensity region without enhancement; and (7) the presence of calcification. Additionally, the abdominal aortic enhancement level at the level of renal artery on the arterial phase was measured to rule out the effect of cardiovascular status.
Statistical analysis
All statistical data analyses were performed with SPSS (version 20, SPSS, Chicago, USA) and MedCalc (version 15.6.1, MedCalc Software bvba, Ostend, Belgium). All calculations of CT imaging features were performed on a per-lesion basis. Continuous variables were expressed as mean ± standard deviation and categorical variables were described as proportions. Student’s t test was used for comparison of continuous variables if the data were normally distributed; otherwise, the Mann–Whitney U test was used. Proportions for categorical variables were compared using the χ2 test. For quantitative variables with statistical significance, receiver operating characteristic curve (ROC) analysis and Youdex index were used to obtain the best cutoff values for maximum sensitivity and specificity. Subsequently, the quantitative variables were dichotomized based on the optimal cutoff values and then they were involved in the multivariate analysis. Finally, binary logistic regression analysis using stepwise method based on the likelihood ratio test was performed to identify the risk factors for diagnosis of pheochromocytomas. A diagnostic scoring model was developed based on the odd ratio (OR) of the risk factors for pheochromocytoma. The area under the curve (AUC) was calculated to assess the diagnostic performance and was compared with DeLong test. In addition, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and their 95% confidence interval (CI) were calculated. A two-tailed p value < 0.05 was considered statistically significant.
Results
Patients
The mean patient age of pheochromocytomas and lipid-poor adenomas was 51.7 ± 11.4 years, 49.7 ± 13.4 years, respectively. Hypertension was found in 52.7% of pheochromocytomas (49/93) and 48.1% of lipid-adenomas (62/129). There were no significant differences in age, gender, and hypertension between lipid-poor adenomas and pheochromocytomas (all p > 0.05).
Comparison of CT imaging features
Univariate analysis of CT imaging features is summarized in Table 1. The size of pheochromocytomas (mean, 40.9 ± 22.7 mm; ranges, 10–130 mm) was lager than that of lipid-poor adenomas (mean, 23.5 ± 10 mm; ranges, 10–72 mm) (p < 0.001). 46.4% of pheochromocytomas (45/97) presented cystic degeneration while only 3.0% of lipid-poor adenomas (4/132) did (p < 0.001). Both hemorrhage and calcification within tumor were uncommon in the two groups and no significant differences were found between them (p = 0.288, p = 0.344, respectively). More than half of pheochromocytomas (59.8%, 58/97) and lipid-poor adenomas (68.2%, 90/132) showed peak enhancement level on venous phase. Furthermore, 17.4% (23/132) of lipid-poor adenomas and 24.7% of (24/97) pheochromocytomas showed peak enhancement level on arterial phase. There were no significant differences in peak enhancement phase between the two groups (p = 0.348).
The mean values of CTU, CTA, and CTV of pheochromocytomas were 38.6 HU, 96.5 HU, and 102.7 HU, respectively, which were significantly higher than that of lipid-poor adenomas (CTU: 25.4 HU; CTA: 65.8 HU; CTV: 81.6 HU) (all p < 0.001). ERA and ERV of pheochromocytomas were significantly lower than that of lipid-poor adenomas (ERA:1.58 vs 1.78; ERV: 1.76 vs 2.64; all p < 0.05).
For quantitative variables with significant difference at the p < 0.05 level, ROC analysis was performed to obtain the optimal cutoff values for dichotomy. The cutoff values for lesion size, CTU, CTA, CTV, ERA, and ERV were as follows: lesion size, 29 mm; CTU, 32 HU; CTA, 81 HU; CTV, 97 HU; ERA, 1.05; ERV, 1.5 (Table 2). Of these variables, the AUC of CTU was higher than that of lesion size, CTA, CTV, ERA, and ERV (Fig. 2).
Multivariate logistic regression analysis
According to univariate analysis, seven variables—lesion size, CTU, CTA, CTV, ERA, ERV, and cystic degeneration, were involved in the multivariate logistic regression analysis. Finally, five variables—lesion size, CTA, CTV, ERV, and cystic degeneration, were considered as independent factors to distinguish lipid-poor adenomas from pheochromocytomas. The OR of these variables is listed in Table 3. Lesion size > 29 mm (OR: 5.74; 95% CI 2.51–13.16; p < 0.001), the presence of cystic degeneration (OR: 6.22, 95% CI 1.74–22.25; p = 0.005), CTA > 81 HU (OR: 2.54; 95% CI 1.04–6.17; p = 0.04), CTV > 97 HU (OR: 11.19; 95% CI 3.21–38.97; p < 0.001), and ERV ≤ 1.5 (OR: 20.23; 95% CI 6.30–64.87; p < 0.001) were risk factors for the diagnosis of pheochromocytomas.
A diagnostic scoring system was established for differentiation of lipid-poor adenomas from pheochromocytomas. Based on OR of the risk factors, the scores were assigned as follows: 1 point for CTA > 81 HU, 2 points for lesion size > 29 mm and the presence of cystic degeneration and CTV > 97 HU, and 3 points for ERV ≤ 1.5. Diagnostic performances with different cutoff values are listed in Table 4. An optimal cutoff value of ≥ 5 points yielded a sensitivity of 70.1% (95% CI 60.0%-79.0%) and a specificity of 90.9% (95% CI 84.7%-95.2%). The AUC was 0.911 (95% CI 0.866–0.945) (Fig. 3). Additionally, a lesion with score less than 3 points was highly suggestive of lipid-poor adenoma with likelihood of 96.6%, and a lesion with score equal or greater than 6 points was highly suggestive of pheochromocytomas with likelihood of 96.1%. Examples are given in Figs. 4, 5, 6.
Adrenal pheochromocytoma in a 55-year-old woman. Biphasic contrast-enhanced CT imaging of a unenhanced phase, b arterial phase, and c venous phase showed a 31-mm mass in right adrenal. Attenuation values on unenhanced, arterial, and venous phase were 43 HU, 133 HU, and 175 HU, respectively. The enhancement ratio on venous phase was 3.07. No cystic degeneration was observed within the tumor. The lesion got a score of 5 points, indicating diagnosis of pheochromocytoma
Adrenal pheochromocytoma in a 23-year-old woman. Biphasic contrast-enhanced CT imaging of a unenhanced phase, b arterial phase, and c venous phase showed a 53-mm mass in right adrenal. Attenuation values on unenhanced, arterial, and venous phase were 36 HU, 93 HU, and 156 HU, respectively. The enhancement ratio on venous phase was 3.33. Cystic degeneration was seen within the tumor. The lesion got a score of 7 points, indicating diagnosis of pheochromocytoma
Adrenal adenoma in a 52-year-old woman. Biphasic contrast-enhanced CT imaging of a unenhanced phase, b arterial phase, and c venous phase showed a 28-mm mass in right adrenal. Attenuation values on unenhanced, arterial, and venous phase were 25 HU, 68 HU, and 94 HU, respectively. The enhancement ratio on venous phase was 2.76. No cystic degeneration was seen within the tumor. The lesion got a score of 0 point, indicating diagnosis of lipid-poor adenoma
Discussion
Adenomas are the most common masses in adrenal incidentalomas and show rapid washout features compared with non-adenomas [18, 19]. Hence, a dedicated adrenal CT protocol consisted of unenhanced phase, venous phase, and 15-min delayed phase is recommended for characterization of adrenal masses [18, 19]. However, several studies have reported the limited value of adrenal CT protocol due to overlapping washout features between adenomas and non-adenomas [11,12,13]. A pooled proportion of 35% of pheochromocytomas met washout criteria of adenomas in a meta-analysis, leading to misdiagnosis [12]. Additionally, the delayed scan increases radiation dose and medical cost for patients with adrenal incidentalomas found on biphasic contrast-enhanced CT scan, which further limit its clinical application. In our study, we investigated the value of biphasic contrast-enhanced CT in differentiation of lipid-poor adenomas from pheochromocytomas and found that lesion size, CTA, CTV, ERV, and the presence of cystic degeneration were risk factors for identifying pheochromocytomas. A diagnostic scoring model combining these risk factors showed good diagnostic performance for differentiation of lipid-poor adenomas from pheochromocytomas, with AUC of 0.911.
Tumor size has been considered as a vital factor for characterization of adrenal masses. In our study, lesion size was an independent factor to identify pheochromocytomas. Compared with lipid-poor adenomas, pheochromocytomas were larger, in line with previous studies [16, 20]. A cutoff value of > 29 mm showed a sensitivity of 71.1% and a specificity of 71.9% for the diagnosis of pheochromocytomas. However, there were some overlaps in size between lipid-poor adenomas and pheochromocytomas. Additionally, cystic degeneration within tumor was another important factor for differentiation of lipid-poor adenomas from pheochromocytomas. The frequency of cystic degeneration was more common in pheochromocytomas than in lipid-poor adenomas [20, 21].
Attenuation on CT provides important values for characterization of adrenal masses. Compared with lipid-poor adenomas, pheochromocytomas showed significantly higher attenuation values on unenhanced phase in our study, as well as higher enhancement level on arterial and venous phase, in line with the hypervascular nature of pheochromocytomas. The results were consistent with prior studies [14,15,16]. Northcutt et al. [14] suggested arterial enhancement level greater than 110 HU prompted the diagnosis of pheochromocytomas rather than adenomas. Mohammed et al. [16] found venous enhancement level provided additional values for identification of pheochromocytomas, especially those that demonstrated adenoma-like washout features. A cutoff value of 85 HU on venous phase afforded a sensitivity of 88.2% and a specificity of 83.7% for the diagnosis of pheochromocytomas. However, in our study, 32 of 97 (33.0%) pheochromocytomas and 7 of 132 (6.3%) lipid-poor adenomas enhanced greater than 110 HU on arterial phase, and 62.9% (61/97) of pheochromocytomas and 37.9% of lipid-poor adenomas enhanced greater than 85 HU on venous phase. Moreover, the diagnostic performances of CTA and CTV in our study were moderate. When the cutoff values were set at 81 HU and 97 HU for CTA and CTV, respectively, it yielded a sensitivity of 53.6% and 51.5% and a specificity of 78.0% and 81.1% for the diagnosis of pheochromocytomas. The contradiction between studies may result from the differences of study cohort. The sample size of previous studies was small, especially for pheochromocytomas [14,15,16]. Furthermore, more than 50% of adenomas in previous studies were lipid-rich [14,15,16]. Studies revealed lipid-rich adenomas had lower enhancement level on arterial and venous phases compared with lipid-poor adenomas [14, 22].
Enhancement ratio on arterial and venous phase reflects wash-in characteristics of adrenal masses. In our study, enhancement ratio on arterial and venous phases of pheochromocytomas was significantly lower than that of lipid-poor adenomas. Multivariate analysis showed that enhancement ratio on venous phase was an independent risk factor for the diagnosis of pheochromocytomas (OR: 20.23; 95% CI: 6.3–64.87). Although wash-in characteristics in adrenal masses have been explored in previous studies [8, 17, 23], few studies investigated the difference of wash-in characteristics between adenomas and pheochromocytomas. Foti et al. [23] found that there was no significant difference of enhancement ratio on venous phase between adenomas and metastases. In the study of Goroshi et al. [17], enhancement ratio on venous phase of pheochromocytomas was significantly higher than that of other non-benign adrenal masses (cortical carcinomas, primary lymphomas, and metastases). Further studies are needed to focus on the differences of wash-in characteristics in adrenal masses, especially between adenomas and pheochromocytomas.
In our study, 68.2% of lipid-poor adenomas showed higher enhancement level on venous phase, in line with previous studies [14, 23]. However, there were some discrepancies on peak enhancement phase of pheochromocytomas and the difference of peak enhancement phase between adenomas and pheochromocytomas. Northcutt et al. [14] found that there was significant difference on enhancement pattern between pheochromocytomas and adenomas. No adenomas enhanced greatly on arterial phase, while 25% of pheochromocytomas (3/12) did [14]. In the study of Goroshi et al. [17], 75.8% of pheochromocytomas (25/33) showed higher enhancement level on arterial phase. However, the sample size of their studies was relatively small. In condition of the same methods for the evaluation of peak enhancement phase with previous studies [14, 17], our study found that 59.8% of pheochromocytomas (58/97) showed higher enhancement level on venous phase, and 24.7% (24/97) of pheochromocytomas and 17.4% (23/132) of lipid-poor adenomas showed peak enhancement level on arterial phase. No difference was found on peak enhancement phase between adenomas and pheochromocytomas on biphasic contrast-enhanced CT.
Previous studies solely focused on individual CT imaging features for the differentiation of adenomas from pheochromocytomas. However, an accurate diagnosis should based on multiple imaging features. The strength of our study was identification of independent risk factors using multivariate analysis with relatively large sample and establishment of a diagnostic scoring model combining these risk factors according to the OR. The diagnostic scoring mode showed good performance for the differentiation of lipid-poor adenomas from pheochromocytomas with an AUC of 0.911, which was higher than that of every individual variables.
There were several limitations in our study. First, the study was a retrospective analysis and thus there may be some selection bias. However, we have included a consecutive series of patients meeting inclusion criteria in our study. Second, a variety of CT scanners were used due to the retrospective nature of the study. The amount and injection rate of contrast material, cardiovascular status of patients may impact the enhancement level. However, the abdominal aortic enhancement level on the arterial phase between lipid-poor adenomas and pheochromocytomas was comparable. Besides, it could be regarded as the strength of our study because it accorded with reality in work practice and offered some potential generalizability. Third, we did not calculate washout characteristics due to lack of delayed phase.
In conclusion, biphasic contrast-enhanced CT showed good diagnostic performance in differentiation of lipid-poor adenomas from pheochromocytomas. Lesion size, CTA, CTV, ERV, and cystic degeneration were independent risk factors for the differentiation of lipid-poor adenomas from pheochromocytomas. A diagnostic scoring model combining these risk factors showed an AUC of 0.911.
References
Young WJ. (2007) Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 356:601-610. https://doi.org/10.1056/NEJMcp065470
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. (2016) Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 175:G1-G34. https://doi.org/10.1530/EJE-16-0467
Lam AK. (2017) Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol 28:213-227. https://doi.org/10.1007/s12022-017-9484-5
Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, et al. (2009) Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 161:355-361. https://doi.org/10.1530/EJE-09-0384
Yu R, Pitts A, Wei M. (2012) Small pheochromocytomas: significance, diagnosis, and outcome. J Clin Hypertens (Greewich) 14:307-315. https://doi.org/10.1111/j.1751-7176.2012.00604.x
Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. (1998) Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 171:201-204. https://doi.org/10.2214/ajr.171.1.9648789
Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. (2002) Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 222:629-633. https://doi.org/10.1148/radiol.2223010766
Kamiyama T, Fukukura Y, Yoneyama T, Takumi K, Nakajo M. (2009) Distinguishing adrenal adenomas from nonadenomas: combined use of diagnostic parameters of unenhanced and short 5-minute dynamic enhanced CT protocol. Radiology 250:474-481. https://doi.org/10.1148/radiol.2502080302
Schieda N, Alrashed A, Flood TA, Samji K, Shabana W, McInnes MD. (2016) Comparison of quantitative MRI and CT washout analysis for differentiation of adrenal pheochromocytoma from adrenal adenoma. AJR Am J Roentgenol 206:1141-1148. https://doi.org/10.2214/AJR.15.15318
Liu T, Sun H, Zhang H, Duan J, Hu Y, Xie S. (2019) Distinguishing adrenal adenomas from non-adenomas with multidetector CT: evaluation of percentage washout values at a short time delay triphasic enhanced CT. Br J Radiol 92:20180429. https://doi.org/10.1259/bjr.20180429
Patel J, Davenport MS, Cohan RH, Caoili EM. (2013) Can established CT attenuation and washout criteria for adrenal adenoma accurately exclude pheochromocytoma? AJR Am J Roentgenol 201:122-127. https://doi.org/10.2214/AJR.12.9620
Woo S, Suh CH, Kim SY, Cho JY, Kim SH. (2018) Pheochromocytoma as a frequent false-positive in adrenal washout CT: A systematic review and meta-analysis. Eur Radiol 28:1027-1036. https://doi.org/10.1007/s00330-017-5076-5
Altinmakas E, Perrier ND, Grubbs EG, Lee JE, Prieto VG, Ng CS. (2020) Diagnostic performance of adrenal CT in the differentiation of adenoma and pheochromocytoma. Acta Radiol 61:1080-1086. https://doi.org/10.1177/0284185119889568
Northcutt BG, Raman SP, Long C, Oshmyansky AR, Siegelman SS, Fishman EK, et al. (2013) MDCT of adrenal masses: Can dual-phase enhancement patterns be used to differentiate adenoma and pheochromocytoma? AJR Am J Roentgenol 201:834-839. https://doi.org/10.2214/AJR.12.9753
Northcutt BG, Trakhtenbroit MA, Gomez EN, Fishman EK, Johnson PT. (2016) Adrenal adenoma and pheochromocytoma: comparison of multidetector CT venous enhancement levels and washout characteristics. J Comput Assist Tomogr 40:194-200. https://doi.org/10.1097/RCT.0000000000000343
Mohammed MF, ElBanna KY, Ferguson D, Harris A, Khosa F. (2018) Pheochromocytomas versus adenoma: role of venous phase CT enhancement. AJR Am J Roentgenol 210:1073-1078. https://doi.org/10.2214/AJR.17.18472
Goroshi M, Jadhav SS, Sarathi V, Lila AR, Patil VA, Shah R, et al. (2019) Radiological differentiation of phaeochromocytoma from other malignant adrenal masses: importance of wash-in characteristics on multiphase CECT. Endocr Connect 8:898-905. https://doi.org/10.1530/EC-19-0198
Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, et al. (2017) Management of incidental adrenal masses: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 14:1038-1044. https://doi.org/10.1016/j.jacr.2017.05.001
Glazer DI, Mayo-Smith WW. (2020) Management of incidental adrenal masses: an update. Abdom Radiol (NY) 45:892-900. https://doi.org/10.1007/s00261-019-02149-2
Jun JH, Ahn HJ, Lee SM, Kim JA, Park BK, Kim JS, et al. (2015) Is preoperative biochemical testing for pheochromocytoma necessary for all adrenal incidentalomas? Medicine (Baltimore) 94:e1948. https://doi.org/10.1097/MD.0000000000001948
Kang S, Oh YL, Park SY. (2020) Distinguishing pheochromocytoma from adrenal adenoma by using modified computed tomography criteria. Abdom Radiol (NY). https://doi.org/10.1007/s00261-020-02764-4
Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. (2000) Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol 175:1411-1415. https://doi.org/10.2214/ajr.175.5.1751411
Foti G, Faccioli N, Manfredi R, Mantovani W, Mucelli RP. (2010) Evaluation of relative wash-in ratio of adrenal lesions at early biphasic CT. AJR Am J Roentgenol 194:1484-1491. https://doi.org/10.2214/AJR.09.3636
Funding
This research was supported by Zhejiang Province Public Welfare Technology Application Research Project (NO. LGF21H030004) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (NO. 2018KY410).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The study was approved by the Institutional Review Board, and informed consent was waived due to retrospective analysis of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
An, Yy., Yang, GZ., Lin, B. et al. Differentiation of lipid-poor adenoma from pheochromocytoma on biphasic contrast-enhanced CT. Abdom Radiol 46, 4353–4361 (2021). https://doi.org/10.1007/s00261-021-03121-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03121-9