Abstract
Purpose
To determine the long-term natural history of size change in SBRT-treated HCC to identify an imaging biomarker to help assess treatment response.
Methods
This was a retrospective cohort study of consecutive HCCs treated with SBRT from January 2008 to December 2016 with either 2 years post-treatment MRI follow-up or post-treatment resection histology. Size, major features for HCC, and mRECIST and LI-RADS v.2018 treatment response criteria were assessed at each post-treatment MRI. Local progression, distant progression, and survival were modeled with Kaplan Meier analyses.
Results
56 HCCs met inclusion criteria. Mean baseline HCC diameter was 30 mm (range: 9–105 mm). At 3 months, 76% (N = 43) of treated HCCs decreased in size (mean reduction: 8 mm, range: 5–99 mm) and 0% (N = 0) increased in size. By 24 months, 11% (N = 5) had increased in size and were considered local progression. APHE remained in 77% (43/56) at 3 months, 38% (19/50) at 12 months, and 23% (11/47) at 24 months. mRECIST-defined viable disease was observed in 77% (43/56) at 3 months and 20% (9/47) at 24 months. LI-RADS v.2018 criteria identified viable or equivocal disease in 0% at 3 months and 10% (5/47) at 24 months.
Conclusion
Gradual loss of APHE and slow decrease in size are normal findings in HCCs treated with SBRT, and persistent APHE does not indicate viable disease. mRECIST is not accurate in the assessment of HCC after SBRT due to an overreliance on APHE to define viable disease. Increasing mass size or new nodular APHE at the treatment site may indicate local progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver [1]. Despite recent advances in therapy, 5-year survival rates remain low (< 10%– ~ 50%).[2, 3]. There are multiple treatment options for HCC, and treatment selection depends on tumor stage, liver function, and patient and provider preference. Since biopsy is not standard of care before or after treatment for most HCCs, as endorsed by the AASLD [2], radiologists must understand post-treatment imaging features suggesting viable disease in order to effectively manage these patients.
All imaging-based post-treatment tumor response assessment algorithms (i.e., European Association for the Study of Liver Disease (EASL) [4], Modified Response Evaluation Criteria in Solid Tumors (mRECIST) [5], Liver Reporting and Data System (LI-RADS) treatment response algorithm (TRA) v.2018 [6]) utilize enhancement as a key imaging biomarker indicating viable disease. Unfortunately, residual enhancement does not predict response in HCCs treated with stereotactic body radiation therapy (SBRT) because the majority of successfully treated HCCs will exhibit residual arterial phase hyperenhancement (APHE) for 3 months or more [7,8,9,10,11,12,13,14,15,16] Thus, radiological criteria designed for assessing response to ablation or transarterial chemoembolization (TACE) (i.e., EASL [4] and mRECIST [5]) do not accurately determine local response to SBRT, particularly in the early post-treatment period.
One advantage of the LI-RADS TRA is that if residual APHE in an HCC treated with SBRT is considered “treatment-specific expected enhancement pattern,” it could possibly be classified as non-viable [6]. However, there is lack of radiology–pathology correlation data confirming true pathologic non-viability which would indicate effective treatment. Validated imaging-based biomarkers are therefore needed to determine response post-SBRT because the presence or absence of residual APHE is not reliable for treatment response assessment in these patients [8, 9]. One alternative might be to couple APHE with size of the treated mass. HCC doubling time in a cirrhotic liver has been reported to be 86–204 days when untreated and 21–412 days (mean: 73 days) following locoregional therapy [17,18,19,20,21,22,23,24,25]. These data might be useful when determining response following SBRT. If an SBRT-treated HCC has residual APHE but is unchanged or decreasing in size, it may indicate effective local treatment [8, 9].
The purpose of this study was to determine the long-term natural history of size change in SBRT-treated HCC. These data would inform use of post-treatment size as a potential imaging biomarker to predict non-viable HCC. Secondary aims were to (a) corroborate prior studies describing the expected MR imaging appearance of HCC treated with SBRT in the cirrhotic liver [8, 9] and (b) assess the performance of mRECIST [5] and LI-RADS TRA [6] to predict viability of HCC after SBRT.
Materials and methods
Institutional review board approval was obtained and informed consent waived for this Health Insurance Portability and Accountability Act compliant retrospective cohort study.
Subjects
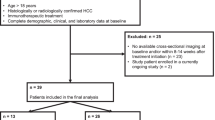
All patients (n = 183) undergoing SBRT for HCC between 1/1/2008 and 12/31/2016 were identified retrospectively using an IRB-approved prospectively maintained institutional SBRT registry. The following inclusion criteria were used: (1) hepatocellular carcinoma based on LI-RADS 5 imaging criteria [6] or biopsy-proven HCC, (2) hepatocellular carcinoma treated with SBRT, (3) multiphasic MRI performed within 3 months prior to initiation of SBRT, (4) at least one multiphasic MRI performed within 3 months following completion of SBRT, (5) ≥ 2 years follow-up MRI or explant histology post-SBRT, and (6) cirrhosis determined by imaging or biopsy. Exclusion criteria included use of locoregional therapy (e.g., TACE, ablation, Y-90 radioembolization) to the SBRT-treated HCC within 3 months of SBRT or systemic therapy prior to SBRT or at any time point during follow-up. This was done to ensure the imaging findings were likely a result of SBRT. SBRT of viable disease following locoregional therapy was allowed as long as the previous therapy was > 3 months prior to initiating SBRT.
Forty-five patients with 56 HCCs met inclusion and exclusion criteria (Fig. 1). All pre-treatment HCCs were classified as definite HCC by Organ Procurement and Transplantation Network imaging criteria (i.e., OPTN 5) [26], LI-RADS v2018 criteria (i.e., LI-RADS 5) [6] or by biopsy.
Imaging
All patients underwent multiphasic contrast-enhanced MRI within 3 months prior to SBRT. MRI was performed on a 1.5- or 3.0-T magnet using a 16- or 32-channel phased array coil and included the following sequences: axial and coronal T2-weighted single-shot fast spin echo (FSE), axial T1-weighted dual echo gradient recalled echo (GRE), axial T2-weighted respiratory-triggered FSE with fat saturation, axial T1-weighted spoiled GRE with fat saturation pre-contrast and dynamically post-contrast (i.e., arterial [20–30 s], venous [60–90 s], and late dynamic [120–150 s]) images. Only some patient had axial diffusion-weighted imaging (DWI) with b-values of 0 and 800 s/mm2 secondary to the retrospective nature of the study. All subjects received a weight-based dose of gadobenate dimeglumine (0.2 mmol/kg, maximum dose 20 mL) that was power-injected at 2 mL/sec followed by a 20 mL saline chaser injected at 2 mL/s.
Stereotactic body radiation therapy procedure
The decision to use SBRT was made by a weekly multidisciplinary hepatobiliary tumor board that consisted of diagnostic radiologists, interventional radiologists, hepatologists, medical oncologists, hepatic surgeons, and radiation oncologists. Generally, SBRT was considered for masses that were difficult to access percutaneously and for patients with contraindications to other forms of locoregional therapy. A controlled breath-hold technique was used, and for patients who could not suspend respiration, a 4D-CT was acquired to generate an internal target volume (ITV) and account for tumor excursion. CT and MRI images were co-registered for delineation of gross tumor volume (GTV) by the radiation oncologist for treatment planning. Expansion of the GTV to the planning target volume (PTV) for patients able to breath-hold was 5 mm in the axial dimension and 8 mm in the craniocaudal dimension. Patients were treated to 21–60 Gy dose in 3–5 fractions.
Retrospective image review
Imaging interpretation was performed on a picture archiving and communications system workstation (McKesson, Richmond BC) in consensus by two board-certified abdominal fellowship-trained radiologists with 9 and 3 years of experience in liver imaging. The goal was to establish the natural history of imaging findings post-SBRT and not individual radiologist diagnostic accuracy. Therefore, consensus reads were used rather than independent reads. Both radiologists were aware of the diagnosis of HCC and that the HCCs were treated with SBRT, but were blinded to the imaging reports.
The following details were recorded for each HCC on the pre-treatment and post-treatment imaging examinations: HCC location; maximum diameter (on pre-treatment studies measured on T1-weighted pre-contrast or delayed-phase post-contrast sequences and not on the arterial phase sequence [done per LI-RADS v.2018 guidelines to avoid overestimating tumor size from shunting related to neovascularity]; post-treatment HCC was measured on arterial phase sequence because tumor response criteria use size of APHE as the biomarker for viability); relative signal intensity of the HCC to untreated background parenchyma on T2-weighted and diffusion-weighted imaging; dynamic post-contrast imaging findings including presence of APHE, “washout,” and “capsule” [6].
Persistent APHE of the treated tumor was distinguished from off-target parenchymal APHE by closely comparing the location and size of the pre-treatment MRI to the post-treatment MRI, and by correlating with information from other pulse sequences. Pre-treatment HCCs were assessed by OPTN and LI-RADS v.2018 for the major features of HCC [6, 26], while lesion-level response using mRECIST (complete response [CR], partial response [PR], progressive disease [PD], stable disease [SD]) [5] and LI-RADS TRA (viable [LR-TR-viable], equivocal [LR-TR-equivocal], non-viable [LR-TR-non-viable]) [6] were recorded at each post-treatment time point following SBRT. In its current form, LI-RADS v2018 suggests treatment response assessment of HCC after SBRT as LR TR Equivocal [6]. However, one key defining criteria for LR TR Non-viable is “treatment-specific expected enhancement pattern.” Lack of data has been a limiting factor in understanding the natural evolution of imaging findings after SBRT. Thus, in this study, we use LR TR Non-viable for TRA after SBRT, even in a lesion with persistent APHE, albeit no increase in size.
Data analysis
Demographic data (age, sex, reason for cirrhosis, Child–Pugh score) were summarized with counts and percentages. Tumor size (mm), change in tumor size (mm) at follow-up, and total SBRT dose (Gy) were summarized with means and ranges. Qualitative features for HCC such as LI-RADS v.2018 score, OPTN score, relative signal intensity on diffusion-weighted imaging, APHE, “washout,” “capsule,” and tumor response (mRECIST, LI-RADS TRA) were summarized at the lesion level pre- and post-treatment. All time-to-event outcomes were calculated from the start of treatment with censoring at the date of last clinical follow-up. Time to local progression was summarized at the lesion level using the Kaplan–Meier method and associated 95% confidence intervals were calculated. Overall survival and time to progression elsewhere in the liver were summarized at the patient level. The analysis was conducted in R version 3.4.2.
Results
Study population
Details of the study population are provided in Table 1 and Figs. 2, 3. Mean patient age was 65 years (22–90 years). Most patients (53% [29/56]) had a Child Pugh score of ‘5.’ The majority of HCCs were greater than 2 cm (Fig. 2). Forty-four of 56 HCCs (79%) were LR-5; the rest were biopsy-confirmed HCC (N = 4), LR-tumor-in-vein (N = 2), or LR-TR-Viable (viable HCC in a prior treatment zone, N = 6) (Fig. 3). Seven (13%) had resection or explant histology after SBRT and 49 (87%) had at least 2 years of MRI follow-up.
Distribution of included hepatocellular carcinomas (HCC; N = 56) by OPTN (Organ Procurement and Transplantation Network) class. Non-OPTN class 5 (hepatocellular carcinoma by LI-RADS v.2018 or by biopsy), OPTN 5a (hepatocellular carcinoma 1.0–1.9 cm), OPTN 5b (hepatocellular carcinoma 2.0–5.0 cm), OPTN 5x (hepatocellular carcinoma greater than 5 cm or tumor in vein), OPTN 5a-g (hepatocellular carcinoma with threshold growth), OPTN 5T (treated hepatocellular carcinoma)
Change in size following SBRT
Mean baseline HCC diameter was 30 mm (range: 9–105 mm) (Table 1). At 3 months, 80% (N = 45) of treated HCCs decreased in size (mean reduction: 8 mm, range: 5–99 mm), 16% (N = 9) were unchanged in size, 0% (N = 0) increased in size, and 4% (N = 2) were not measurable because of insufficient tumor-to-background contrast. By 24 months, only 11% (N = 5) had increased in size (Table 2, Fig. 4). Change in size over time of every treated HCC in the cohort is shown in Fig. 4.
Arterial phase hyperenhancement following SBRT
At baseline, nearly all (96% [54/56]) HCCs had APHE (Tables 2 and 3). Of the two without baseline APHE, one was a hypoenhancing LR-M that was biopsy-proven HCC and the other was an enlarging LR-TR-Viable HCC. Enhancement of the treated HCC steadily declined at 3 months (77% [43/56]), 12 months (38% [19/50]), 24 months (23% [11/47]), and > 24 months (7% [1/15]) (Table 3, Fig. 5). Median time to loss of APHE was 7.6 months (95% CI: 7.5–18.0 months) (Fig. 6). Non-enhancement was observed in 23% (13/56) at 3 months and 77% (36/47) at 24 months. Change in APHE over time is shown in Fig. 5.
A 73-year-old male with HCC. a 10.8 cm HCC with APHE (arrow) and “washout” (not shown) pre-treatment. After SBRT there is gradual decrease in size over time, with persistent APHE and increasing central necrosis (*). The treated tumor measures 9.8 cm at 3 months (b), 9.0 cm at 12 months (c), 8.6 cm at 36 months (d), and 5.2 cm at 60 months (e). At all times points, this lesion would be considered Partial Response based on mRECIST. However, if APHE is considered a ‘treatment-specific enhancement,’ this tumor could be classified as LR TR Equivocal and eventually Non-viable
Washout, capsule, and impeded diffusion following SBRT
At baseline, “washout” (79% [44/56]), “capsule” (71% [50/56]), and impeded diffusion (68% [36/56]) were common. Over time, these features progressively resolved (Table 3). By 24 months, they were uncommon (“washout”: 4% [2/47], “capsule”: 2% [1/47], impeded diffusion: 8% [4/47]) (Table 3).
Differences in treatment response criteria following SBRT
mRECIST criteria commonly differed from LI-RADS TRA criteria (Table 2) because increase in size post-SBRT was uncommon and APHE can be considered by LI-RADS TRA but not mRECIST criteria to be a “treatment-specific expected enhancement pattern.” mRECIST-defined viable disease was observed in 77% (3 months [43/56]), 36% (12 months [18/50]), and 20% (24 months [9/47]). However, when using LI-RADS v.2018 criteria, viable or equivocal disease was only observed in 0% (3 months [0/56]), 4% (12 months [2/50]), and 10% (24 months [5/47]) (Fig. 4). Of the 2 HCCs considered LR-TR-equivocal at 12 months, both were deemed LR-TR-viable at 15 months and 22 months based on increased size and new APHE (Fig. 6).
Fifteen treated HCCs had MRI follow-up for at least 4 years. All 15 decreased in size; 93% (14/15) eventually became non-enhancing and 7% (1/15) had residual APHE for the entire length of follow-up.
Metastasis and mortality following SBRT
Median overall survival was 5.7 years (Fig. 5). Eight (14%) demonstrated local progression between 12 and 31 months post-SBRT; 5 increased in size and all 8 demonstrated new or increased APHE (Fig. 5). Thirteen patients (28%) developed progression elsewhere within the liver (median: 3.4 years post-SBRT; range: 1–4 years) (Fig. 5). No patient developed extrahepatic metastasis.
Explant pathology
Seven HCCs (all with APHE) had post-SBRT histology, all greater than 12 months after SBRT (range 12–29 months post-SBRT). Six were unchanged or decreased in size and had persistent APHE that had not resolved from baseline: all had 100% necrosis at histology. One converted from non-enhancement to new APHE prior to explant and was classified LR-TR-Viable; viable tumor was confirmed at explant.
Discussion
We have shown the history of HCC treated with SBRT is gradual loss of APHE, loss of “washout” and “capsule,” loss of impeded diffusion, and decrease in size over a 1- to 2-year period. It is rare for HCC to become non-enhancing or lose APHE at 3 months post-SBRT. It is common for SBRT-treated HCCs to remain visible and retain APHE at 2 years. Persistent APHE is not a specific indicator of viable disease when treated with SBRT. HCCs treated with SBRT with residual APHE that are unchanged or decreasing in size on follow-up imaging, and are subsequently explanted, often are non-viable at histology. Use of mRECIST or EASL criteria to determine local treatment response soon after SBRT (both of which rely on detection of APHE) will be inaccurate because residual APHE is common in successfully treated HCCs. Thus, LI-RADS TRA criteria maybe preferred since residual APHE can be considered a “treatment-specific expected enhancement pattern.” However, as mentioned above, in its current version, LI-RADS TRA suggests the use of LR TR Equivocal for HCC treated with SBRT which demonstrates persistent APHE. In this study, we show that if residual APHE is considered an expected imaging finding in HCC treated with SBRT, without interval growth, then use of LR TR Non-viable category predicts treatment response assessment accurately. Several years after treatment, LI-RADS TRA and mRECIST may demonstrate similar accuracy [12, 27, 28]. Until new imaging-based biomarkers can be developed that adequately differentiate successful early treatment response post-SBRT, interpretation of SBRT-treated HCCs will require serial follow-up and knowledge of the natural history. New or increasing intensity of APHE or increase in size of a treated HCC are each potential indicators of local treatment failure.
Accumulating evidence indicates SBRT likely has good local treatment efficacy for intrahepatic HCC, a good safety profile, and—like other forms of local therapy (e.g., ablation, TACE)—may act as a bridge for hepatic transplantation [7, 12, 29]. Assessment of treatment response to SBRT is challenging due to counterintuitive imaging findings in successfully treated masses (i.e., residual APHE, lack of non-enhancement, persistent visibility) and adjacent parenchymal reaction related to off-target radiation. Insufficient radiology–pathology correlation, lack of head-to-head comparison trials between SBRT and conventional ablative therapy, relative novelty of SBRT, and (potentially overstated) fears of hepatic toxicity are challenges for widespread acceptance of SBRT into HCC treatment algorithms [7, 12, 29].
Early studies suggesting that persistent APHE may be an expected finding in a successfully treated HCC post-SBRT are confirmed in our study [8,9,10,11,12,13,14]. We have shown that APHE is commonly seen for up to 2 years. Rather than simple detection of residual APHE, radiologists should consider new nodular APHE, increasing intensity of APHE within the treated mass, or an increase in size to indicate potential local treatment failure. In our study, the large majority of HCCs progressively decreased in size over 2+ years of follow-up. Given that the HCC doubling time after other forms of locoregional therapy reportedly ranges from 21 to 412 days (mean: 73 days) [17,18,19,20,21,22,23,24,25], a follow-up period of 2 years should be sufficient to determine whether local progression has occurred. Unfortunately, excellent local control from SBRT as suggested in our study does not necessarily prevent progression elsewhere in the liver. We found that progression elsewhere in the liver was much more common than progression at the treatment site, and median overall survival in our cohort was only 5.7 years.
Our study has several limitations. First, there is a relatively small sample size. Second, it was retrospective, thus subject to selection bias, and lacked a reference standard for treatment efficacy for most of the treated masses. For masses lacking a histologic reference standard, lack of growth over 2+ years was used as an indirect sign of absent local progression. In SBRT-treated HCC, where persistent APHE is a common and expected imaging finding, histologic confirmation would be of added value to confirm the lack of viable tissue. Future studies with radiology–pathology correlation are needed. Additional limitations given the retrospective nature include different imaging follow-up times, not all lesions had DWI sequences, and history of prior locoregional therapy, albeit greater than 3 months prior to SBRT could result in altered vascularity of the lesions. Furthermore, this study focuses exclusively on treatment response post-SBRT using MRI. Although it is predicted that CT imaging findings should be interpreted similarly, scientific confirmation of that hypothesis will be important. Finally, mRECIST criteria are designed to be used at the patient level, while LI-RADS TRA criteria are designed for use at the lesion level. For practical purposes they were both applied at the lesion level in our study.
In conclusion, SBRT is an effective option with excellent local control for treatment of primary HCC. Persistent APHE is a common finding for up to 2 years. Therefore, lack of growth of a treated mass (and not absent APHE) may be the best indicator of successful treatment. Increasing size or new nodular APHE at the treatment site may indicate local progression. mRECIST and EASL criteria should not be used to determine local treatment response following SBRT because they are overly reliant on APHE. LI-RADS TRA is likely more accurate following SBRT and should be used instead.
References
Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clinc Gastroenterol, 2014; 28:753-770.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology, 2011; 3:1020-1022.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med, 2011; 365:1118-1127.
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. J Hepatol 2001; 35:421-430.
Lencioni R, Llovet JM. Modified RECIST (mRECIST assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30:52-60.
Kielar A, Fowler K, Lewis S, Yaghmai V, Miller F, Yarmohammadi H, Kim C, Chernyak V, Yokoo T, Meyer J, Newton I, Do R. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdominal Radiology, 2018; 43 (1): 218-230.
Oldrini G, Huertas A, Renard-Oldrini S, Taste-George H, Guillaume V, Laurent V, Salleron J, Henrot P. Tumor response assessment by MRI following stereotactic body radiotherapy for hepatocellular carcinoma. Plos One, 2017; e01766118
Mendiratta-Lala M, Masch W, Shankar PR, Hartman HE, Davenport MS, Schipper MJ, Maurino C, Cuneo KC, Lawrence TS, Owen D. Magnetic resonance imaging evaluation of hepatocellular carcinoma treated with stereotactic body radiation therapy: long term imaging follow-up. Int J Radiat Oncol Biol Phys, 2019; 103(1):169-179.
Mendiratta-Lala M, Gu E, Owen D, Cuneo KC, Bazzi L, Lawrence TS, Hussain H, Davenport MS. Imaging findings within the first 12 months of hepatocellular carcinoma treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys, 2018; 102(4):1063-1069.
Herfarth KK, Hof H, Bahner ML, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003;57:444-51.
Yip C, Cook GJR, Owczarczyk K, Goh V. Challenges in imaging assessment following liver stereotactic body radiotherapy: pitfalls to avoid in clinical practice. Chinese Clinical Oncology, 2017; 6(2):S11.
Sanuki, N, Takeda, A, Mizuno, T. Tumor response on CT following hypofractionated stereotactic ablative body radiotherapy for small hypervascular hepatocellular carcinoma with cirrhosis. AJR Am J Roentgenol. 2013;201(6):W812–W820.
Park, MJ, Kim, SY, Yoon, SM. Stereotactic body radiotherapy-induced arterial hypervascularity of non-tumorous hepatic parenchyma in patients with hepatocellular carcinoma: potential pitfalls in tumor response evaluation on multiphase computed tomography. PLoS One. 2014;9(2):e90327.
Kimura, T, Takahashi, S, Takahashi, I. The time course of dynamic computed tomographic appearance of radiation injury to the cirrhotic liver following stereotactic body radiation therapy for hepatocellular carcinoma. PLos One. 2015;10(6):e0125231.
Yang JF, Lo CH. Is stereotactic body radiotherapy better than radiofrequency ablation for the treatment of hepatocellular carcinoma? J Clin Oncol 2016; 34 (23): 3797-3798.
Price TR, Perkins SM, Sandrasegaran K, et al. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer, 2012; 118:3191-3198.
Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology, 1985; 89:259-266.
Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Digestive Diseases and Sciences, 2003; 48(3):581-586.
An C, Choi YA, Choi D, Paik YH, et al. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clinical and molecular hepatology, 2015; 21:279-286.
Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992; 16:132–137.
Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci 2003; 48:581–586.
Okazaki N, Yoshino M, Yoshida T, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time: a preliminary report. Cancer 1989; 63:2207–2210.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Longterm survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002; 235:373–382.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
Tezuka M, Hayashi K, Kubota K, et al. Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig Dis Sci 2007; 52:783–788.
Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS Policy for liver transplant allocation: Standardization of liver imaging, diagnosis, classification and reporting of hepatocellular carcinoma. Radiology 2013; 266:376-382.
Vincenzi, B, Di Maio, M, Silletta, M. Prognostic relevance of objective response according to easl criteria and mrecist criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLos One. 2015;10(7): e0133488.
Mannina, EM, Cardenes, HR, Lasley, FD. Role of stereotactic body radiation therapy before orthotopic liver transplantation: retrospective evaluation of pathologic response and outcomes. Int J Radiat Oncol Biol Phys. 2017;97(5):931–938.
Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol. 2016 Feb 10;34(5):452-9. doi: 10.1200/JCO.2015.61.4925. Epub 2015 Nov 30. PMID: 26628466; PMCID: PMC4872011.
Funding
This study was not funded by a grant; however, a few authors (Mishal Mendiratta-Lala, Dawn Owen, Kyle Cuneo, Theodore S. Lawrence, Matthew J. Schipper) are Co-PI’s on grant: P01 CA59827 (NIH funded).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work, or acquisition, analysis, or interpretation of data; drafted work or revised it critically; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity are appropriately resolved.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Institutional review board approval was obtained and informed consent waived for this Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective cohort study. This research was conducted retrospectively from data obtained for clinical purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mendiratta-Lala, M., Masch, W., Owen, D. et al. Natural history of hepatocellular carcinoma after stereotactic body radiation therapy. Abdom Radiol 45, 3698–3708 (2020). https://doi.org/10.1007/s00261-020-02532-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02532-4