Abstract
Stereotactic body radiation therapy (SBRT) is an emerging locoregional treatment (LRT) modality used in the management of patients with hepatocellular carcinoma (HCC). The decision to treat HCC with LRT is evaluated in a multidisciplinary setting, and the specific LRT chosen depends on the treatment intent, such as bridge-to-transplant, down-staging to transplant, definitive/curative treatment, and/or palliation, as well as underlying patient clinical factors. Accurate assessment of treatment response is necessary in order to guide clinical management in these patients. Patients who undergo LRT need continuous imaging evaluation to assess treatment response and to evaluate for recurrence. Thus, an accurate understanding of expected post-SBRT imaging findings is critical to avoid misinterpreting normal post-treatment changes as local progression or viable tumor. SBRT-treated HCC demonstrates unique imaging findings that differ from HCC treated with other forms of LRT. In particular, SBRT-treated HCC can demonstrate persistent APHE and washout on short-term follow-up imaging. This brief review summarizes current evidence for the use of SBRT for HCC, including patient population, SBRT technique and procedure, tumor response assessment on contrast-enhanced cross-sectional imaging with expected findings, and pitfalls in treatment response evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although the gold standard of care for hepatocellular carcinoma (HCC) is surgical resection, only 15–30% of patients with HCC qualify for surgery. High tumor burden and underlying liver dysfunction [1] often preclude patients from definitive resection [2]. For patients who are not surgical candidates and who have disease confined to the liver, treatment with locoregional therapy (LRT) remains the most effective treatment option. Patients with HCC should be evaluated in a multidisciplinary setting, and LRT is chosen depending on the intent of treatment, which could include bridge-to-transplant, down-staging to transplant, definitive/curative treatment, and/or palliation [3, 4]. Furthermore, treatment choice varies depending on a host of clinical scenarios, including stage (extent of disease), performance status, and underlying liver function. While ablation or liver transplantation are favored in patients with early-stage HCC, locoregional therapies such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and stereotactic body radiation therapy (SBRT) have conventionally been reserved to treat more advanced unresectable tumors [2].
Previous to recent technologic advances, radiation therapy (RT) was not typically used in the treatment of liver tumors due to the lack of daily image guidance, computed tomography (CT)-based/magnetic resonance imaging (MRI)-based radiation planning, and lack of understanding of liver radiation tolerance, which resulted in high doses of radiation to large volumes of adjacent uninvolved hepatic parenchyma [5]. Historically, the resulting dose splash to the liver using 3D conformal radiation caused moderate-to-severe toxicity manifested as compromised liver function post radiotherapy. However, advances in the field of radiation oncology have allowed for refinement of radiation dose delivery and target definition, resulting in the development of stereotactic body radiation therapy (SBRT). SBRT is used for the delivery of high dose radiation in a highly targeted fashion, and with rapid dose drop off farther from the center of the radiation zone [6]. This spares large portions of adjacent liver parenchyma, while simultaneously providing ablative doses to the tumor. SBRT is typically delivered in 3–5 fractions, with a relatively low risk of radiation-induced liver disease (RILD). Numerous clinical trials have found SBRT to be highly effective in providing local control for small HCC tumors [7,8,9], although outcomes vary depending on baseline liver function and data on long-term follow-up are pending [10]. Although SBRT is not included in the current Barcelona Conference (BCLC) guidelines, it is included in the most recent version of the National Comprehensive Cancer Center guidelines for primary liver cancer as a treatment option for unresectable disease or medically inoperable patients [11,12,13].
Patient selection and technique
SBRT can be used as definitive therapy, salvage therapy, or as a bridge to transplantation [3, 4] and important considerations for case selection include the extent of disease, site of the tumor, and history of prior treatments. SBRT has historically been indicated for patients who are not eligible for surgical resection or other forms of LRT. However, with emerging evidence demonstrating good local control and overall survival, there is a trend to increased use of SBRT for treatment of HCC. In general, patient with moderately good liver function are candidates for SBRT [7,8,9] due to a lower risk of hepatic decompensation compared to patients with more advanced cirrhosis. More recent studies suggest that patients with poorer liver function may be treated with SBRT but caution needs to be exercised given that these patients are extremely sensitive to radiation associated liver dysfunction [14, 15]. Tumors that are not amenable to percutaneous ablation, e.g., dome/subdiaphragmatic lesions, lesions directly adjacent to large vessels, or lesions with associated tumor in vein (TIV) can be treated with radiotherapy. The delivery of high doses of radiotherapy can be limited by tumor location next to hollow viscus which are more sensitive to post-radiation complications. In these cases, SBRT in 3–5 fractions may not be ideal but these patients can be treated with hypofractionated regimens which is safer for adjacent bowel or with the use of laparoscopically placed tissue expanders which displace bowel away from the target lesion. Further, in cases where liver tolerance cannot be met with photon radiation, protons may demonstrate superior liver sparing and a much reduced risk of radiation associated liver toxicity [16,17,18,19,20,21].
The key to the safe delivery of liver radiation is predicated on the following: evaluation of the patient’s baseline liver function and extent of disease (recent calculation of Child Pugh score, recent diagnostic imaging and staging), a detailed CT and MR simulation scan for clear delineation of the tumor, respiratory control for minimizing tumor motion, designing a radiation plan that respects known liver tolerances to mitigate the risk of acute and late toxicity, and daily image guidance for precise and accurate alignment of the target [16, 17]. With respect to the first item, as discussed above, baseline liver function directly predicts the risk of hepatic decompensation post liver radiation as does the ratio of tumor burden to that of normal/uninvolved liver parenchyma. Delineation of the gross tumor volume (GTV; Fig. 1), demonstrable visualized tumor extent, requires at least a triple-phase contrast CT and/or an MR liver with contrast. To limit the amount of normal liver parenchyma in the radiation field, it is imperative to consider motion management as the liver typically moves 1–3 cm in the superior/inferior direction with respiration. Treating a moving target with extreme motion requires the treatment of more normal liver. Strategies such as fiducial markers for gated treatment and alignment, abdominal compression, or controlled breath-hold techniques should be utilized to limit liver and tumor motion [17, 22, 23]. The planning target volume (PTV) is a geometric expansion to account for setup variability during treatment and is dependent on the radiation machine, the imaging guidance for treatment, and the immobilization technique used for the patient. For patients who are able to hold their breath consistently in a controlled setting, the planning target volume is expanded from the GTV. In the setting of free breathing treatment where there is motion of the tumor, an internal target volume (ITV) is constructed to reflect the possible positions of the tumor prior to expansion to the PTV. (Fig. 1). Creation of the radiation plan requires knowledge of liver tolerance in patients without liver cirrhosis or dysfunction who may be treated with SBRT for liver metastases. It is critical to understand the implications of the mean liver dose as well as consideration of the low dose splash to the uninvolved liver parenchyma [8, 9, 24, 25]. Finally, the use of daily cone beam CT or MR-linac is required to ensure safe alignment of the lesion prior to delivery of an ablative radiation dose.
Simulation CT scan for liver radiation therapy. a Gross tumor volume (GTV) is outlined in red, and the yellow clip is a fiducial marker placed by interventional radiology for localization at the time of radiation therapy. b Radiation plan. This plan used protons to limit parenchymal dose. Blue edge is the 50% isodose line. c SBRT plan using photons. Dark blue denotes GTV, green denotes internal target volume (ITV), and pink denotes PTV. The loculated fluid density next to the liver is a tissue expander to displace the stomach from the left hepatic lobe for SBRT
Current clinical evidence
There is encouraging evidence from a growing number of studies evaluating the use of SBRT in the treatment of HCC, including well-designed phase II trials [26, 27], to support the safety and feasibility of SBRT for primary HCC. However, since SBRT is not included in the BCLC guidelines, the inclusion criteria across these studies were heterogeneous and SBRT was most commonly evaluated as a primary treatment modality when patients were not appropriate candidates for other LRTs. Furthermore, only few retrospective studies comparing SBRT with other LRTs are available [28,29,30].
Even so, data from multiple phase II trials showed 1-year local tumor control rates with SBRT at 82%–96% and 1-year overall survival rates of 36%–78% [26, 27, 31,32,33]. Moreover, in a large retrospective cohort of 221 HCC patients, acute toxicities of ≥ grade 3, based on the Common Terminology Criteria for Adverse Events V3.0 (CTCAE), were observed in only 24 patients (13%), of which only three complications (1.6%) persisted and the rest eventually recovered to grade 1–2. Grade 5 toxicity with acute liver failure occurred was observed in only two patients [34]. When compared to other LRTs, SBRT appears to have a favorable toxicity profile with at least comparable local tumor control and overall survival rates. A recent retrospective study of 209 patients compared outcomes of TACE and SBRT in patients with 1–2 tumors while adjusting for imbalances in treatment assignment. While there was no difference in overall survival between the two groups, 1-year local control rate was 97% for SBRT and 47% for TACE (P < 0.001), while acute toxicities of ≥ grade 3 occurred in 13% TACE treatments and only 8% of SBRT treatments (P = 0.05) [30]. Thus, SBRT of HCC has a very good safety profile with a small incidence of complications.
Expected imaging findings post SBRT
Treatment response assessment after LRT is necessary in order to evaluate tumor response and to assess for residual viable tumor. As mentioned, many of these patients are being downstaged or bridged to liver transplant, and thus accurate characterization of viable tumor is essential for identifying tumor burden. SBRT has historically been used as last-line therapy for HCC treatment, often in patients who have already undergone other LRTs or are ineligible for surgical resection, and only recently has emerged as first-line treatment. Therefore, there is a paucity of explant/resection data in this cohort, resulting in limited radiology–pathology correlation in SBRT-treated HCC. Thus, the significance of the imaging findings after SBRT are still unknown, although, as mentioned above, outcomes data suggest overall good efficacy after SBRT, with overall survival comparable or better than other forms of LRT, and response rates allowing for bridging and down-staging for transplant. [30, 35,36,37,38]
Imaging features that are used to evaluate for treatment response after LRT for HCC vary depending on the treatment response classification system being used, and can be applied to imaging using multiphasic CT or dynamic post-contrast MRI. These imaging criteria include arterial phase hyperenhancement (APHE), washout (WO) appearance, enhancement similar to pretreatment, and change in size. MRI-specific ancillary features of diffusion restriction and T2-weighted hyperintensity are not strict criteria used for treatment response assessment based on current treatment response classification systems. However, a combination of these imaging characteristics are often used to assess HCC response to SBRT. In the post-SBRT setting, it is critical to understand that imaging characteristics evolve over time and the interval between radiation and time to imaging study must be considered when assessing SBRT response [39]. Furthermore, radiation-induced changes in the parenchyma adjacent to the treated tumor add a layer of complexity when interpreting post-SBRT imaging, as it is often difficult to distinguish between treated tumor, viable tumor, and the surrounding parenchyma.
After LRT, follow-up imaging can be with either contrast-enhanced CT or MRI to evaluate for treatment response. Timing for follow-up after SBRT varies, but in general, should be every 3 months after treatment. Imaging less than 3 months after treatment can be confusing because of the microvascular radiation-induced venoocclusive changes which occur early post treatment resulting in extensive arterial phase hyperenhancement in the entire treatment zone, frequently obscuring evaluation of the treated lesion [40, 41].
Most HCCs effectively treated with SBRT exhibit a slow decrease in size, and thus a measurable change in size between short interval imaging studies is not always appreciable [42]. One study showed a measurable decrease in size of 35% at 3 months, 48% at 9 months, and 54% at 12 months following SBRT. [43] Another report noted that of 67 HCCs treated with SBRT, all either remained unchanged or decreased in size in the first 12 months post treatment (34% unchanged and 66% decreased) with none demonstrating an increase in size during this time [44]. Thus, lesions that are unchanged or decreasing in size post SBRT should be cautiously interpreted for viability, despite enhancement characteristics; these lesions should not be considered viable because they are unchanged in size even if there is persistent enhancement in the early post-treatment period [45]. On the contrary, treated tumor demonstrating increased size post SBRT is highly suggestive of residual or recurrent, viable disease [45].
Enhancement patterns of SBRT-treated HCC also evolve over time. One study noted that 75% of SBRT-treated HCCs can demonstrate persistent APHE 3–6 months after therapy, as well as persistent washout. These features slowly resolve after 6 months, although they can occasionally be seen in the treated lesion 1 year post SBRT (Figs. 2 and 3) [44, 46]. Eventually, most successfully treated tumors are shown to convert to non-enhancement [44, 46]. A study by Sanuki et al. demonstrated that the median time for complete resolution of APHE was 5.9 months (range 1.2–34.2 months) in a cohort of 38 SBRT-treated HCCs [47]. In this same cohort, 76% of SBRT-treated HCCs had persistent enhancement at 3 months, 33% at 6 months, and 29% at 12 months [47]. Another study by Kimura et al., with 55 lesions, showed persistent APHE in 25.3% of SBRT-treated lesions at 3 months and 2% residual APHE at 6 months, with no lesions demonstrating increase in size [48]. Price et al. demonstrate similar findings in a cohort of 26 patients, in which 41% of tumors had persistent APHE at 3 months, 31% at 6 months, 19% at 9 months, 8% at 12 months, with tumors demonstrating progressive decrease in size of the tumor over each time interval [48].
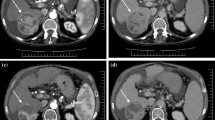
Expected post-treatment changes of HCC treated by SBRT seen on CT within first 4 months. a (i) Axial arterial phase CT from a multiphasic study shows an enhancing observation in segment VII measuring 1.1 cm (arrow). (ii) Delayed phase CT shows an enhancing capsule and central washout of contrast within the observation (arrow). The imaging features are diagnostic of hepatocellular carcinoma. LR 5. b Multiphasic CT was obtained one month post stereotactic body radiation therapy (SBRT). (i) Arterial phase image shows a decrease in size and degree of enhancement of the segment VII hepatocellular carcinoma (arrow). (ii) Delayed phase CT shows enhancement in segment VII similar to background hepatic parenchyma with no washout or capsule. Findings are typical of radiation therapy with no evidence of recurrent tumor. c Multiphasic CT was obtained four months post SBRT. (i) Arterial phase CT shows a geographic area of enhancement (arrow) in the radiation field. (ii) Delayed phase CT shows enhancement in segment VII similar to background hepatic parenchyma with no washout or capsule. Dilated bile ducts (arrow) within the treated segment are compatible with fibrosis. Findings are typical of radiation therapy with no evidence of recurrent tumor
Expected post-treatment changes of HCC treated by SBRT seen on CT within first 12 months. a (i) Axial arterial phase CT from a multiphasic study shows an enhancing observation in segment VII (arrow). (ii) Delayed phase CT shows central washout of contrast within the observation. Imaging characteristics are diagnostic of hepatocellular carcinoma, LR 5 (arrow). b Multiphasic CT was obtained 6 months post SBRT. (i) The hepatocellular carcinoma in segment VII (arrow) appears similar to the pretreatment CT. (ii) The hepatocellular carcinoma in segment VII again shows washout on delayed phase imaging (arrow). The observation is minimally decreased in size from the pretreatment study. LR-TR Equivocal c multiphasic CT was obtained 9 months post SBRT. (i) The hepatocellular carcinoma in segment VII is smaller with less arterial enhancement (arrow) compared to the pretreatment images. (ii) The hepatocellular carcinoma is decreased in size with persistent washout (arrow). LR-TR Equivocal Findings are typical of radiation therapy with no evidence of recurrent tumor. d Multiphasic CT was obtained 12 months post SBRT. (i) The treated segment VII lesion shows no arterial enhancement. LR-TR Non-viable (ii) Progressive, geographic enhancement of the treated liver on delayed phase CT is compatible with fibrosis (arrow). There is no washout. Findings are typical of radiation therapy with no evidence of recurrent tumor
Another study, which included 35 subjects, found APHE with portal or delayed phase washout, T2-weighted tissue hyperintensity, and diffusion-weighted hyperintensity to be key features seen 3 months post SBRT [49]. APHE was present in all patients at baseline, 45.7% at 3 months, 25.7% at 6 months, and 8.6% at 12 months. T2-weighted hyperintensity was present in 74.3% at baseline, 36.9% at 3 months, and 5.1% at 12 months. Restricted diffusion was seen in 62.9% of lesions at baseline, 31.7% at 3 months, and 6.2% at 12 months [49]. Thus, there is ample emerging evidence that the presence of persistent APHE on short-term follow-up is an expected imaging finding post SBRT and that there is continued temporal evolution of APHE after SBRT, although some cases may show persistent internal APHE even at one year post SBRT. Therefore, persistent APHE does not necessarily indicate the presence of clinical significant viable tumor.
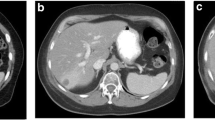
There is limited research identifying imaging features which suggest local recurrence after SBRT. However, it has been reported that an increase in size of a treated lesion or increasing or new nodular APHE within a lesion should raise suspicion for SBRT failure and viable tumor [45] (Fig. 4).
Recurrent HCC seen on MRI following SBRT treatment. a Dynamic contrast-enhanced MR was performed. (i) Axial T1-weighted arterial phase image shows an enhancing observation in segment VII (arrow). (ii) Axial T1-weighted delayed phase image shows an enhancing capsule and central washout of contrast within the observation (arrow). The imaging features are diagnostic of hepatocellular carcinoma, LR 5. (iii) The lesion (arrow) is hyperintense on axial T2-weighted imaging with fat saturation. (iv) The lesion (arrow) restricts diffusion. b Dynamic contrast-enhanced MR was performed 3 months after SBRT to segment VII lesion. (i) Axial T1-weighted arterial phase image shows interval decrease in size of the hepatocellular carcinoma in segment VII (arrow). There is mild peripheral and internal enhancement, a non-specific finding in the setting of recent SBRT. (ii) Axial T1-weighted delayed phase image shows persistent enhancement of the lesion with no washout (arrow). LR-TR Equivocal. Geographic enhancement adjacent to the treated lesion is an expected finding post SBRT. (iii) The lesion remains hyperintense on axial T2-weighted imaging with fat saturation (arrow). (iv) Restricted diffusion (arrow) has decreased compared to the pretreatment exam. C) Dynamic contrast-enhanced MR was performed 9 months after SBRT to segment VII lesion. (i) Axial T1-weighted arterial phase image shows interval increase in size of the treated hepatocellular carcinoma in segment VII (arrow). Peripheral and internal enhancement are similar to increased in intensity. (ii) Axial T1-weighted delayed phase image shows persistent central enhancement of the lesion with no washout (arrow). Geographic enhancement adjacent to the treated lesion is a sequela of SBRT. (iii) The lesion has increased in size and remains hyperintense on axial T2-weighted imaging with fat saturation (arrow). (iv) Restricted diffusion has increased (arrow). Overall, findings are compatible with recurrent hepatocellular carcinoma, LR-TR Viable
The SBRT treatment zone not only includes the targeted tumor, but also a surrounding rim of adjacent hepatic parenchyma (PTV as described above). As a result, the surrounding hepatic parenchyma also demonstrates characteristic imaging features which evolve over time. Early post treatment, there is geographic APHE in off-target parenchyma adjacent to the treated HCC, which has been seen to persist for about 6 months [50]. The histopathological changes have been shown to include hyperemia, small vessel venous congestion, microhemorrhages, venoocclusion, and a possible giant cell reaction, all of which lead to architecture changes [44, 51]. In response to decreased venous inflow, there is increased arterial inflow that manifests on contrast-enhanced imaging as early arterial phase hyperenhancement (APHE) [51]. Differentiating this from tumor progression can sometimes be challenging. Lack of washout or other ancillary features (e.g., T2-weighted or diffusion-weighted hyperintensity) in this part of the liver is helpful to differentiate expected post-radiation perfusional changes from infiltrative tumor [52]. Subsequent cell death, necrosis, and fibrosis contribute to the change in imaging findings from APHE to portal venous and delayed phase hyperenhancement, usually 6 months post SBRT. Additional features seen months after SBRT include overlying capsular retraction and upstream biliary ductal dilatation, as a result of radiation-induced fibrosis.
Pitfalls in treatment response assessment: RECIST, mRECIST, LI-RADS
European Association for the Study of the Liver (EASL), Modified Response Evaluation Criteria in Solid Tumors (mRECIST), and Liver Reporting and Data System (LI-RADS) treatment response algorithm (TRA) v.2018 are several of the algorithms used to gauge tumor response following LRT, all of which use the imaging finding of enhancement as a predictor of residual or recurrent disease [1, 53, 54]. EASL uses bidimensional measurements to evaluate the residual enhancing tumor [55]. mRECIST utilizes the single largest diameter of the enhancing (during arterial phase) tumor component [2]. LI-RADS TRA considers a tumor non-viable (LR-TR non-viable) when there is no appreciable lesion or treatment-specific expected enhancement, viable (LR-TR viable) if there is nodular or mass-like washout or arterial phase enhancement associated with the lesion or if enhancement is similar to pretreatment, and equivocal (LR-TR equivocal) if the pattern of enhancement is atypical for the treatment-specific expected enhancement and does not meet criteria for probably or definitely viable [54, 56].
Since all of these classification systems use APHE as an imaging biomarker for detection of viable disease post treatment, use of these algorithms for treatment response assessment following SBRT could lead to incorrect characterization of tumor as viable since persistent APHE has been shown to be an expected imaging feature in successfully treated HCC with SBRT. Such miscategorization could lead to unnecessary retreatment or negatively impact management, such as disqualifying a patient for liver transplant or unnecessary start of systemic therapy. However, when residual APHE is considered a “treatment-specific expected enhancement pattern” using the LI-RADS TRA criteria, this system could result in a more accurate characterization of these lesions as non-viable [45]. Currently, LI-RADS TRA suggests deeming an SBRT-treated HCC as LR-TR Equivocal if there is persistent APHE early post SBRT, and eventual conversion to LR-TR Non-viable with temporal evolution. Although this may result in an increase in follow-up imaging studies, as well as the potential of leaving viable tumors untreated, the risk is generally mitigated by the slow growth of HCC which typically demonstrates a doubling time of 85.7–117 days [57, 58]. Thus, caution must be taken when using the existing treatment response algorithms for interpretation of HCC treated with SBRT. Importantly, decision-making for management of patients following SBRT should be made by a multidisciplinary tumor board for best patient care.
Conclusion
Post-SBRT imaging generally demonstrates predictable temporal evolution of imaging characteristics. The misinterpretation of these findings has the potential to impact clinical management, including unnecessary additional treatments. It is important for radiologists to understand the expected post-SBRT findings, especially the presence of early post-treatment persistent APHE.
Classification systems such as EASL, mRECIST, and LI-RADS TRA should be cautiously applied when evaluating HCC treated with SBRT. Close collaboration with the referring clinical team is essential in the assessment of equivocal findings, taking into consideration clinical findings and tumor markers. Further studies are needed to assess the clinical utility of current treatment response classification systems related to outcomes data, imaging features such as restricted diffusion and T2 signal in treatment response evaluation, and radiology–pathology data to validate current imaging characteristics.
References
Bruix J, Sherman M, Llovet JM, et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430. https://doi.org/10.1016/s0168-8278(01)00130-1
European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer (2012) EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 55:908–943
Gerum S, Jensen AD, Roeder F (2019) Stereotactic body radiation therapy in patients with hepatocellular carcinoma: A mini-review. World J Gastrointest Oncol 11:367–376. https://doi.org/10.4251/wjgo.v11.i5.367
Zeng Z-C, Seong J, Yoon SM, et al (2017) Consensus on Stereotactic Body Radiation Therapy for Small-Sized Hepatocellular Carcinoma at the 7th Asia-Pacific Primary Liver Cancer Expert Meeting. Liver Cancer 6:264–274. https://doi.org/10.1158/000475668
Tanguturi SK, Wo JY, Zhu AX, et al (2014) Radiation therapy for liver tumors: ready for inclusion in guidelines? The oncologist 19:868
Kellock T, Liang T, Harris A, et al (2018) Stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma: imaging evaluation post treatment. Br J Radiol 91:20170118
Bujold A, Massey CA, Kim JJ, et al (2013) Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J Clin Oncol 31:1631–1639. https://doi.org/10.1200/JCO.2012.44.1658
Lasley FD, Mannina EM, Johnson CS, et al (2015) Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol 5:e443–e449. https://doi.org/10.1016/j.prro.2015.02.007
Cárdenes HR, Price TR, Perkins SM, et al (2010) Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 12:218–225. https://doi.org/10.1007/s12094-010-0492-x
Rim CH, Kim HJ, Seong J (2019) Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother Oncol 131:135–144. https://doi.org/10.1016/j.radonc.2018.12.005
Liu E, Stenmark MH, Schipper MJ, et al (2013) Stereotactic Body Radiation Therapy for Primary and Metastatic Liver Tumors. Transl Oncol 6:442–446
Benson AB, D’Angelica MI, Abbott DE, et al (2017) NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Cancer Netw JNCCN 15:553–563. https://doi.org/10.6004/jnccn.2017.0058
Schaub SK, Hartvigson PE, Lock MI, et al (2018) Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current Trends and Controversies. Technol Cancer Res Treat 17:13033818790217. https://doi.org/10.1177/13033818790217
Jackson WC, Tang M, Maurino C, et al (2021) Individualized Adaptive Radiation Therapy Allows for Safe Treatment of Hepatocellular Carcinoma in Patients With Child-Turcotte-Pugh B Liver Disease. Int J Radiat Oncol 109:212–219. https://doi.org/10.1016/j.ijrobp.2020.08.046
Lee P, Ma Y, Zacharias I, et al (2020) Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma in Patients With Child-Pugh B or C Cirrhosis. Adv Radiat Oncol 5:889–896. https://doi.org/10.1016/j.adro.2020.01.009
Hong TS, Wo JY, Yeap BY, et al (2016) Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol Off J Am Soc Clin Oncol 34:460–468. https://doi.org/10.1200/JCO.2015.64.2710
Crane CH, Koay EJ (2016) Solutions that Enable Ablative Radiotherapy for Large Liver Tumors: Fractionated Dose Painting, Simultaneous Integrated Protection, Motion Management and CT Image Guidance. Cancer 122:1974–1986. https://doi.org/10.1002/cncr.29878
Sanford NN, Pursley J, Noe B, et al (2019) Protons versus Photons for Unresectable Hepatocellular Carcinoma: Liver Decompensation and Overall Survival. Int J Radiat Oncol Biol Phys 105:64–72. https://doi.org/10.1016/j.ijrobp.2019.01.076
Skinner HD, Hong TS, Krishnan S (2011) Charged-particle therapy for hepatocellular carcinoma. Semin Radiat Oncol 21:278–286. https://doi.org/10.1016/j.semradonc.2011.05.007
Yoon SS, Aloia TA, Haynes AB, et al (2014) Surgical placement of biologic mesh spacers to displace bowel away from unresectable liver tumors followed by delivery of dose-intense radiation therapy. Pract Radiat Oncol 4:167–173. https://doi.org/10.1016/j.prro.2013.07.007
Fukuda K, Okumura T, Abei M, et al (2017) Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci 108:497–503. https://doi.org/10.1111/cas.13145
Yoon K, Kwak J, Cho B, et al (2016) Gated Volumetric-Modulated Arc Therapy vs. Tumor-Tracking CyberKnife Radiotherapy as Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: A Dosimetric Comparison Study Focused on the Impact of Respiratory Motion Managements. PLOS ONE 11:e0166927. https://doi.org/10.1371/journal.pone.0166927
Riou O, Llacer Moscardo C, Fenoglietto P, et al (2017) SBRT planning for liver metastases: A focus on immobilization, motion management and planning imaging techniques. Rep Pract Oncol Radiother J Gt Cancer Cent Poznan Pol Soc Radiat Oncol 22:103–110. https://doi.org/10.1016/j.rpor.2017.02.006
Miften M, Vinogradskiy Y, Moiseenko V, et al (2018) Radiation Dose-Volume Effects for Liver SBRT. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2017.12.290
Toesca DAS, Osmundson EC, von Eyben R, et al (2017) Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract Radiat Oncol 7:173–182. https://doi.org/10.1016/j.prro.2016.10.003
Kang J-K, Kim M-S, Cho CK, et al (2012) Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 118:5324–5331. https://doi.org/10.1002/cncr.273
Moon DH, Wang AZ, Tepper JE (2018) A prospective study of the safety and efficacy of liver stereotactic body radiotherapy in patients with and without prior liver-directed therapy. Radiother Oncol 126:527–3. https://doi.org/10.1016/j.radonc.2018.01.004
Su T-S, Liang P, Liang J, et al (2017) Long-Term Survival Analysis of Stereotactic Ablative Radiotherapy Versus Liver Resection for Small Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 98:639–646. https://doi.org/10.1016/j.ijrobp.2017.02.095
Wahl DR, Stenmark MH, Tao Y, et al (2016) Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 34:452–458. https://doi.org/10.1200/JCO.2015.61.4925
Sapir E, Tao Y, Schipper MJ, et al (2018) Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 100:122–130. https://doi.org/10.1016/j.ijrobp.2017.09.001
Takeda A, Sanuki N, Tsurugai Y, et al (2016) Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122:2041–2049. https://doi.org/10.1002/cncr.30008
Scorsetti M, Comito T, Cozzi L, et al (2015) The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol 141:1301–1309. https://doi.org/10.1007/s00432-015-1929-y
Huang W-Y, Jen Y-M, Lee M-S, et al (2012) Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 84:354–361. https://doi.org/10.1016/j.ijrobp.2011.11.057
Sanuki N, Takeda A, Oku Y, et al (2014) Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol Stockh Swed :399–404. https://doi.org/10.3109/0284186X.2013.820342
Bitterman DS, Sanford NN, Niemierko A, et al (2019) Patterns of Care and Outcomes of Definitive External Beam Radiotherapy and Radioembolization for Localized Hepatocellular Carcinoma: A Propensity Score-adjusted Analysis. Am J Clin Oncol 42:554–562. https://doi.org/10.1097/COC.0000000000000550
Wang L, Ke Q, Huang Q, et al (2020) Stereotactic body radiotherapy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 37:1313–1321. https://doi.org/10.1080/02655636.2020.1843719
Guarneri A, Franco P, Romagnoli R, et al (2016) Stereotactic ablative radiation therapy prior to liver transplantation in hepatocellular carcinoma. Radiol Med (Torino) 121:873–881. https://doi.org/10.1007/s11537-016-0670-1
Sapisochin G, Barry A, Doherty M, et al (2017) Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 67:92–99. https://doi.org/10.1016/j.jhep.2017.02.022
Mastrocostas K, Jang H-J, Fischer S, et al (2019) Imaging post-stereotactic body radiation therapy responses for hepatocellular carcinoma: typical imaging patterns and pitfalls. Abdom Radiol 44:1795–1807
Olsen CC, Welsh J, Kavanagh BD, et al (2009) Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 73:1414–1424. https://doi.org/10.1016/j.ijrobp.2008.07.032
Park MJ, Kim SY, Yoon SM, et al (2014) Stereotactic body radiotherapy-induced arterial hypervascularity of non-tumorous hepatic parenchyma in patients with hepatocellular carcinoma: potential pitfalls in tumor response evaluation on multiphase computed tomography. PLoS One 9:e90327
Brook OR, Thornton E, Mendiratta-Lala M, et al (2015) CT imaging findings after stereotactic radiotherapy for liver tumors. Gastroenterol Res Pract 2015:
Haddad MM, Merrell KW, Hallemeier CL, et al (2016) Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol 41:2061–2077
Mendiratta-Lala M, Masch W, Shankar PR, et al (2019) Magnetic resonance imaging evaluation of hepatocellular carcinoma treated with stereotactic body radiation therapy: long term imaging follow-up. Int J Radiat Oncol Biol Phys 103:169–179
Mendiratta-Lala M, Masch W, Owen D, et al (2020) Natural history of hepatocellular carcinoma after stereotactic body radiation therapy. Abdom Radiol 1–11
Mendiratta-Lala M, Gu E, Owen D, et al (2018) Imaging findings within the first 12 months of hepatocellular carcinoma treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 102:1063–1069
Sanuki N, Takeda A, Mizuno T, et al (2013) Tumor response on CT following hypofractionated stereotactic ablative body radiotherapy for small hypervascular hepatocellular carcinoma with cirrhosis. AJR Am J Roentgenol 201:W812-820. https://doi.org/10.2214/AJR.12.10169
Kimura T, Takahashi S, Kenjo M, et al (2013) Dynamic computed tomography appearance of tumor response after stereotactic body radiation therapy for hepatocellular carcinoma: How should we evaluate treatment effects? Hepatol Res Off J Jpn Soc Hepatol 43:717–727. https://doi.org/10.1111/hepr.12007
Oldrini G, Huertas A, Renard-Oldrini S, et al (2017) Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PloS One 12:e0176118. https://doi.org/10.1371/journal.pone.0176118
Mendiratta-Lala M, Masch WR, Shampain K, et al (2020) MRI Assessment of Hepatocellular Carcinoma after Local-Regional Therapy: A Comprehensive Review. Radiol Imaging Cancer 2:e190024
Takamatsu S, Kozaka K, Kobayashi S, et al (2018) Pathology and images of radiation-induced hepatitis: a review article. Jpn J Radiol 36:241–255. https://doi.org/10.1007/s11604-018-0728-1
Tétreau R, Llacer C, Riou O, Deshayes E (2017) Evaluation of response after SBRT for liver tumors. Rep Pract Oncol Radiother J Gt Cancer Cent Poznan Pol Soc Radiat Oncol 22:170–175. https://doi.org/10.1016/j.rpor.2015.12.004
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. In: Seminars in liver disease. \copyright Thieme Medical Publishers, pp 052–060
Kielar A, Fowler KJ, Lewis S, et al (2018) Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol 43:218–230
Llovet JM, Di Bisceglie AM, Bruix J, et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711
Hussein RS, Tantawy W, Abbas YA (2019) MRI assessment of hepatocellular carcinoma after locoregional therapy. Insights Imaging 10:8
An C, Choi YA, Choi D, et al (2015) Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol 21:279
Kubota K, Ina H, Okada Y, Irie T (2003) Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci 48:571–576
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shampain, K.L., Hackett, C.E., Towfighi, S. et al. SBRT for HCC: Overview of technique and treatment response assessment. Abdom Radiol 46, 3615–3624 (2021). https://doi.org/10.1007/s00261-021-03107-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03107-7