Abstract
Purpose
To evaluate the efficacy and safety of covered stent placement for the treatment of hepatic artery pseudoaneurysm (HAP).
Methods
Between March 2006 and March 2019, 17 consecutive patients underwent emergency covered stent placement for treatment of HAP. There were 12 men and 5 women aged 24–71 years, with an average age of 49.4 years. Eleven patients had undergone Whipple procedure, 3 had hepatic abscess following hepatectomy, 2 had undergone hepatectomy under extracorporeal circulation, and 1 had received surgical exploration after a car accident. The average interval from surgical intervention to massive bleeding was 15.3 days (range: 6–35 days). After HAP was confirmed by angiography, 1–3 covered stent grafts (3–8 mm in diameter and 13 mm–5 cm in length) were implanted. Adequate drainage, anti-infection treatment, and symptomatic treatment were offered after stent placement, and no anticoagulation or antiplatelet drug was used.
Results
The interventions were successful in all 17 patients. Angiography revealed pseudoaneurysms in common hepatic artery in 16 patients (in gastroduodenal artery stumps in 4 patients) and hemorrhage from a ruptured right hepatic artery in 1 patient. All patients were successfully implanted with 1–3 covered stent grafts. Bleeding was completely controlled in 12 patients (stent diameter: 4.5–8 mm). Four patients (stent diameter: 3–4.5 mm) experienced bleeding recurrence 1 h to 3 days after stent implantation, and type 1 endoleaks were identified during second angiography. Finally, these 4 patients died of multiple organ failure 2–10 days after embolization/blockage. The remaining patient suffered from abdominal hemorrhage again 2 weeks after stent implantation, and second angiography showed hemorrhage from a branch of the superior mesenteric artery; no bleeding occurred after embolization. Thirteen patients survived at discharge, and the average length of hospital stay was 26.53 days (range: 11–58 days). The average follow-up time was 23 months (range: 16–37 months), during which 6 patients died of tumor progression. No bleeding recurred during the follow-up period, and routine color Doppler ultrasound revealed that the common hepatic artery was patent and the blood flow was smooth at the stent implantation site.
Conclusion
Covered stent placement is a safe and effective alternative for treating HAP patients with high risk of severe complications after hepatic artery embolization. Larger stent grafts (> 4 mm in diameter) may achieve better prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hepatic artery pseudoaneurysm (HAP) is a serious complication of injury to the hepatic artery. It usually occurs after acute/chronic severe pancreatitis, blunt abdominal trauma, or surgical treatment. Since HAP may lead to sudden life-threatening hemorrhage, active treatment is required [1, 2]. Surgical treatment used to be the mainstream approach for managing visceral aneurysms, and in recent years, embolization has become the preferred treatment for such lesions [3,4,5]. However, for patients with a high risk of complications (e.g., liver failure and liver abscess) following hepatic artery embolization, implantation of covered stent grafts can be another treatment option. In this article, we report a group of HAP patients treated with stent graft implantation.
Materials and methods
This retrospective study was approved by the Chinese PLA general Hospital Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (the Chinese PLA general Hospital Ethics Committee) and with the 1964 Helsinki declaration and its later amendments.
We enrolled 17 patients with massive abdominal hemorrhage who were treated in our center between March 2006 and March 2019. These patients included 12 men and 5 women aged 24 to 71 years old, with an average age of 49.4 years old. Eleven patients had undergone Whipple procedure, 3 had hepatic abscess following hepatectomy, 2 had undergone hepatectomy under extracorporeal circulation, and 1 had received surgical exploration after a car accident. The average interval from surgical intervention to massive bleeding was 15.3 days (range: 6–35 days).
Before the emergency interventional treatment, the patients’ family members or designated agents have signed informed consent for the operation. Adequate fluid replacement and transfusion were given to ensure a safe intervention. After a routine preoperative preparation, interventional procedure was performed under sterile conditions, with the patient under local anesthesia. The femoral artery was punctured using the standard Seldinger’s technique, and a 4F vascular sheath was introduced for abdominal angiography and treatment.
The 4F pigtail catheter was inserted into the abdominal aorta until the 12th thoracic vertebral level. Abdominal aortic angiography was performed on a digital subtraction angiography system, followed by selective angiography of the celiac trunk, hepatic artery, splenic artery, and superior mesenteric artery using 4F Cobra, Yasiro, or RH hepatic artery catheters (Terumo Corp., Japan; Cordis Corp., USA). Blood flow in the hepatic artery and portal vein was assessed to locate the bleeding site and to rule out any portal vein thrombosis/injury or other arterial injuries.
Then, the 6–8F femoral arterial sheaths, with the length of 45–70 cm (Super Arrow-Flex sheath introducer sets, Arrow International Inc., USA; Flexor Check-Flo Introducer, Cook Company, USA) were alternately introduced up to the celiac trunk and an angiogram through the sheath was performed to measure the diameter and length of the hepatic artery at the bleeding site. Guided by guide wire/microwire (0.014″ HI-TORQUE; Abbott, USA, 0.035″ RADIFOCUS STIFF TYPE; Terumo Corp., Japan, depending on the stent platform), the covered stent graft was advanced into the bleeding site through the arterial sheath to ensure the stent was positioned to cover the abnormal area with the proximal and distal end of the stent positioned in a normal segment of vessel on either side, where 1–3 stent grafts were implanted. The stent grafts used included Viabahn (5–8 mm in diameter, 2.5–5 cm in length; Gore Company, USA), Fluency (8 mm × 4 cm, Bard Company, USA), JOSTENT Graft Master (3–3.5 mm in diameter, 16–19 mm in length, Abbott, USA), and Willis (3.5–4.5 mm in diameter, 13–16 mm in length, MicroPort, China), mainly depending on the size of hepatic artery at the bleeding site we measured during procedure. Hepatic angiography was repeated to confirm that the original pseudoaneurysm was no longer visible, as well as no contrast extravasation and the patency of hepatic artery.
Eight patients underwent CT-guided abscess/pancreatic fistula drainage after stent implantation. In two patients with diffuse infections, Percutaneous nephroscopic necrosectomy was performed to remove necrotic tissue, followed by re-placement of the drainage tube. Symptomatic treatment was offered after interventional procedures, and no anticoagulation or antiplatelet drug was used.
During hospitalization after stent implantation, liver color Doppler ultrasound was performed on a daily basis to detect the blood flow at the stent implantation site. Ultrasound scan was performed every 3 months after discharge.
Results
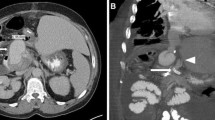
The interventions were successful in all 17 patients. Angiography revealed pseudoaneurysms in the common hepatic artery in 16 patients (in gastroduodenal artery stumps in 4 cases) and hemorrhage from a ruptured right hepatic artery in 1 patient. All patients were successfully implanted with 1–3 stent grafts.
Stable hemodynamics (no recurrent bleeding, and active bleeding was completely controlled) was achieved in 12 patients (stent diameter: 4.5–8 mm). Four patients (stent diameter: 3–4.5 mm) experienced bleeding recurrence 1 h to 3 days after stent implantation, and type 1 endoleaks were identified during second angiography. Three of them underwent coil embolization (entire hepatic artery embolization); one of them, who had undergone portal vein ligation, was temporarily blocked with a balloon (Fogarty, Edwards Lifesciences Corp., USA) in celiac trunk. The balloon was intermittently deflated inside the intensive care unit to ensure the intermittent blood supply to the liver, during which the blood supply from the collateral vessels of the liver was expected to form. Intermittent blood transfusion and symptomatic treatment were also provided (Fig. 1, 2,3, 4, 5). Finally, these 4 patients died of multiple organ failure 2–10 days after embolization/blockage. The other patient suffered from abdominal hemorrhage again 2 weeks after stent implantation, and second angiography showed hemorrhage from a branch of the superior mesenteric artery, whereas no bleeding occurred after embolization.
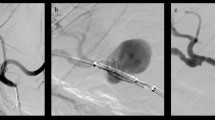
Pseudoaneurysm rupture of the hepatic artery–duodenal artery root. A 23-year-old woman underwent laparotomy and portal vein ligation after a car accident. She had abdominal hemorrhage 15 days after operation. Bleeding was observed after empirical splenic artery and inferior pancreaticoduodenal artery embolization. Repeated abdominal angiography showed bleeding from a pseudoaneurysm rupture of the hepatic artery–duodenal artery root (→)
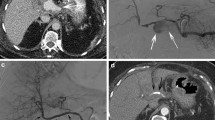
Thirteen patients survived at discharge, and the average length of the hospital stay was 26.53 days (range: 11–58 days). The average follow-up time was 23 months (range: 16–37 months), during which six patients died of tumor progression. No bleeding recurred during the follow-up period, and regular color Doppler ultrasound revealed that the common hepatic artery was patent and the blood flow was smooth at the stent implantation site (Fig. 6, 7, 8).
Discussion
HAPs are fatal and mostly iatrogenic. Once a diagnosis of HAP is established, active and effective treatment should be offered as soon as possible. Embolization remains the mainstream treatment for HAPs, especially in patients with massive bleeding due to aneurysm rupture [6, 7]. However, the blood supply to the peripheral of the hepatic artery may be difficult to establish if the patient has portal occlusion or if the liver is mobilized during surgery (e.g., liver transplantation, or extracorporeal hepatectomy). In addition, embolization of the hepatic artery may lead to serious complications such as liver failure in the presence of liver abscess or other conditions [8]. Therefore, the hepatic artery should be kept patent when blocking HAP and achieving immediate hemostasis. In these patients, stent implantation should be the preferred treatment. Recent studies have shown that patency of the hepatic artery is closely related to the incidence of postoperative complications [9]. Therefore, the role of hepatic artery stent grafts in treating HAPs requires further investigation.
HAPs caused by surgery are usually fragile. Due to possible infection, bile leakage, or pancreatic fistula after surgery, the arterial wall becomes fragile. Therefore, small-sized covered stent grafts are often used. In some studies, the stents were even placed inside the proper hepatic artery, with a diameter below 4 mm [10, 11]. In our series, larger stent grafts were used in the later stage (after 2010), the diameter we chose is at least 150% of the measured value, and the diameters of the stent grafts implanted in the common hepatic artery could reach 8 mm. The purposes were as follows. [1] The diameter of a blood vessel measured during hemorrhage is smaller than its real diameter due to vasospasm, a stent graft selected according to the measured value might become undersized when the spasm resolved and the type 1 endoleak developed thereafter. The placement of larger stent grafts can effectively reduce the incidence of endoleaks. [2] After the stent graft is implanted, anticoagulant and antiplatelet therapies for at least 6 months are generally required to avoid intra-stent thrombosis; however, bleeding may recur at the surgical field following the use of anticoagulation and antiplatelet drugs due to the poor coagulation function after surgery. Thus, in our opinion, the use of larger stent grafts is more feasible as they have fewer requirements for postoperative anticoagulation and antiplatelet therapies. In our study, we did not find hepatic artery occlusion without anticoagulant and antiplatelet therapies during the follow-up. Based on the small number of cases, to determine whether anticoagulation and antiplatelet therapies are needed or not, a larger sample is still needed.
Large-sized stent grafts were used in our study; there were no serious complications such as arterial rupture (even in patients with accompanying pancreatic leakage and/or infections). This may have been because the stented area that we selected was long enough (> 1 cm above the proximal and distal ends of the pseudoaneurysm) and all the stented areas were within the normal vessel. However, the risk of implanting a large stent graft still should not be neglected. Onishi et al. reported a case of arterial dissection caused by guiding sheath during the release of the Viabahn stent for the treatment of HAP [12].
Although stent graft implantation can effectively control bleeding, other complications such as peripancreatic infection and pancreatic leakage should still be actively treated. In our current series, anti-infection treatment was administered after the stent graft was implanted, and effective drainage was performed for patients with pancreatic leakage and infection, which may also explain the good prognoses of our patients.
Our study was limited by its small sample size and retrospective design, and thus, we could not conclude that placement of the covered hepatic artery stent could be used as the preferred treatment for HAP. However, with the accumulation of cases and the extension of follow-up time, covered stent placement may become an alternative to hepatic artery embolization, especially for patients with massive hemorrhage from ruptured common hepatic artery complicated with portal occlusion. The implantation of a covered stent can effectively treat HAP while maintaining patency of the hepatic artery. According to our study, larger stent (> 4 mm in diameter) may provide better prognosis in these patients.
References
Harrison J, Harrison M, Doria C. Hepatic Artery Pseudoaneurysm Following Orthotopic Liver Transplantation: Increasing Clinical Suspicion for a Rare but Lethal Pathology. Ann Transplant. 2017 Jul 7;22:417-424.
Teixeira C, Ribeiro SM, Alves AL, Cremers I. Haemobilia due to hepatic artery pseudoaneurysm. BMJ Case Rep. 2017 May 29;2017. pii: bcr-2017-220575. https://doi.org/10.1136/bcr-2017-220575.
Hasegawa T, Ota H, Matsuura T, Seiji K, Mugikura S, Motoi F, et al. Endovascular Treatment of Hepatic Artery Pseudoaneurysm after Pancreaticoduodenectomy: Risk Factors Associated with Mortality and Complications. J Vasc Interv Radiol. 2017 Jan;28(1):50-59.e5. https://doi.org/10.1016/j.jvir.2016.04.004. Epub 2016 Jun 16.
Zhang J, Khalifeh A, Santini-Dominguez R, Barth RN, Bruno D, Desikan S, et al. Endovascular Reconstruction of the Hepatic Arterial System for the Management of Mycotic Pseudoaneurysm in a Liver Transplant Patient. Ann Vasc Surg. 2019 Aug 5. pii: S0890-5096(19)30556-4. https://doi.org/10.1016/j.avsg.2019.05.060.
Oppenheimer DC, Jones L, Sharma A. Percutaneous Thrombin Injection for Treatment of a Hepatic Arterial Pseudoaneurysm after the Placement of a Transjugular Intrahepatic Portosystemic Shunt. J Clin Imaging Sci. 2019 May 24;9:20. doi: 10.25259/JCIS_87_18. eCollection 2019
Senthilkumar MP, Battula N, Perera M, Marudanayagam R, Isaac J, Muiesan P, et al. Management of a pseudo-aneurysm in the hepatic artery after a laparoscopic cholecystectomy. Ann R Coll Surg Engl. 2016 Sep;98(7):456-60.
Mine T, Murata S, Yasui D, Yokota H, Tajima H, Kumita S. Glue Embolization of a Blunt Traumatic Hepatic Arteriovenous Fistula under Inflow and Outflow Control. J Nippon Med Sch. 2016; 83(1):27-30. doi: 10.1272/jnms.83.27
de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Lameris JS, van Gulik TM, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241(1):85–91.
Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, et al. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg. 2007 Apr;11(4):432-8.
Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Stent-grafts placement for treatment of massive hemorrhage from ruptured hepatic artery after pancreaticoduodenectomy. World J Gastroenterol. 2010 Aug 7;16(29):3716-22.
Aoun C, El Rassi Z. Stent graft placement for an incidental finding of hepatic artery pseudoaneurysm post hepatectomy, a case report and literature review. Int J Surg Case Rep. 2018;49:191-193. https://doi.org/10.1016/j.ijscr.2018.06.038
Onishi Y, Kimura H, Kanagaki M, Oka S, Fukumoto G, Otani T, et al. Placement of a Viabahn stent-graft for hepatic artery pseudoaneurysm complicated by arterial dissection caused by a guiding sheath. Radiol Case Rep. 2019 Apr 2;14(6):711-713.
Acknowledgements
We would like to extend our sincere gratitude to our departmental chair for all these support. Also, we would like to give many thanks to our physicians, engineers, nurses, as well as other staff of the department.
Funding
Funding is not applicable.
Author information
Authors and Affiliations
Contributions
FD contributed to the study conception and design. Material preparation, data collection and analysis were performed by LC, LK, YB, and JH. The interventional treatments were performed by FD, XL, and XW. The first draft of the manuscript was written by LC, LK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, L., Kong, L., Bai, YH. et al. Covered stent placement for hepatic artery pseudoaneurysm. Abdom Radiol 45, 3337–3341 (2020). https://doi.org/10.1007/s00261-020-02452-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02452-3