Abstract
Purpose
Hepatic artery pseudoaneurysms are a rare but potentially life-threatening complication of major pancreaticobiliary surgery. We evaluated the safety and efficacy of endovascular stentgraft implantation for the management of such vascular lesions.
Materials and Methods
Between May 2013 and October 2015, ten patients with postoperative hepatic artery pseudoaneurysm, of which eight presented with active hemorrhage, were treated with endovascular stentgraft implantation. All patients had undergone major pancreatic or hepatic surgery before (pylorus-preserving pancreaticoduodenectomy, pancreatectomy, hemihepatectomy, extended hemihepatectomy). The pseudoaneurysms were diagnosed 13–202 days after surgery and were associated with postsurgical complications (e.g., leakage of pancreaticojejunal anastomosis).
Results
In 9/10 patients, the pseudoaneurysm was completely excluded via stentgraft implantation. In 1/10 patient, the pseudoaneurysm ruptured during the procedure and was successfully treated by immediate open surgery. In 1/10 patient, a second intervention was performed after 6 days because of rebleeding; this was successfully treated by implantation of a second overlapping stentgraft. Mean follow-up time is 51 days. None of the patients died due to stentgraft- or aneurysm-related complications. Further episodes of hemorrhage were not observed. In one patient, clinically asymptomatic complete occlusion of the stentgraft was discovered at follow-up imaging.
Conclusion
Stentgraft implantation is a safe and effective technique to treat hepatic artery pseudoaneurysms related to major pancreatic or hepatic surgery, especially in the setting of acute hemorrhage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Splanchnic artery aneurysms are a rare vascular pathology, with a documented prevalence of 0.1–2 % [1]. Hepatic artery aneurysms (HAA) are the second most common location of splanchnic artery aneurysm, accounting for up to 20 % of these lesions [2]. HAA can be either true aneurysms or pseudoaneurysm. In true aneurysm, there is an enlargement of at least 1.5 times the arterial diameter, with all parts of the arterial wall dilated, whereas pseudoaneurysms present a leaking hole in the artery wall, covered only by adventitia or by perivascular tissue.
Hepatic artery pseudoaneurysms are one of the most dangerous complications associated with major pancreatic or hepatic surgery [3–5]. Different factors like digestion of the arterial wall caused by bile or pancreatic juice from anastomotic leakage or mechanical injury during the operation weaken the arterial wall structure, by means of visceral inflammation, which damages the adventitia and leads to thrombosis of the vasa vasorum, resulting in arterial rupture or enlargement [4].
Pseudoaneurysms should always be treated, independent of their diameter, associated symptoms or lack thereof, and independent of their location [6], due to the high risk of rupture resulting in acute and potentially life-threatening hemorrhage.
Although surgery has always been considered the gold standard for hepatic artery pseudoaneurysms treatment, endovascular treatment has gained ground and become the treatment of first choice in centers that can offer such procedures [6, 7]. Among the possible interventional treatments, the minimal-invasive stentgraft implantation has the advantage of excluding the aneurysm, controlling its bleeding and preserving hepatic artery perfusion [7].
Therefore, the objective of this study is to report on our experiences with stentgraft implantation for treatment of postoperative pseudoaneurysms of the hepatic artery in patients after major hepatic or pancreatic surgery.
Patients, Materials and Methods
Patients
All patients, who were referred to our department for the minimal-invasive treatment of postoperative hepatic artery pseudoaneurysm between May 2013 and October 2015, were included in this retrospective analysis. A total of ten patients (all male, mean age 63 ± 11 years) were treated due to postoperative hepatic pseudoaneurysm during this period and exclusively underwent stentgraft implantation to exclude the pseudoaneurysm. All included patients had undergone major pancreatic or hepatic surgery (pylorus-preserving pancreaticoduodenectomy: n = 4; pancreatectomy: n = 3, one hemihepatectomy: n = 1; extended hemihepatectomy: n = 1; pylorus-preserving pancreaticoduodenectomy with right extended hemihepatectomy: n = 1) to treat an oncologic or inflammatory disease (Klatskin tumor: n = 3; bile duct tumor: n = 1, pancreatic head carcinoma: n = 3; pancreatic intraductal papillary mucinous neoplasms: n = 2, necrotizing pancreatitis: n = 1). Postsurgical complications were found in all cases; in particular, six patients developed anastomotic leakage of pancreaticojejuno- or hepaticojejunostomy, and one patient developed an abscess in the perihepatic space, for which six of them received a CT-guided percutaneous drainage. The other complications included abscess, abdominal hematoma and drainage hemorrhage. Eight pseudoaneurysms were located in the common hepatic artery, one pseudoaneurysm in the proper hepatic artery and one pseudoaneurysm in the left hepatic artery. For details on patients, see also Table 1.
Indications
Eight patients presented in hypovolemic shock at the moment of the treatment due to active bleeding of the pseudoaneurysm, as confirmed by contrast-enhanced CT. The remaining two pseudoaneurysms were incidentally detected during the postoperative CT follow-up, without active hemorrhage.
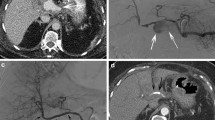
All patients underwent contrast-enhanced CT imaging before the angiographic treatment (Fig. 1).
A Contrast-enhanced CT before the procedure. Large pseudoaneurysm (6.5 cm) of the common hepatic artery (arrow). B Follow-up contrast-enhanced CT image (5 months after stentgraft implantation). Pseudoaneurysm was successfully treated. The stentgraft (arrow) and the left hepatic artery (arrow head) were patent
Technique
All procedures were performed under sterile conditions and local anesthesia. Depending on condition of the patient, additional sedation was used during the procedure.
The right common femoral artery was chosen as access in 9/10 cases, whereas in 1/10 case, the left common femoral artery was punctured; in one case, the left brachial artery was punctured in addition to the right common femoral artery.
Via a long visceral introducer sheath (5–7 F, Cook, Bloomington, IL, USA), the celiac trunk and the hepatic artery were catheterized using 5-F cobra-2 catheter (Cook, Bloomington, IL, USA), or a 4-F glide catheter (Terumo, Tokyo, Japan), a 5-F vertebral catheter (Merit Medical, South Jordan, UT, USA) or a 5-F Shepherd Hook catheter (Merit Medical, South Jordan, UT, USA) depending on the individual anatomy and access route. Thereafter, a 2.7-F microcatheter (Progreat, Terumo, Tokyo, Japan) was used to cross the pseudoaneurysm and place a stiff 0.018-inch guidewire (Steelcore, Abbott vascular, Abbott GmbH, Wiesbaden, Germany) in a peripheral branch of the hepatic artery. Then, GORE® VIABAHN® (Gore & Associates, Inc., Flagstaff, AZ, USA) or Advanta™ (Atrium/Maquet Cardiovascular, Hudson, NH, USA) polytetrafluoroethylene-covered stentgrafts were placed (Figs. 2, 3). For further material details, see also Table 1.
In one patient, the aneurysm sac of the common hepatic artery involved the origin of the gastroduodenal artery. In this patient, the GDA was embolized with 6 microcoils (Cook, Bloomington, IL, USA) (Fig. 3) using a retrograde approach via the superior mesenteric artery arcade.
After successful stentgraft placement, anticoagulation with i.v. administration of heparin (1000 I.U./h) was continued for 24 h, and dual antiplatelet therapy by clopidogrel 75 mg/day for 6 weeks and lifelong aspirin 100 mg/day was recommended.
Follow-up
Patient underwent close follow-up by CT angiography to check for successful exclusion of the pseudoaneurysm, patency of the parent vessel and possible procedure- or surgery-related complications.
Technical success was defined as the total exclusion of the aneurysm via stentgraft placement. In symptomatic patients, i.e., patients with active hemorrhage, clinical success was achieved in case the bleeding was successfully stopped, with preserved patency of the hepatic artery. We report on technical and clinical success rates, as well as early and delayed complications.
Results
Technical success was achieved in 9/10 patients. In 1/10 patient, the aneurysm ruptured during the intervention. In this case, 2 overlapping stents (6 × 25 and 5 × 25 mm VIABAHN) were placed to treat a 20-mm aneurysm of the proper hepatic artery. After the placement of the second stentgraft, the artery ruptured distally of the edge of this graft and the hemorrhage was controlled by means of a 5 × 20 mm balloon catheter that was immediately inflated at the site of rupture. The reparation via stentgraft was excluded because of the tortuous anatomy of the hepatic artery; the patient was transferred to the operating theater for emergency surgery, and the aneurysm was treated surgically.
Among these 9 successfully endovascular treated patients, the exclusion was obtained at the first interventional attempt in 6/9 cases; in 1/9 patient, we did not succeed in crossing the aneurysm during the first DSA session, which was successfully performed a few days later during a second attempt. In 1/9 patient, a reintervention was necessary 6 days after stentgraft placement because of hemodynamically significant rebleeding. The angiography revealed a pseudoaneurysm arising from the proximal edge of the previously placed stentgraft, which was successfully treated with a proximally overlapping stentgraft.
In 8 symptomatic patients who underwent emergency stentgraft placement because of acute hemorrhage of the pseudoaneurysm, bleeding was controlled in 7/8 cases directly after the intervention.
The mean follow-up time is 51 ± 52 days, ranging from 2 to 190 days. Three patients died at 9, 66 and 163 days after stentgraft implantation, respectively. The first patient died because of multiple organ failure, the second patient died because of the progression of his oncological disease, and the third patient died because of septic shock arising from multiple refractory liver abscesses. In none of the cases, mortality was attributable to or related to the pseudoaneurysm or the placed stentgraft.
Among the 9 patients who had been successfully treated by endovascular stentgraft placement, the last follow-up CT revealed patency of the stentgraft in 8/9 cases, whereas in 1/9 patient, complete occlusion of the stentgraft was observed at a follow-up CT 3 days after the intervention, which, however, remained clinically asymptomatic.
Discussion
Intra-abdominal hemorrhage represents up to 38 % of the mortality causes after pancreatic and hepatobiliary surgery [8]. Therefore, due to the high risk of rupture and consequent bleeding, pseudoaneurysm of the hepatic artery is one of the most life-threatening complications for these patients [4].
There are several ways, which can lead to pseudoaneurysm formation in patients after major abdominal surgery: First of all, the tumor or the inflammation itself can provoke vessel erosion. The mechanical manipulation, mainly during the lymph node dissection, can directly injure the arterial wall. Moreover, there are two predisposing factors for pseudoaneurysm formation which can occur after pancreatic and hepatic surgery: anastomotic leakage and abscess presence in the hepatic spaces. In particular, the bile from the anastomotic insufficiency and the infectious material of the abscess can cause irritation and tryptic necrosis of the arterial wall [4].
In our case series, six patients presented leakage from the pancreaticojejuno- or hepaticojejunostomy and one patient developed an abscess in the perihepatic space and six of them received a percutaneous drainage to handle this complication. These data confirm the strong association between anastomotic leakage and development of pseudoaneurysm of the hepatic artery.
Since up to 76 % of the pseudoaneurysms of the hepatic artery rupture and since mortality rates of ruptured pseudoaneurysm of the hepatic artery range from 35 to 50 %, an aggressive management is mandatory [9]. The rupture of pseudoaneurysm of hepatic artery occurs commonly into the biliary tree and can present clinically with jaundice, biliary colic and signs of gastrointestinal hemorrhage, a condition also known as Quincke’s triad [3, 7]. In our experience, postoperative HAAs are most commonly a midterm complication after abdominal surgery. In 9/10 cases, the pseudoaneurysm was diagnosed within 2 months from the surgical procedure; in the remaining case, the aneurysm was diagnosed 202 days after surgery.
Surgery had traditionally been advocated to represent the treatment of first choice. Surgical management depends on the location of the aneurysm, the presence of collateral flow and the general medical condition of the patient [7]. Operating options include ligation, ligation with bypass, hepatic resection and, rarely, orthotopic liver transplantation [10].
The endovascular approach is nowadays considered the treatment of choice in centers that can offer such procedures, in both elective and emergency cases. Nevertheless, current literature is limited to case reports or case series, mainly on coil embolization of HAAs [11–13]. For years, coiling has been the only endovascular treatment option in patients with HAA. It consists of the closure of the artery by means of coils, alone or in combination with glue, in both the afferent and efferent vessel of the aneurysm, in order to cut the blood supply to the aneurysm and promote clotting. The main complications of this procedure are delayed reperfusion of the aneurysm, coil migration, intra-procedural aneurysm rupture and nontarget embolization [14]. Moreover, closure of the hepatic artery reduces hepato-portal blood flow and may not be tolerable in patients who underwent major liver resection or who exhibit portal hypertension. In any case, patency of the portal vein and sufficient antegrade portal venous blood flow are necessary prerequisites for both surgical ligation and coiling of proper hepatic artery [15].
Besides embolisation and stentgraft placement, another endovascular treatment option is the use of a flow-diverting bare stent. This device changes the local flow patterns by decreasing the turbulent flow in the aneurysm sac while improving laminar flow in the main artery and collateral branches [6, 16]. However, this technique does not allow an immediate exclusion of the aneurysm, making it an unsuitable option for patients with acute bleeding or for aneurysms that are at high risk of rupture.
Exclusion of pseudoaneurysm by stentgraft placement has several advantages when compared to other interventional techniques, leading our choice to this technique. It excludes the pseudoaneurysm immediately from perfusion; this was considered very important for our patients, since eight of them underwent emergency treatment due to active bleeding. In seven of these eight cases, the stentgraft allowed immediate control of the bleeding, making this technique very suitable to be used in emergency conditions.
Besides, using a stentgraft preserves the antegrade arterial flow to the liver and can therefore be offered even in patients with limited portal venous flow or patients who underwent major liver resection. This is another significant point in our decision-making process, since three of our patients had undergone major hepatic surgery (hemihepatectomy or extended hemihepatectomy) for tumor resection. Therefore, preserving arterial blood supply of the liver remnant was considered a priority to maintain liver function.
Moreover, stentgraft implantation is associated with less fluoroscopy time during the intervention [14].
Open surgery for the treatment of visceral aneurysms has been shown to be associated with a morbidity rate as high as 100 % and a mortality rate as high as 33 % [7]. In contrast, endovascular stentgraft placement is minimally invasive, thus substantially reducing morbidity and mortality rates. In our case series, stentgraft placement was associated with a morbidity rate of 1/10 (10 %) and a mortality rate of 0 %.
After stentgraft implantation, an antiplatelet therapy (clopidogrel 75 mg/day for 6 weeks and lifelong aspirin 100 mg/day) is recommended. No one of our patients presented absolute contraindications to the double antiplatelet therapy, and no one of them develops an antiplatelet therapy-related complication. Another potential complication well described in the literature is stent infection, consequently to the contact of the gastrointestinal content or the infected necrotic tissue with the stentgraft [17]. This is very important in our context, in which the presence of anastomotic leakage or abscesses can cause the chronicization of such a process. In our series, no patient presented stentgraft infection-related signs. However, a prompt drainage insertion in the site of leakage or abscesses could be protective from this complication.
Moreover, further complications of stentgraft implantations are endoleaks and stent thrombosis [18, 19]. In our experience, only 1/9 patient who underwent successful stentgraft placement developed stent occlusion, which, however, remained clinically asymptomatic. Follow-up CTs of the remaining patients showed patency of the implanted stentgraft. Of course, our follow-up period is still limited, with an average of 64 days, such that we cannot provide data on long-term results. The latter, however, will be difficult to provide anyway due to the limited prognosis of the oncological patients in our cohort, which reflects the lack of data in the literature regarding long-time patency of stentgraft implantation in visceral arteries.
Moreover, there are anatomical contraindications of this technique: short- or wide-neck aneurysm and tortuous or small vessels, which would interfere with safe stentgraft placement. In addition, aneurysms with clinically important vessels originating from its sac are not amenable to stentgraft implantation, due to the risk of splenic or hepatic infarction and type II endoleak [6, 16].
Preinterventional CT angiography is useful to plan the appropriate treatment strategy in each patient case because it helps evaluating the anatomical features of the aneurysm (dimension, neck and possible arising branches) in order to assert its suitability for this technique.
All these advantages and disadvantages were considered in our decision-making procedure, and during the time lapse of our study (May 2013/October 2015), no other interventional treatments were performed in our clinics to handle a pseudoaneurysm of the hepatic artery.
The stentgraft approach to handling pseudoaneurysm of visceral arteries is not well studied. Except Lü et al., who in 2013 described their experience with 8 patients treated with stentgraft to handle a pseudoaneurysm in the hepatic artery, the literature is limited to case reports [20]. Lü et al. reported on the successful use of the Jostent graftmaster coronary stent graft (Abbott Vascular, Redwood City, California, USA), which is a balloon-expandable stainless steel stentgraft, that is available with a diameter up to 4.8 mm and a length up to 26 mm. However, in our experience, we mainly used a self-expandable stentgraft, which many interventional radiologists used and is also available in larger dimensions. In the one patient with major complication due to aneurysm rupture, surgical repair of aneurysm was necessary. In this case, an inflated balloon was used to cover the rupture site, thus minimizing blood loss during surgery, a fact that greatly contributed to the success of the subsequent emergency surgical procedure. This underscores once more how important a close cooperation between interventional radiologists and surgeons is to successfully control even critical procedural complications.
Conclusion
According to our experience, stentgraft implantation is a safe and effective therapy option to treat postoperative hepatic artery pseudoaneurysms, especially if they present with acute active bleeding. Long-term follow-up and larger series are needed to further evaluate this technique and to assess its indication in treatment of this vascular pathology. A multidisciplinary team approach and close cooperation of interventional radiologists and abdominal surgeons are mandatory to manage possible complications like vessel rupture.
References
Hossain A, Reis ED, Dave SP, Kerstein MD, Hollier LH. Visceral artery aneurysms: experience in a tertiary-care center. Am Surg. 2001;67(5):432–7.
O’Driscoll D, Olliff SP, Olliff JF. Hepatic artery aneurysm. Br J Radiol. 1999;72(862):1018–25.
Berceli SA. Hepatic and splenic artery aneurysms. Semin Vasc Surg. 2005;18(4):196–201.
Iswanto S, Nussbaum ML. Hepatic artery pseudoaneurysm after surgical treatment for pancreatic cancer: minimally invasive angiographic techniques as the preferred treatment. N Am J Med Sci. 2014;6(6):287–90.
Chen J, Weinstein J, Black S, Spain J, Brady PS, Dowell JD. Surgical and endovascular treatment of hepatic arterial complications following liver transplant. Clin Transplant. 2014.
Belli AM, Markose G, Morgan R. The role of interventional radiology in the management of abdominal visceral artery aneurysms. Cardiovasc Intervent Radiol. 2012;35(2):234–43.
Sachdev-Ost U. Visceral artery aneurysms: review of current management options. Mt Sinai J Med. 2010;77(3):296–303.
Spiliopoulos S, Sabharwal T, Karnabatidis D, Brountzos E, Katsanos K, Krokidis M, et al. Endovascular treatment of visceral aneurysms and pseudoaneurysms: long-term outcomes from a multicenter European study. Cardiovasc Intervent Radiol. 2012;35(6):1315–25.
Abbas MA, Fowl RJ, Stone WM, Panneton JM, Oldenburg WA, Bower TC, et al. Hepatic artery aneurysm: factors that predict complications. J Vasc Surg. 2003;38(1):41–5.
Sachdev U, Baril DT, Ellozy SH, Lookstein RA, Silverberg D, Jacobs TS, et al. Management of aneurysms involving branches of the celiac and superior mesenteric arteries: a comparison of surgical and endovascular therapy. J Vasc Surg. 2006;44(4):718–24.
Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45(2):276–83.
Kasirajan K, Greenberg RK, Clair D, Ouriel K. Endovascular management of visceral artery aneurysm. J Endovasc Ther. 2001;8(2):150–5.
Carrafiello G, Rivolta N, Fontana F, Piffaretti G, Mariscalco G, Bracchi E, et al. Combined endovascular repair of a celiac trunk aneurysm using celiac-splenic stent graft and hepatic artery embolization. Cardiovasc Intervent Radiol. 2010;33(2):352–4.
Pasha SF, Gloviczki P, Stanson AW, Kamath PS. Splanchnic artery aneurysms. Mayo Clin Proc. 2007;82:472–9.
Balderi A, Antonietti A, Pedrazzini F, Sortino D, Vinay C, Grosso M. Treatment of visceral aneurysm using multilayer stent: two-year follow-up results in five consecutive patients. Cardiovasc Intervent Radiol. 2013;36:1256–61.
Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7(10):1081–92.
Greenhalgh RM, Powell JT. Endovascular repair of abdominal aortic. N Engl J Med. 2008;358(5):494–501.
de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241(1):85–91.
Suzuki K, Mori Y, Komada T, Matsushima M, Ota T, Naganawa S. Stent-graft treatment for bleeding superior mesenteric artery pseudoaneurysm after pancreaticoduodenectomy. Stent-graft treatment for bleeding superior mesenteric artery pseudoaneurysm after pancreaticoduodenectomy. Cardiovasc Interv Radiol. 2009;32(4):762–6.
Lü PH, Zhang XC, Wang LF, Chen ZL, Shi HB. Stent graft in the treatment of pseudoaneurysms of the hepatic arteries. Vasc Endovascular Surg. 2013;47(7):551–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pedersoli, F., Isfort, P., Keil, S. et al. Stentgraft Implantation for the Treatment of Postoperative Hepatic Artery Pseudoaneurysm. Cardiovasc Intervent Radiol 39, 575–581 (2016). https://doi.org/10.1007/s00270-015-1274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1274-1