Abstract
Hepatocarcinogenesis is a multi-step process characterized by progressive cellular and molecular dedifferentiation of hepatocytes and culminating in the emergence of hepatocellular carcinoma (HCC). Knowledge of hepatocarcinogenesis is important because familiarity with the associated imaging features can lead to improved diagnosis of HCC at its early stages. The article reviews the alterations that accumulate leading to HCC result in abnormal imaging features, many of which are included in LI-RADS v2017 as major and ancillary features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocarcinogenesis is a multi-step process characterized by progressive cellular and molecular dedifferentiation of hepatocytes and culminating in the emergence of hepatocellular carcinoma (HCC). Knowledge of hepatocarcinogenesis is important because familiarity with the associated imaging features can lead to improved diagnosis of HCC at its early stages, as well as differentiation from other malignant entities that may arise in the chronically diseased liver, thereby resulting in more successful therapeutic outcomes. In this article, we will describe hepatocarcinogenesis and relate its molecular and histologic changes with observed imaging features in the Liver Imaging Reporting and Data System (LI-RADS) v2017.
Molecular alterations
Hepatocarcinogenesis occurs in a milieu of injured liver tissue. Chronic inflammation plays a pivotal role in the development of HCC by causing repeated cycles of cellular injury, death, and regeneration. This cycle promotes aberrant cell signaling and frequent genetic and epigenetic mutational events.
Within the inflammatory microenvironment, genetic and epigenetic mutational events may be introduced by reactive oxygen species or nitrogen intermediates released by inflammatory infiltrates. These molecular alterations occur during a prolonged preneoplastic phase that begins years or decades before cirrhosis is established. Hepatocyte proliferation in response to injury leads to clonal propagation of these mutations and genomic instability. Interestingly, mature adult hepatocytes retain the ability to dedifferentiate into progenitor states capable of regeneration of both the hepatocyte and cholangiocyte components of hepatic parenchyma [1]. Because of the plasticity of adult hepatocytes, malignant cells may demonstrate features in common with progenitor cells (“stemness”) or cholangiocytes [2]. This helps to explain why many primary malignant tumors in the cirrhotic liver express “stem”-like molecular markers, or contain mixed cell populations, with some resembling pure HCC and others resembling cholangiocarcinoma (so-called hepatocholangiocarcinoma).
Eventually, genetic and epigenetic alterations escalate during a neoplastic phase, in which chromosomal deletions and rearrangements, aneuploidy, gene amplifications and mutations, and DNA methylation changes are seen. These genetic and epigenetic alterations combine to result in cells with autonomous growth potential. Commonly implicated processes include inactivation of the tumor suppressor genes p53 and Rb, activation of the Wnt/β-catenin and EGFR cellular proliferation signaling pathways (an epithelial-to-mesenchymal transition), and host immunosuppression [3]. Therefore, the chronically diseased liver contains clonal populations of molecularly aberrant cells that may ultimately progress to malignancy. Although HCC occurs most often in cirrhotic livers, cirrhosis is probably not a premalignant condition per se, but a parallel process that occurs in response to the same insults as hepatocarcinogenesis.
Despite the variety of conditions that can contribute to HCC, the inflammatory microenvironment results in common pathogenic mechanisms mentioned above. An exception to these pathways is the direct involvement of the hepatitis B virus in hepatocarcinogenesis. Specifically, integration of the HBV genome directly contributes to hepatocarcinogenesis by causing host DNA microdeletions and transcriptional activation of cellular proliferation genes by the viral protein HBx [3]. Therefore, patients with chronic hepatitis B viral infection may develop HCC prior to the long period of inflammation that leads to cirrhosis. Another exception is the specific mutagenic effect of aflatoxin-B1, which mutates a particular amino acid of the p53 tumor suppressor protein. The specific aflatoxin-B1-associated mutation is rarely detected in HCC cases occurring the United States, but is present in 30% to 60% of HCC tumor samples collected from aflatoxin-endemic areas such as eastern Asia and sub-Saharan Africa [4].
Histologic alterations
During hepatocarcinogenesis, hepatocellular nodules progress in stages from benignity to overt malignancy. Although the progression spans a biological continuum, nodules along five stages of this spectrum are categorized for clinical use according to their histologic features [5]: (1) regenerative nodule, (2) low-grade dysplastic nodule (LGDN), (3) high-grade dysplastic nodule (HGDN), (4) early HCC, and (5) progressed HCC. The molecular alterations that accompany hepatocarcinogenesis are not applied in defining these stages, as molecular characterization is rarely performed clinically.
A regenerative nodule, formally known as a cirrhotic regenerative nodule, is a well-defined nodular lesion surrounded by fibrotic scar tissue. Regenerative nodules are indistinguishable from background liver parenchyma on histology and imaging. However, they can progress to dysplastic nodules that have abnormal cytologic features. Regenerative nodules can occur in the absence of cirrhosis, particularly in vascular disorders such as diffuse nodular regenerative hyperplasia or Budd–Chiari syndrome. In these cases, regenerative nodules are not initially surrounded by fibrotic scar tissue unless the underlying condition progresses to cirrhosis.
Dysplastic nodules are classified into LGDNs and HGDNs. LGDNs are difficult to distinguish from regenerative nodules based on morphology alone, as they lack cellular atypia [5] by definition. Fortunately, this distinction is of little clinical consequence, as LGDNs tend to have an indolent course with only slight elevated risk of progression to malignancy compared to background regenerative nodules. However, HGDNs are premalignant nodules demonstrating cellular and architectural atypia, albeit insufficient for the diagnosis of HCC. HGDNs can also display altered vascular supply, like that seen in HCC, as described below. Therefore, HGDNs may be difficult to distinguish from well-differentiated HCC.
Evolution from a dysplastic nodule to HCC is defined by stromal invasion and accelerated neoangiogenesis. Stromal invasion refers to growth of tumor cells into the fibrous tissue (stroma) of the portal tracts. Neoangiogenesis, the formation of new blood vessels within a tumor, is characterized in HCC by growth of unpaired arteries and sinusoidal capillarization. Unpaired (or non-triadal) arteries are isolated arteries that are not accompanied by bile ducts (or portal veins) because they were not formed during normal hepatic development. Sinusoidal capillarization refers to alterations in the sinusoidal epithelium, such as loss of fenestrae and deposition of a basement membrane. HCC is divided into early (or vaguely nodular) HCC and progressed (or distinctly nodular) HCC. Early HCCs are usually smaller than 1.5 cm but occasionally may be as large as 2 cm or more. Progressed HCCs that have arisen from within early HCCs, by comparison, tend to be larger than 1.5 cm. As discussed later, some progressed HCCs are intrahepatic metastases from a primary mass elsewhere in the liver. Metastatic HCCs may be any size, depending on when they are detected. Unlike early HCC which has little capacity for vascular invasion or metastasis, progressed HCC is frankly malignant, commonly displaying microvascular invasion and a tumor capsule. Thus, while tumor cells in both early HCC and progressed HCC invade the stroma around the portal tracts, only the tumor cells of progressed HCCs are expected to invade through the vessel wall into the lumen. While progressed HCC is distinctly nodular and characterized by expansile growth initially, further dedifferentiation may allow the cells to directly infiltrate the surrounding stroma and exhibit a permeative growth pattern.
Imaging features
While molecular and histologic alterations during hepatocarcinogenesis have specific implications for the imaging diagnosis of HCC (Table 1), it is important to recognize that histologic and LI-RADS categories do not have a 1:1 correspondence (Fig. 1). In this section, we will describe how LI-RADS features relate to hepatocarcinogenesis.
Each LI-RADS category does not correspond to one histologic grade. LI-RADS categories reflect the radiologist’s degree of diagnostic suspicion based on observed imaging features, but those categories do not correspond exactly to the histologic categorization described by pathologists. For example, all LI-RADS 5 observations meet imaging criteria for HCC and are very likely to also meet histologic criteria for HCC. However, not all HCCs are LI-RADS 5, some can be categorized as LI-RADS 3 or 4 based on their imaging features
Major features
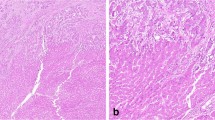
Arterial hyperenhancement, which is enhancement unequivocally greater than background liver during the hepatic arterial phase, is a major feature in LI-RADS. Although normal liver, regenerative nodules, and dysplastic nodules derive the majority of their blood supply from the portal vein, HCC derives a higher proportion of its blood supply from the hepatic artery (Fig. 2B). HCCs arterially hyperenhance because of formation of numerous unpaired arteries through neoangiogenesis. Unpaired arteries within an HCC (Fig. 3) differ from undistorted vessels coursing through an observation that is not space-occupying (see Fig. 17 of Chernyak et al. [11]). The number of unpaired arteries found in nodular HCC correlates to the degree of arterial phase hyperenhancement [6]. Poorly differentiated HCC, especially those with an infiltrative imaging appearance, may switch from aerobic to glycolytic metabolism, with subsequent underexpression of angiogenic signaling pathways. Thus, this type of HCC may show no arterial phase hyperenhancement, preventing its categorization as LI-RADS 5.
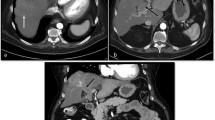
Major diagnostic features of HCC include arterial phase hyperenhancement, washout appearance, and capsule appearance. A 62-year-old woman with hepatitis-C cirrhosis underwent an abdominal MRI with extracellular contrast agent gadobutrol for follow-up imaging on a suspicious nodule. A A 21 mm observation in segment 6 is hypointense to the liver parenchyma in the pre-contrast phase. B The observation demonstrates arterial phase hyperenhancement and C washout appearance in the portal venous phase. C A hyperenhancing rim around the observation indicates capsule appearance
Formation of unpaired arteries is a key feature of progression from dysplastic nodule to HCC. A 56-year-old man with HCC underwent an abdominal CT with intravenous contrast. A An observation in segment 7 demonstrates hyperenhancement in the arterial phase due to unpaired arteries within an HCC. B The tubular structure of the arteries is better shown using a maximum intensity projection of 4 mm thickness at the same level
Enhancing capsule appearance refers to a peripheral rim of smooth hyperenhancement unequivocally thicker or more conspicuous than fibrotic tissue surrounding background nodules on portal venous, delayed, or transitional phase imaging (Fig. 2C). The enhancing “capsule” has a strong positive predictive value for HCC in at-risk patients and may correlate histologically to either a true capsule or a pseudocapsule [7]. True fibrous capsules are made up of collagen produced by myofibroblast-like cells and are surrounded by prominent sinusoids. A pseudocapsule contains varying amounts of fibrosis and dilated sinusoids without the discretely layered architecture of a true capsule.
Threshold growth in LI-RADS is defined as a diameter increase of an observation by at least 5 mm that represents either a ≥ 50% diameter increase over ≤ 6 months or ≥ 100% diameter increase over > 6 months. Also, a new ≥ 10 mm observation when compared to exams within the last 24 months is considered to represent threshold growth. Growth in the size of a mass is a feature of all solid malignancies, including HCC.
Washout appearance refers to a visually assessed temporal reduction in enhancement in whole or in part of the observation relative to composite liver tissue from earlier to later phase resulting in extracellular phase hypoenhancement (see Fig. 2C and Fig. 3 of Santillan et al. [8]). The mechanism that results in washout appearance is still a matter of debate. Some suggest that the “washout” is due to rapid venous drainage from the HCC [9]. Alternatively, washout appearance may be caused by the background liver achieving peak parenchymal enhancement during the portal venous phase. Since an HCC has a relatively diminished portal venous supply, the tumor receives less contrast-enhanced blood during the venous phase than background liver. This results in apparent hypoenhancement although the tumor is not actually de-enhancing. Historically, the evaluation of washout appearance has been subjective rather than quantitatively based on kinetics or absolute values reflecting decreased attenuation or signal intensity.
Ancillary features
Hepatobiliary contrast agents (such as gadoxetate disodium) cause hyperintensity of the liver due to progressive uptake of the agents into hepatocytes by organic anion transporter polypeptides (OATP). Hepatocarcinogenesis is associated with decreased expression of these transporters, accounting for hepatobiliary phase hypointensity seen in HCC [10] (see Fig. 9 of Chernyak et al. [11]). However, genomic abnormalities acquired during hepatocarcinogenesis can also cause overexpression of OATP in a minority of HCCs, resulting in hyperintensity on hepatobiliary phase imaging. Multidrug resistance-associated proteins (MRPs) are responsible for excretion of hepatobiliary contrast agents, and cirrhosis causes upregulation of MRPs to facilitate elimination of various exogenous substances. Changes in the expression levels of MRPs during hepatocarcinogenesis have not yet been studied systematically. These variations in genomic alterations likely account for inconsistent results in studies that have attempted to correlate hepatobiliary phase intensity with tumor grade. HBP hypointensity can occur earlier in hepatocarcinogenesis than arterial hyperenhancement (Fig. 4). Therefore, nodules that demonstrate hepatobiliary phase hypointensity without abnormal dynamic contrast enhancement should be followed closely for the subsequent development of arterial phase hyperenhancement that may indicate malignant transformation and more definitive diagnosis as HCC (Fig. 5).
Alterations to vascular supply and OATP expression during multi-step hepatocarcinogenesis. Hepatocarcinogenesis is characterized by successive selection and expansion of less-differentiated subnodules within more well-differentiated parent nodules. The subnodules grow and eventually replace (blue arrows) the parent nodules. Progressed HCCs show expansile growth (red arrows) and characteristically are encapsulated with fibrous septa. Earlier nodules lack these structures and show replacing growth. During hepatocarcinogenesis, the density of portal triads diminishes while the density of unpaired arteries increases. The net effect is that intranodular arterial supply diminishes initially and then increases (bottom graph); progressed HCCs typically show arterial hypervascularity compared with background liver, while earlier nodules typically do not. OATP expression usually diminishes progressively (top graph); progressed HCCs, early HCCs, many high-grade dysplastic nodules, and some low-grade dysplastic nodules show OATP underexpression compared with background liver. Not all nodules exhibit the illustrated characteristics. Also note that during tumor development some stages may be skipped and not all HCCs arise from histologically definable precursor lesions.
Hepatobiliary phase hypointensity can precede arterial phase hyperenhancement. A 76-year-old man with HCV-induced cirrhosis underwent multiple abdominal MRIs with hepatobiliary contrast agent (gadoxetate) for HCC surveillance. A An observation in segment 5 demonstrates hepatobiliary phase hypointensity on the initial examination. B The observation does not demonstrate arterial phase hyperenhancement. The same observation 16 months later demonstrates C hepatobiliary phase hypointensity and D arterial phase hyperenhancement (arrow). Observations demonstrating hepatobiliary phase hypointensity should be observed for the subsequent development of other imaging features of HCC
Corona enhancement refers to a zone or rim of peri-observation enhancement in the late arterial phase or early portal venous phase occurring after rapid dissipation of contrast material from an arterial phase hyperenhancing mass. This feature is observed because blood from HCCs tends to drain into the surrounding parenchyma rather than into the hepatic venous system. During hepatocarcinogenesis, hepatic veins are occluded by compression and invasion, so drainage shifts from hepatic veins to hepatic sinusoids, and then subsequently to portal veins [12, 13]. In a nodule with a capsule, drainage may occur via sinusoids within the capsule. This pattern of shifting venous drainage during hepatocarcinogenesis explains why intrahepatic metastases are often detected as “satellites” within the parenchyma surrounding a primary tumor (Fig. 6). Due to the high risk of metastatic foci in the adjacent liver, ablative therapies often need to include treatment of tissue beyond the visible margin of the HCC.
Satellite nodules reflect intrahepatic metastasis and shifting venous drainage patterns during hepatocarcinogenesis. A A 60-year-old man underwent an MRI with gadoxetate. Hyperenhancing satellite nodules (arrows) in segment 6 can be seen surrounding the primary tumor in the arterial phase. B A different 60-year-old man underwent abdominal CT for HCC surveillance. Hyperenhancing satellite nodules (arrows) in segment 4a can be seen surrounding the primary tumor in the arterial phase
“Nodule-in-nodule” appearance refers to the presence of a small nodule within a larger nodule (Fig. 7A). When multiple smaller nodules are present within a larger nodule, it may constitute part of “mosaic architecture” [14] (Fig. 7B, C). Any clonal expansion of a hepatocyte population that has acquired a new mutation conferring enhanced proliferative capacity may result in a “nodule-in-nodule” appearance, often manifesting as differences in fat, iron, or water concentration. This imaging appearance classically indicates the development of a progressed HCC within a dysplastic nodule or well-differentiated HCC. However, a nodule-in-nodule appearance could also represent a HGDN forming within a LGDN. Hence, nodule-in-nodule appearance is not diagnostic of HCC but is considered an ancillary feature favoring HCC, and reflects progressive dedifferentiation of nodules during hepatocarcinogenesis. Importantly, nodule-in-nodule does not occur in the development of cholangiocarcinoma. Thus, in problematic cases, nodule-in-nodule architecture virtually excludes cholangiocarcinoma from diagnostic consideration.
Nodule-in-nodule and mosaic appearance. A A 67-year-old man underwent an abdominal MRI with gadoxetate for HCC surveillance. An observation in segment 6 of the hepatobiliary phase demonstrates nodule-in-nodule appearance. “Nodule-in-nodule” refers to the presence of a small nodule (arrow) within a larger nodule which often indicates a progressed HCC. B A 55-year-old man with HCC underwent an abdominal CT scan to evaluate treatment response after chemoembolization. An observation spanning segments 4a, 7, and 8 in the arterial phase demonstrates mosaic architecture (arrow). Mosaic architecture refers to the presence of multiple smaller nodules within a larger nodule. C A 62-year-old male underwent an abdominal MRI with gadobenate for HCC and cirrhosis surveillance. An observation in segment 8 of the arterial phase demonstrates mosaic architecture (arrow). Note the satellite metastasis near the tumor at the 2 o’clock position (arrowhead)
The vessels in HCC that develop during neoangiogenesis are leaky and abnormal. Therefore, despite formation of new blood vessels, regions of the tumor can remain relatively hypoxic compared to normal liver. Hypoxia induces vascular endothelial growth factor (VEGF) expression, which acts as an angiogenic factor and cytokine that increases the permeability of existing vessels [15]. This increase in vascular permeability throughout the tumor likely results in increased extracellular free water and is thought to cause mild or moderate T2 hyperintensity relative to background liver (see Fig. 7 of Chernyak et al. [11]).
Fat in mass (also known as intralesional fat) refers to the presence of lipid within a mass in higher concentration than in background liver (Fig. 8). This ancillary imaging feature is specific for hepatocellular neoplasia and, like nodule-in-nodule architecture, can be used to distinguish HCC from cholangiocarcinoma. Intralesional fat identified radiologically correlates to intracellular fatty change found histologically. The pathogenesis of intracellular fatty change is not well understood. Some investigators have postulated that fat may accumulate in response to the development of hypoxia within dysplastic nodules and early HCC as their blood supply shifts from portal vein to artery during hepatocarcinogenesis. However, the mechanism by which hypoxia induces fat accumulation remains unclear. Regardless, fatty change is a frequent histologic feature of early hepatocellular neoplasia, evident in as many as 40% of early HCCs (usually measuring ~ 1.1–1.5 cm) [16]. As HCC progresses, fatty change regresses. Therefore, intralesional fat is observed far less frequently in progressed HCCs. Fat-containing HCCs tend to be more indolent because intralesional fat is a feature of well-differentiated or early HCC, with the exception of steatohepatitic HCC. Associated with non-alcoholic steatohepatitis, this HCC variant has histologic evidence of steatosis and inflammation and can be aggressive despite its fat content [17] (Fig. 9). Thus, a heterogeneous fatty mass that meets LI-RADS 5 criteria is likely to be a steatohepatitic HCC; by comparison, a homogeneously fatty mass without arterial phase hyperenhancement (i.e., meets LI-RADS 3 or 4 criteria depending on its exact features) is likely a dysplastic nodule or early HCC.
Fat in mass (intralesional fat) is an ancillary feature of LI-RADS because it is often present in early HCC. A 59-year-old woman underwent an abdominal MRI with gadobenate. A An observation in segment 2 is inconspicuous on the in-phase image but B displays prominent signal loss on the opposed-phase image. C The observation demonstrates hyperintense T2 signal. D The observation displays high proton-density fat fraction, indicating a steatotic nodule
Probable steatohepatitic HCC. A 66-year-old woman underwent an abdominal MRI with gadobutrol for surveillance. A An observation measuring 3.2 cm in segment 5/6 enhances in the arterial phase and B demonstrates faint washout appearance in the portal venous phase. C The proton-density fat fraction map indicates the presence of fat in the nodule. The mass meets LI-RADS 5 criteria, and likely represents a steatohepatitic HCC
In cirrhotic livers without diffuse iron deposition, a nodule that has preferentially accumulated iron may be referred to as a “siderotic nodule” (Fig. 10). Siderotic nodules typically correspond to low-grade dysplastic nodules, and their imaging appearance is due to clonal proliferation of hepatocytes with increased iron uptake activity [18]. However, as dedifferentiation continues and HCC develops, the expression pattern of iron regulatory genes changes, resulting in decreased accumulation of iron. Therefore, most high-grade dysplastic nodules and HCCs have low iron content, including subnodules of dysplasia and malignancy developing within precursor siderotic nodules. The relative paucity of iron in a solid mass relative to background liver is referred to as iron sparing, and reflects the development of a focus of high-grade dysplasia or malignancy (Fig. 11).
Siderotic nodule. A 50-year-old man with HCC underwent an MRI with gadobutrol for post-TABE surveillance. Three observations were found in segments 4b and 6. A The observations demonstrate hypoenhancement in the arterial phase and B portal venous phase. C The observations also demonstrate hypointense T2 signal. D The observations are bright on the R2* map (indicating high R2* relaxivity decay rates) due to the presence of iron. These are likely low-grade dysplastic nodules unrelated to the treated HCC (not shown)
Iron sparing. A 60-year-old man underwent an MRI with gadobutrol for HCC surveillance. An observation was found in segment 5. A The observation displays arterial phase hyperenhancement and B portal venous phase “washout” with “capsule.” C The observation demonstrates hyperintensity on T2-weighted image. D The observation has low R2* decay compared to the background iron-overloaded liver on R2* map, which indicates an iron-sparing nodule
Conclusion
Hepatocarcinogenesis is a complex, multi-step process characterized by successive stages of dedifferentiation through dysplasia to malignancy. The molecular and histologic alterations that accumulate during this process result in abnormal imaging features, some of which are specific to HCC, and others that suggest malignancy in general. Study of hepatocarcinogenesis through molecular and clinical perspectives can enhance our understanding of HCC’s pathophysiology, and thereby enable more accurate and earlier diagnosis using LI-RADS.
References
Tarlow BD, Pelz C, Naugler WE, et al. (2014) Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15:605–618
Sia D, Villanueva A, Friedman SL, Llovet JM (2016) Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. https://doi.org/10.1053/j.gastro.2016.11.048
Farazi PA, DePinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6:674–687
El-Serag HB, Kanwal F (2014) Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 60:1767–1775
International Consensus Group for Hepatocellular Neoplasia (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49:658–664
Kim CK, Lim JH, Park CK, et al. (2005) Neoangiogenesis and sinusoidal capillarization in hepatocellular carcinoma: correlation between dynamic CT and density of tumor microvessels. Radiology 237:529–534
Ishigami K, Yoshimitsu K, Nishihara Y, et al. (2009) Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 250:435–443
Santillan C, Fowler K, Kono Y, Chernyak V (2017) LI-RADS major features: CT, MRI with extracellular agents, and MRI with hepatobiliary agents. Abdom Radiol. https://doi.org/10.1007/s00261-017-1291-4
Marrero JA, Hussain HK, Nghiem HV, et al. (2005) Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl 11:281–289
Kitao A, Matsui O, Yoneda N, et al. (2011) The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 21:2056–2066
Chernyak V, Tang A, Flusberg M, et al. (2017) LI-RADS ancillary features on CT and MRI. Abdom Radiol. https://doi.org/10.1007/s00261-017-1220-6
Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y (2009) Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography—radiologic-pathologic correlation. Radiology 252:605–614
Matsui O, Kobayashi S, Sanada J, et al. (2011) Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 36:264–272
Stevens WR, Gulino SP, Batts KP, Stephens DH, Johnson CD (1996) Mosaic pattern of hepatocellular carcinoma: histologic basis for a characteristic CT appearance. J Comput Assist Tomogr 20:337–342
Park YN, Kim Y-B, Yang KM, Park C (2000) Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med 124:1061–1065
Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M (2017) Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 33:282–289
Salomao M, Remotti H, Vaughan R, et al. (2012) The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol 43:737–746
Zhang J, Krinsky GA (2004) Iron-containing nodules of cirrhosis. NMR Biomed 17:459–464
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by a grant.
Conflicts of interest
Kazim Narsinh declares that he has no conflict of interest. Jennifer Cui declares that she has no conflict of interest. Demetri Papadatos declares that he has no conflict of interest. Claude Sirlin receives grant funding from Bayer, Guerbet, Siemens, General Electric, Supersonic, and Arterys. He has consulting and service agreements with Alexion, AstraZeneca, Bioclinica, BMS, Bracco, Celgene, Fibrogen, Galmed, Genentech, Genzyme, Gilead, Icon, Intercept, Isis, Janssen, NuSirt, Perspectum, Pfizer, Profil, Sanofi, Shire, Synageva, Tobira, Takeda, and Virtual Scopics. Cynthia Santillan declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Narsinh, K.H., Cui, J., Papadatos, D. et al. Hepatocarcinogenesis and LI-RADS. Abdom Radiol 43, 158–168 (2018). https://doi.org/10.1007/s00261-017-1409-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1409-8