Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide and within the United States. Liver transplant or partial liver resection is the definitive treatment of choice for HCC; however, the majority of cases are detected in advanced stages due to its early-stage asymptomatic nature, often precluding surgical treatment. Locoregional therapy plays an essential role in HCC management, including curative intent, as a bridge to transplant, or in some cases palliative therapy. Radiologists play a critical role in assessing tumor response following treatment to guide further management that may potentially impact transplantation eligibility; therefore, it is important for radiologists to have an understanding of different locoregional therapies and the variations of imaging response to different therapies. In this review article, we outline the imaging response to ablative therapy (AT), transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), and stereotactic body radiation therapy (SBRT). We will also briefly discuss the basic concepts of these locoregional therapies. This review focuses on the imaging features following locoregional treatment for hepatocellular carcinoma following AT, TACE, SIRT, and SBRT.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the 4th leading cause of cancer death and the 6th most diagnosed cancer worldwide for males and females of all ages, with Hepatocellular Carcinoma (HCC) the most common hepatic malignancy [1]. Within the United States, liver cancer is the 5th most common cause of death among men and the 7th among women as of 2019; the increase in death rate has been associated with the high prevalence of hepatitis C infection and the obesity epidemic [2]. Liver transplantation (LT) remains the definitive treatment as it removes both the tumor and cirrhosis, with 4-year survival of 75% for patients transplanted within Milan criteria (single tumor less than 5 cm; no more than three tumors each not exceeding 3 cm; no angioinvasion; no extrahepatic involvement) [3]. However, geographic disparities still exist in access to LT for HCC patients with recent increasing waiting list registration, increased time from listing to LT, and increased probability of drop-out while on the waiting list [4]. In addition, the majority of HCC cases are detected in advanced stages, with lack of symptoms in the early stages, making transplant or surgical resection a non-viable option [5]. Even among patients meeting initial criteria and listed for transplantation, up to 43% will eventually drop off the list due to reasons ranging from tumor progression beyond transplant criteria to death prior to transplant [6]. The major international guidelines that provide clinicians an overview of the care for patients with HCC are the European Association for the Study of Liver Disease (EASL), the American Association for the Study of Liver Disease (AASLD), and the Asian Pacific Association for the Study of Liver Disease (APASL) [7,8,9]. A well-known staging system for HCC that has been externally validated is the Barcelona Cancer Liver Clinic (BCLC), endorsed by the AASLD and EASL and has been discussed in previous publications [7, 8, 10]. Locoregional therapy (LRT) has made many advancements and has become the cornerstone for bridge to transplant (accepted transplant candidate within Milan criteria while on the waiting list), downstaging to within Milan criteria, and treatment of intermediate-stage HCC [7, 8]. Both the AASLD and EASL suggest bridging to transplant in early-stage HCC to decrease the risk of progression of tumor burden and subsequent drop-out from the waiting list, with no recommendation of one LRT over another [7, 8]. For downstaging, the AASLD and EASL suggest that HCC beyond Milan criteria be considered for a liver transplant after successful downstaging to within Milan criteria with LRT [7, 8]. The radiologist has a central role in guiding therapy selection, as well as evaluating tumor response to different locoregional/ablative therapies in patients with HCC. In this review article, we will discuss different locoregional therapies, their technical approach, indications and contraindications, tumor response assessment, pitfalls encountered in interpretation, and treatment complications, focusing on imaging characteristics. Currently, both MRI and CT are used to evaluate HCC treatment response and the use of either is appropriate by international guidelines. This article will focus only on MRI features, however, multi-phasic post-contrast CT findings are analogous to MRI.
Response criteria
Response classification using specific response criteria allows for research standardization, streamlined interdisciplinary dialog, and standardization of terminology used. The commonly used response criteria are the modified Response Criteria in Solid Tumor (mRECIST) and the European Association for the Study of the Liver (EASL) criteria, which use dynamic contrast-enhanced Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) during arterial enhancement as a marker of tumor viability (no enhancement being a marker of necrosis, and arterial enhancement a marker for residual or viable tumor) [11, 12], these has been discussed in previous publications.
Liver imaging reporting and data system (LI-RADS)
LI-RADS latest version was published in 2018 (LI-RADS v2018) and integrated by the AASLD as an image-based diagnosis for HCC [7, 13]. LI-RADS v2018 diagnostic algorithm for CT and MRI has a 4-step approach with 8 categories and intended for untreated observations without histological diagnosis in high-risk patients. It also provides suggested management for each category from the AASLD 2018 consensus [13]. For treated observations, LI-RADS treatment response algorithm v2017 was developed to asses these observations regardless of the type of locoregional and loco-ablative therapies used while taking in to account locoregional therapy done for curative intent, bridge to transplantation, or treatment of advanced disease [14]. Viability is defined by LI-RADS v2017 based on imaging characteristics and developed for consistent reporting and guiding management, keeping in mind that the assessment of tumor viability by imaging may not be concordant with pathological viability [14]. The major metric of viability is arterial phase hyperenhancement and/or washout [14].
Ablation
Clinical evidence, indications, and contraindications
Ablative procedures can be broadly divided into non-energy ablation (chemical ablation) and energy-based ablation (thermal and non-thermal) [15]. This includes radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, ethanol injection, and irreversible electroporation. Other than liver transplant, the AASLD recommendation for resectable HCC (1 to 3 unilobar lesions up to 5 cm for single lesion or 3 cm for ≥ 1 lesion, without extrahepatic spread or vascular invasion, and BCLC stage 0 or A) is surgical resection over RFA while EASL recommends RFA as first-line therapy for BCLC 0 in favorable location or as an alternative to hepatic resection (HR) for BCLC A for single tumors 2–3 cm [7, 8]. However, not all patients are able to undergo resection which can be due to poor hepatic reserve or multilobar distribution of tumor with only 9–29% able to tolerate resection [16]. Recent meta-analysis has demonstrated higher overall survival and disease-free survival for surgical resection compared to RFA in very early and early HCC, but with a shorter duration of hospital stay for RFA. [16, 17]. RFA is still the standard of care when percutaneous ablation is considered. Contraindications include bleeding diastasis, ECOG > 3, Child–Pugh C, tumor invasion of major hepatic bile ducts, and proximity to hallow viscus [18]. The choice between HR and ablation procedures is dependent on institutional protocol, operators experience, resources available, size and number of tumors, multidisciplinary discussions, and overall liver reserve.
Assessment of HCC response to ablation
The mechanism of action for RF, MWA, and cryoablation is beyond the scope of this article and has been described elsewhere [19,20,21]. RFA and MWA have similar post-ablation image findings. The ablation zone is usually 5–10 mm larger than the tumor to account for micrometastasis and microvascular invasion not seen on pre-ablation images and technical success is dependent on the ablation zone being larger than the tumor [15] (Fig. 1). Expected early (1–6 months) post-ablation MRI findings include foci of air which usually resolve on follow-up and heterogenous T1 hyperintensity and T2 hypointensity from hemorrhagic products and coagulation necrosis, which can be easily identified with subtraction sequences [22]. T2 hyperintensity indicates either liquefactive necrosis or biloma formation [22]. Expected early (1–6 months) periablation changes include peripheral rim enhancement from reactive hyperemia or T2 hyperintense rim from edema, this should progressively decrease over 4–9 months [22]. Expected chronic (> 6 months) post-ablation MRI include an ablation zone that is iso or hypointense on T2 [22, 23]. Disease progression is described as residual unablated tumor (persistent viable tumor after ablation) or local tumor progression (Development of new tumor after 1 prior image showing no viable tumor) [15]. Residual unablated tumor and local progression occur at the periphery and appear as an eccentric, focal, nodular T2 hyperintensity with enhancement on T1 arterial phase [24]. Residual unablated tumor is common at the periphery from causes of inadequate ablation due to nearby large vessels (heat sink effect), insufficient safety margin, or suboptimal technique [25]. Advantage of cryoablation is real-time monitoring of ice ball to insure adequate ablation margin ≥ 5 mm [26]. In the first 24 h after cryoablation, the 2 prognostic factors for local tumor progression are minimal ablation margin ≤ 3 mm and ≥ 3 mm blood vessel adjacent to the ablation margin [26]. Unlike RFA and MWA, persistent tumor enhancement in the first 24 h following a technically successful cryoablated margin is common and not prognostic of tumor progression (Likely freeze injury causing vasodilation of coursing vessels with leakage followed by reperfusion) [26]. The tumor enhancement eventually decreases over 2–7 months.
Cryoablation complete response. a–c Segment 6 peripheral arterially enhancing lesion (a white arrow) with T2 FS hyperintensity (b yellow arrow) and restricted diffusion (c red arrow), this was suspicious and multidisciplinary discussion deciding to ablate the lesion. d Cryoablation with monitoring of ice ball, ice ball covering ≥ 5 mm of lesion and considered a technical success. e, f Three months post cryoablation shows pre-contrast T1 mixed signal intensity (e white arrowhead) and no residual enhancement on subtraction images (f red arrowhead)
Pitfalls in tumor response evaluation after ablation
Post ablation inflammatory edema and vascular fibrous tissue are common. This appears as T2 hyperintense ablation zones with enhancement that mimic residual tumor. In this case, a determination of adequate tumor margin ablation is done by comparing pre-ablation, procedural, and immediate post-ablation images. If partial ablation is detected then the chances of residual tumor are high and discussions with multidisciplinary team are warranted for further treatment [24]. Like RFA and MWA, early periablation arterial enhancement may be evident from cryoablation due to reactive hyperemia. This usually resolves on follow-up imaging or may develop (as early as 3 months) a peripheral ring enhancement that is T1 and T2 hypointense with enhancement on delayed imaging indicating a fibrous capsule [22].
Complications of ablative procedures
RFA, MWA, and Cryoablation are generally safe procedures [27, 28]. Complications can be classified into vascular, biliary, extrahepatic, infectious, and tumor seeding with each subclassified as minor or major [27, 28]. Vascular complications include portal vein thrombosis, hepatic infarction, subcapsular or intraparenchymal hemorrhage from mechanical injury, and mechanical injury to vessels causing pseudoaneurysm or arterial–portal shunt [29]. Biliary complications include bile duct leakage leading to biloma formation, biliary stricture, acute cholecystitis for ablated lesions near the gallbladder, and rarely gallbladder perforation [29]. Extrahepatic complications include mechanical injuries such as diaphragm perforation, pneumothorax, and rarely bowel perforation [29]. Infectious complications include abscess formation that appears as foci of air with clinical symptoms of fever and abdominal pain to support the diagnosis [29]. Tumor seeding is rare and appears as enhancing irregular lesions along the tracks of ablation electrode, it is common with superficial and subcapsular tumors [24].
Selective arterial embolization
Clinical evidence, indications, and contraindications
Transarterial embolization has gained wide acceptance and is the first-line therapy for asymptomatic intermediate-stage HCC without portal vein thrombosis and with preserved liver function (Child–Pugh B and less), early-stage HCC as bridge to transplantation, downstaging to within Milan criteria, or when curative resection and ablative therapies are contraindicated [8]. This treatment is based on super-selective arterial occlusion of tumor feeding vessels with embolic material with or without chemotherapeutic agent delivery, to achieve tumor devascularization and complete tumor necrosis. Different techniques and approaches exist; this includes transcatheter arterial embolization (TAE), conventional transarterial chemoembolization (cTACE) by arterial embolization and delivery of chemotherapeutic agent by ethiodized oil, and Transarterial Chemoembolization with drug-eluting beads and super absorbent polymer microspheres loaded with doxorubicin (DEB-TACE). The choice between TAE, cTACE, and DEB-TACE is influenced by a number of factors including operator preference and institutional protocol.
Assessment of HCC response to TACE
When using cTACE, lipiodol usually stains the treated lesion for months while DEB-TACE usually washes out within hours. Non-contrast CT is obtained within 48 h to assess technical success as lipiodol uptake in the entire tumor (the desired scenario) is correlated with complete necrosis [30]. If incomplete retention is seen, another session of cTACE can be scheduled [31]. If no retention is seen, then an aberrant supply needs to be considered and repeated angiography to identify variant anatomy [31]. A set of two TACE procedures is performed 2–8 weeks apart depending on treatment efficacy and need to re-treat, tolerance of treatment, and reaching complete response. Expected successful embolization within 1 month is the absence of enhancement on imaging that is the hallmark of a fully treated lesion (Fig. 2) [32]. Coagulative necrosis with proteinaceous/hemorrhagic debris appears hyperintense on T1 with no enhancement on subtraction sequence and hypointense on T2, if liquefactive necrosis it appears hyperintense on T2 [33]. A complete thin rim of peripheral enhancement embolization zone is likely benign. Expected residual/recurrent appears as incomplete ring, nodular, or satellite enhancement particularly if there is washout on venous or delayed phase imaging (Fig. 3) [32]. If suspicious enhancement is present, it can be re-assessed on a follow-up study with increasing size of enhancement or increasing nodularity indicating residual or recurrent tumor [32]. Diffusion-weighted imaging (DWI) can be used to as a secondary tool to evaluate tumor response where restricted diffusion (High DWI, low ADC) indicates intact tumor cells and increased diffusivity (Low DWI, high ADC) indicates necrosis [34]. Although there have been conflicting data in the use of DWI and ADC values in early response, a recent meta-analysis evaluating DWI and ADC in diagnosing residual or recurrent tumor found DWI had a sensitivity of 85% and specificity of 83% [35]. There is promise in using DWI and ADC values in early response assessment, however, it should be used as an adjunct and not a replacement for traditional response assessment until further research is undertaken. Currently, no standardized follow-up routine exists with a paucity of evidence to dictate a suitable follow-up method. At authors institution, follow-up MRI imaging is routinely performed at the end of the 1st month after embolization, 3rd month, and every 6 months thereafter.
Different patterns of enhancement after TACE treatment. a Rim-like smooth peripheral enhancement is usually benign and resolves on follow-up imaging. b–d Incomplete peripheral ring enhancement, nodular-like enhancement, or nodular foci of enhancement adjacent to the zone of embolization are suspicions for local recurrence or viable tumor. Note that geographic foci enhancement with no washout are often seen which maybe benign vascular shunts
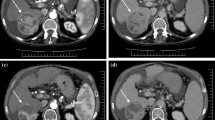
Recurrence adjacent to the zone of ablation. A 64-year-old male with chronic hepatitis C and infiltrative HCC on imaging. Underwent primary treatment with TACE. a, b Pre-TACE multi-phase T1 scan shows a large arterially enhancing lesion in the right lobe (a) with heterogeneous washout on portal venous phase (b). c 2 months post-TACE multi-phase T1 arterial subtraction image with no residual enhancement of the embolized region. d 8 months post-TACE shows arterial enhancement surrounding the previous embolized lesion, classified as LR-TR
Pitfalls in tumor response evaluation after TACE
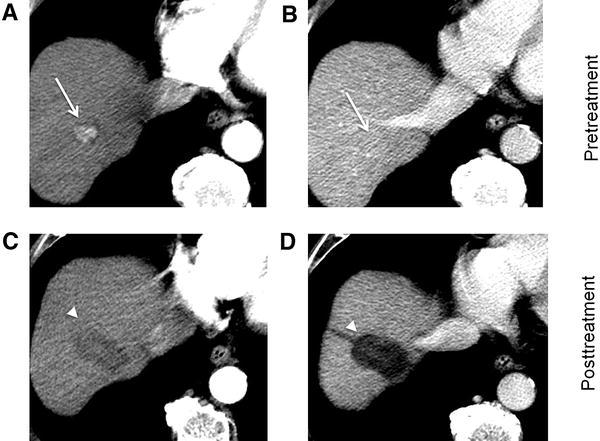
Well-circumscribed homogenous enhancement of a treated lesion that persists on arterial and portal venous phases can occur post-TACE on early post-treatment MRI imaging; this finding is thought to reflect evolving granulation tissue (Fig. 4) [32]. This should not be confused with residual or recurrent disease as it does not demonstrate typical washout on portal venous or delayed phases, and close follow-up within 3 months is warranted to document resolution/stability. There may also be transient hepatic intensity difference (THID) appearing as wedge-shaped early enhancement. This represents benign perfusional change and can be differentiated from residual tumor, as it is isointense to surrounding parenchyma on portal venous and delayed phases (Fig. 5).
Benign non-nodular peripheral rim enhancement post-TACE. A 72 year old with segment 5 HCC and Pre-TACE AFP 509 ng/dl. a Two months post-TACE dynamic T1 portal venous phase shows a thin non-nodular rim enhancement surrounding the embolized lesion (white arrow). b Five months post-TACE T1 arterial phase subtraction image shows resolution of smooth peripheral rim enhancement. Benign finding and usually resolves on follow-up. AFP normalized to 3 ng/dl at 2 months post-TACE
Perfusional changes post-TACE. A 70-year-old male with chronic hepatitis C and HCC by imaging criteria. Underwent DEB-TACE as bridge-to-therapy. a–c 1 month post-TACE dynamic T1 WI shows regional late arterial hypo-enhancement (a white circle) subsequently isointense to surrounding liver on portal venous phase (b). c 5 months post-TACE dynamic T1 WI images show resolution of regional arterial hypo-enhancement
Complications of TACE
The most common clinical complication of TACE is post-embolization syndrome immediately after treatment, presenting as fever, nausea, vomiting, and abdominal pain; this does not have an imaging correlate. Among complications with an imaging correlate is non-target embolization [36]. Inadvertent embolization of the cystic artery causes imaging characteristics of cholecystitis including pericholecystic fluid and gallbladder wall thickening [33]. If the gastroduodenal artery is embolized this may result in pancreatitis, with imaging findings similar to other causes of pancreatitis [36]. The intrahepatic bile ducts are supplied exclusively by hepatic arterial branches. Microvascular damage to peribiliary plexuses from TACE is hypothesized to cause bile duct necrosis with bile leakage, which can lead to biloma formation [37]. This appears on imaging as a T2 hyperintense collection with no enhancement (Fig. 6), with or without secondary infection or abscess [37]. A rare complication with high mortality following TACE is HCC rupture, with only a few case reports in the literature [38].
Selective internal radiation therapy
Clinical evidence, indications, and contraindications
SIRT involves selectively delivering tumoricidal doses of radiation to target lesions while minimizing radiation to the non-target regions of the liver. This is achieved by super-selectively delivering either glass or non-degradable resin loaded with yttrium-90 (Y-90). Y-90 is a beta emitter that decays to stable zirconium-90 as the primary mode of action and causes microembolization of the vasculature as a secondary mode of action [39]. Treatment simulation and shunt fraction simulation are an important part of successful and safe Y-90 treatment, however, it is beyond the scope of this article and has been described elsewhere [40]. Although radiofrequency ablation (RFA) is recommended for unresectable BCLC A and TACE for BCLC B, SIRT has emerged as an alternative treatment option. The choice between different LRT is influenced by institutional protocol and operator experience. Indications for SIRT based on consensus panel recommendations are intermediate (BCLC B) or advanced stage (BCLC C) HCC who have contraindications to TACE or sorafenib, a life expectancy of at least 3 months, and patients who fail first-line therapies for BCLC A, B, or C [41, 42]. Contraindications to SIRT include a hepatopulmonary shunt that would deliver 30 Gy or more radiation to the lung (defined on a pretreatment 99mTc macro-aggregated albumin (MAA) shunt study), extrahepatic uptake that cannot be corrected with angiographic embolization, excessive tumor burden (tumor volume exceeding 50% of the liver volume in cirrhotic patients and > 70% in non-cirrhotic patients), and elevated total bilirubin > 2 mg/dl in the absence of a reversible cause [41, 42].
Assessment of HCC response to SIRT
SIRT mechanism of action is predominantly caused by radiation effects with minimal microembolization rather than the flow-related ischemic effects of TACE [43]. Expected response occurs over several months [3–6 months] with initial reduction in enhancement before reduction in size and image assessment is recommended at a minimum of 3 months after SIRT (Fig. 7) [44]. Other early post-treatment findings (First 6 months) are pseudoprogression and thin non-nodular peripheral enhancement. Pseudoprogression is seen as initial increase in tumor size with reduced enhancement from necrosis, this occurs at a mean of 29–31 days and persists for several months [44]. Thin non-nodular peripheral enhancement is a benign finding that persists for 1–8 months [45]. Persistent or residual enhancement is a diagnostic challenge; in the first months this can appear as nodular or thick/uneven enhancement [45]. If short-term follow-up imaging (≤ 3 months) shows increase in the enhancing portion then is it viable tumor and re-treatment is warrant [45]. If follow-up imaging shows stable or decrease in enhancement, then it indicates successful treatment as enhancement pattern (Nodular or thick) after Y-90 has no predictive value for viable tumor (Fig. 8) [46]. DWI may have a role in determining response in the early period when no change in size is present. A study showed that an increase in ADC value as early as 30 days may detect response to Y-90 before a change in size [47]. Currently, DWI generally serves as a complementary secondary assessment and not a substitute for primary anatomic/viability assessment criteria [48].
Complete response after SIRT. A 79-year-old male with an HCC lesion and Child–Pugh A cirrhosis. a T1 WI portal venous phase demonstrates the lesion in the dome of the liver. b 3 months post SIRT T1WI arterial phase subtracting the precontrast axial T1WI MR shows similar size of the nodule/lesion with no intralesional enhancement (white arrow). c There is necrosis of surrounding liver parenchyma evidenced by lack of enhancing parenchyma on portal venous phase (white arrowhead). d 8 months post SIRT T1WI hepatic venous phase shows reduction in size of the zone of ablation and no intrazonal enhancement consistent with complete response (white arrow)
Thick/uneven enhancement post SIRT. An 80-year-old female with treated HCC by SIRT on follow-up imaging. a 2 months post SIRT T1 arterial phase subtraction image shows uneven, thick arterial enhancement (white arrow), this was followed closely. b 5 months follow-up shows resolution of uneven enhancement and more prominent thin rim of peripheral enhancement on arterial phase (white arrow). c 9 months post SIRT shows decrease in size and the thin rim enhancement is less prominent on follow-up. SIRT and SBRT responses occur over several months, this highlights the delayed response nature for these treatment modalities
Pitfalls in tumor response evaluation after SIRT
After SIRT, there may be lesional and perilesional changes. Lesional changes include hemorrhagic/proteinaceous debris from tumor necrosis that appear hyperintense on T1 sequence and no enhancement on subtraction images. Perilesional changes include peritumoral geographic enhancement, hepatic atrophy with fibrosis, and capsular retraction. Expected early findings include peritumoral geographic changes that appear as increased parenchymal enhancement on arterial or portal venous phases. DWI can distinguish post-inflammatory changes from viable tumor; increased ADC value indicates inflamed tissue and not residual tumor [44]. An expected finding on later follow-up of ablation zone (≥ 6 months) includes hepatic atrophy with fibrosis (Fig. 9), contralateral lobar hypertrophy, increased portal vein diameter, and splenic volume that can mimic imaging criteria for portal hypertension. Capsular retraction occurs on late follow-up (≥ 6 months) and is caused by treated tumors in subcapsular location undergoing necrosis that distorts the margins [44].
Inflammatory edema, fibrotic changes, and mild capsular retraction post SIRT. A 68-year-old male with an HCC lesion underwent SIRT (receiving a dose of 3.37 GBq). a 1.5 months post SIRT dynamic T1 WI arterial image demonstrates ill-defined wedge-shaped peripheral enhancement (white arrow) and T2 WI b high signal intensity from inflammatory changes and edema (white circle). c 12 months post SIRT T1 WI delayed scan shows enhancement consistent with fibrotic changes and progressive capsular retraction (white arrowhead)
Complications of SIRT
Although SIRT is a well-tolerated procedure, complications can occur. The peribiliary plexuses are susceptible to microsphere occlusion leading to injury, bile leakage, and biloma formation [49]. This may lead to superinfection with hepatic abscess formation [49]. Another complication, especially with bilobar treatment, is radioembolization-induced liver disease (REILD), which manifests 4–8 weeks after treatment with incidence ranging between 0 and 8% [50]. This is similar to the radiation-induced liver disease that can be caused by SBRT, with pathological changes consistent with veno-occlusive disease. Imaging features are non-specific and include heterogeneous enhancement or hypo-enhancement on the portal venous phase. Diagnosis should take into account the clinical picture and laboratory findings supporting the diagnosis of REILD.
Stereotactic body radiation therapy
Clinical evidence, indications, and contraindications
In the past, radiation therapy to the liver was limited due to the low tolerance of the whole liver, as no more than 30–35 Gy can be given without the risk of radiation-induced liver disease [51, 52]. However, in recent years, advances in radiotherapeutic treatment planning allow for delivery of radical radiation doses to tumor while preserving adjacent normal tissue. This allows dose escalation to a target lesion with high conformal ablative radiation dose [51, 53, 54]. Despite the data available for the efficacy and safety of external beam radiation therapy, EASL and AASLD state that further large-scale prospective studies are needed [7, 8]. A consensus on SBRT for HCC at the 7th Asian-pacific primary liver cancer expert meeting in 2017 outlined the indications for SBRT: 1–5 lesions with a maximum diameter of 5 cm, contraindications or refractory to locoregional/other ablative therapies to be used as palliative or bridge to transplant, a minimum distance from gastrointestinal luminal tissue of 5 mm, and preferably Child–Pugh class B7 or less [55].
Assessment of HCC response to SBRT
Assessment of HCC response to SBRT requires evaluation of changes in internal enhancement characteristics and changes in target lesion size [56]. Assessment of tumor response is undertaken with multi-phase contrast-enhanced CT or MRI. Fiducial markers may confound CT evaluation of treatment changes, and MRI may be preferred [57]. In the first 12 months, arterial tumor enhancement with or without portal venous washout may persist in the early months and gradually decreases over time. Persistent arterial enhancement soon after treatment does not necessarily indicate residual tumor. Instead, this may reflect early inflammation as progressive necrosis may take up to 12 months, a finding unique to SBRT and SIRT (Fig. 10) [58, 59]. This is followed by a gradual reduction in size that occurs over 6–12 months. It should be noted that mRECIST and ELAS take into account the viability of the tumor, it is unclear if these criteria apply to HCC treated with SBRT, as the treatment and mechanism of tumor death differ from other locoregional and ablative therapies and may not be well suited for evaluating HCC post-SBRT; more validation studies are needed to define this [59]. In a paper by Oldrini et al. studying MRI predictive factors for tumor response after SBRT, the authors concluded that a reduction in T2 signal intensity and absence of tumor DWI signal intensity three months following treatment correlated with subsequent complete response according to RECIST [60].
Pitfalls in tumor response evaluation after SBRT
The liver parenchyma within the non-ablative field surrounding the target lesion undergoes temporal imaging changes following SBRT, the changes in imaging appearance are referred to as focal liver reaction (FLR). FLR can be divided into acute (1–3 months), subacute (3–6 months), and chronic (> 6 months) and are discussed in the pitfalls section. The acute phase manifests on imaging as arterial phase hyperenhancement conforming to the shape of the non-ablative dose irradiated field that can either persist or show hypo-enhancement on the portal venous phase (Fig. 11) [56, 58]. The subacute phase manifests on imaging as hypo-enhancement on portal venous phase and hyperenhancement on delayed phase [58, 59]. In the chronic phase, there is fibrosis formation with volume loss, and if the lesion is subcapsular in location there may be associated atrophy/capsular retraction of the liver. On imaging, this will appear as minimal arterial enhancement with progressive hyperenhancement on the delayed phase from fibrosis and rim-like loss of signal on in-phase MRI [56]. Regarding lesional changes, a common finding in the short-term follow-up (< 3 months) period after SBRT is increased tumor size with persistent arterial enhancement and portal venous washout similar to SIRT (Fig. 12). This usually resolves in the subacute phase on follow-up and a size decrease will be apparent at 3–6 months [58, 59]. Rim-like enhancement on arterial or portal venous phase imaging can occur surrounding the liver lesion from FLR in the acute and subacute phases; this usually resolves in the chronic phase.
Acute phase FLR arterial enhancement. A 67-year-old female with past medical history of HCV diagnosed with HCC, 1 month post SBRT treatment. a T1 dynamic MRI arterial phase demonstrates perilesional arterial enhancement (white arrow). b Delayed phase demonstrates iso-intensity to surrounding liver parenchyma with no delay in contrast clearance. A common finding in the acute phase (1–3 months) of slow portal flow appearing as perilesional arterial enhancement
Early images after SBRT with temporary increase in tumor size and persistent early enhancement. A 60-year-old male with cirrhosis diagnosed with HCC. Planned for 50 Gy over 5 fractions. a, b pre-SBRT Dynamic T1WI scan arterially enhancing lesion (a) with washout and pseudo-capsule (b). c, d 26 days after SBRT shows persistent arterial enhancement (c) and washout with pseudo-capsule (d), there is an apparent size increase from perilesional focal liver reaction. e 4 months post SBRT shows dynamic T1 WI with no enhancement and a decrease in overall size consistent with complete response
Complications of SBRT
The most common complications are gastrointestinal and hepatic toxicity [61]. The rate of GI and hepatic complications are 4.7% (95% CI 3.4–6.5) and 3.9% (95% CI 2.6–5.6) [61]. Liver toxicity is in the form of RILD which can present as subclincal inflammation of elevated LFT or decline in Child–Pugh score with hepatomegaly, ascites, right upper quadrant pain, and elevated alkaline phosphatase [62]. There are no specific imaging findings and the diagnosis is clinical. If the radiation field is close to bowel, there is risk radiation-induced inflammation [62]. For radiation fields at the hepatic dome, there is risk of radiation-induced pneumonitis [63].
Conclusion
Several locoregional and ablative therapies are available for the management of HCC. Radiology plays a key role in treatment selection, response assessment following therapy, and detection of treatment complications. It is therefore crucial for diagnostic radiologists to understand the indications for therapy, common patterns of treatment response, complications, and imaging pitfalls in order to interpret studies accurately in these patients.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone A-M, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2018;124(13):2785–800.
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis [Internet]. https://doi.org/10.1056/NEJM199603143341104. 2009 [cited 2019 Dec 21]. Available from: https://doi.org/10.1056/NEJM199603143341104?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov
Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol. 2016 May 14;22(18):4438–45.
Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016 Oct 5;3:41–53.
Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2002 Oct;8(10):873–83.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol Baltim Md. 2018;67(1):358–80.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018 Jul 1;69(1):182–236.
Omata M, Cheng A-L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017 Jun 15;11(4):317–70.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018 Mar 31;391(10127):1301–14.
European Association For the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012 Apr;56(4):908–43.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30(1):52–60.
Elsayes KM, Kielar AZ, Elmohr MM, Chernyak V, Masch WR, Furlan A, et al. White paper of the Society of Abdominal Radiology hepatocellular carcinoma diagnosis disease-focused panel on LI-RADS v2018 for CT and MRI. Abdom Radiol N Y. 2018;43(10):2625–42.
Kielar A, Fowler KJ, Lewis S, Yaghmai V, Miller FH, Yarmohammadi H, et al. Locoregional Therapies for Hepatocellular Carcinoma and the New LI-RADS Treatment Response Algorithm. Abdom Radiol N Y. 2018 Jan;43(1):218–30.
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. Radiology. 2014 Jun 13;273(1):241–60.
Li J-K, Liu X-H, Cui H, Xie X-H. Radiofrequency ablation vs. surgical resection for resectable hepatocellular carcinoma: A systematic review and meta-analysis. Mol Clin Oncol. 2020 Jan;12(1):15–22.
Xu X-L, Liu X-D, Liang M, Luo B-M. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287(2):461–72.
Nahon P, Vibert E, Nault J-C, Ganne‐Carrié N, Ziol M, Seror O. Optimizing curative management of hepatocellular carcinoma. Liver Int. 2020;40(S1):109–15.
Hong K, Georgiades C. Radiofrequency Ablation: Mechanism of Action and Devices. J Vasc Interv Radiol. 2010 Aug 1;21(8, Supplement):S179–86.
Erinjeri JP, Clark TWI. Cryoablation: Mechanism of Action and Devices. J Vasc Interv Radiol. 2010 Aug 1;21(8, Supplement):S187–91.
Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave Tumor Ablation: Mechanism of Action, Clinical Results, and Devices. J Vasc Interv Radiol. 2010 Aug 1;21(8, Supplement):S192–203.
Sainani NI, Gervais DA, Mueller PR, Arellano RS. Imaging After Percutaneous Radiofrequency Ablation of Hepatic Tumors: Part 1, Normal Findings. Am J Roentgenol. 2013 Jan 1;200(1):184–93.
MRI assessment of hepatocellular carcinoma after locoregional therapy [Internet]. [cited 2019 Dec 19]. Available from: https://www.readcube.com/library/f4ae90b1-d3ea-46ee-a46b-e45d9fea9705:615eebd9-15fe-4b83-8d37-70045713915e
Sainani NI, Gervais DA, Mueller PR, Arellano RS. Imaging After Percutaneous Radiofrequency Ablation of Hepatic Tumors: Part 2, Abnormal Findings. Am J Roentgenol. 2013 Jan 1;200(1):194–204.
Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency Ablation of Hepatocellular Carcinoma: Correlation Between Local Tumor Progression After Ablation and Ablative Margin. Am J Roentgenol. 2007 Feb 1;188(2):480–8.
Shyn PB, Mauri G, Alencar RO, Tatli S, Shah SH, Morrison PR, et al. Percutaneous Imaging-Guided Cryoablation of Liver Tumors: Predicting Local Progression on 24-Hour MRI. Am J Roentgenol. 2014 Feb 20;203(2):W181–91.
Lahat E, Eshkenazy R, Zendel A, Zakai BB, Maor M, Dreznik Y, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014 Oct;3(5):317–23.
Littrup PJ, Aoun HD, Adam B, Krycia M, Prus M, Shields A. Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series. Abdom Radiol. 2016 Apr 1;41(4):767–80.
Complications from percutaneous microwave ablation of liver tumours: a pictorial review [Internet]. [cited 2020 Aug 7]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6636263/
Dioguardi Burgio M, Ronot M, Bruno O, Francoz C, Paradis V, Castera L, et al. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2016;22(11):1491–500.
Young S, Taylor AJ, Sanghvi T. Post Locoregional Therapy Treatment Imaging in Hepatocellular Carcinoma Patients: A Literature-based Review. J Clin Transl Hepatol. 2018 Jun 28;6(2):189–97.
Chung W-S, Lee K-H, Park M-S, Lee YJ, Kwon J, Baek S-E, et al. Enhancement Patterns of Hepatocellular Carcinoma After Transarterial Chemoembolization Using Drug-Eluting Beads on Arterial Phase CT Images: A Pilot Retrospective Study. Am J Roentgenol. 2012 Aug 1;199(2):349–59.
Chiu RYW, Yap WW, Patel R, Liu D, Klass D, Harris AC. Hepatocellular Carcinoma Post Embolotherapy: Imaging Appearances and Pitfalls on Computed Tomography and Magnetic Resonance Imaging. Can Assoc Radiol J. 2016 May 1;67(2):158–72.
Kamel IR, Bluemke DA, Eng J, Liapi E, Messersmith W, Reyes DK, et al. The Role of Functional MR Imaging in the Assessment of Tumor Response after Chemoembolization in Patients with Hepatocellular Carcinoma. J Vasc Interv Radiol. 2006 Mar 1;17(3):505–12.
Liu Z, Fan J-M, He C, Li Z-F, Xu Y-S, Li Z, et al. Utility of diffusion weighted imaging with the quantitative apparent diffusion coefficient in diagnosing residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: a meta-analysis. Cancer Imaging. 2020 Jan 6;20(1):3.
Brennan IM, Ahmed M. Imaging Features Following Transarterial Chemoembolization and Radiofrequency Ablation of Hepatocellular Carcinoma. Semin Ultrasound CT MRI. 2013 Aug 1;34(4):336–51.
Chung J, Yu J-S, Chung J-J, Kim JH, Kim KW. Haemodynamic events and localised parenchymal changes following transcatheter arterial chemoembolisation for hepatic malignancy: interpretation of imaging findings. Br J Radiol. 2010 Jan;83(985):71–81.
Nishida K, Lefor AK, Funabiki T. Rupture of Hepatocellular Carcinoma after Transarterial Chemoembolization followed by Massive Gastric Bleeding. Case Rep Hepatol [Internet]. 2018 Jun 4 [cited 2020 May 28];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6008880/
Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, et al. Technical Aspects of Radioembolization with 90Y Microspheres. Tech Vasc Interv Radiol. 2007 Mar 1;10(1):12–29.
Vesselle G, Petit I, Boucebci S, Rocher T, Velasco S, Tasu J-P. Radioembolization with yttrium-90 microspheres work up: Practical approach and literature review. Diagn Interv Imaging. 2015 Jun 1;96(6):547–62.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):13–23.
Woerner AJ, Johnson GE. Advances in Y-90 radioembolization for the treatment of hepatocellular carcinoma. Hepatoma Res. 2022 Jan 6;8:2.
Sato K, Lewandowski RJ, Bui JT, Omary R, Hunter RD, Kulik L, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006 Aug;29(4):522–9.
Spina JC, Hume I, Pelaez A, Peralta O, Quadrelli M, Garcia Monaco R. Expected and Unexpected Imaging Findings after 90Y Transarterial Radioembolization for Liver Tumors. RadioGraphics. 2019 Mar 1;39(2):578–95.
Mora RA, Ali R, Gabr A, Abouchaleh N, Asadi AA, Kallini JR, et al. Pictorial essay: imaging findings following Y90 radiation segmentectomy for hepatocellular carcinoma. Abdom Radiol. 2018 Jul 1;43(7):1723–38.
Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Spear GG, Mulcahy MF, et al. Radiologic–pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49(4):1185–93.
Rhee TK, Naik NK, Deng J, Atassi B, Mulcahy MF, Kulik LM, et al. Tumor Response after Yttrium-90 Radioembolization for Hepatocellular Carcinoma: Comparison of Diffusion-weighted Functional MR Imaging with Anatomic MR Imaging. J Vasc Interv Radiol. 2008 Aug 1;19(8):1180–6.
Ludwig JM, Camacho JC, Kokabi N, Xing M, Kim HS. The Role of Diffusion-Weighted Imaging (DWI) in Locoregional Therapy Outcome Prediction and Response Assessment for Hepatocellular Carcinoma (HCC): The New Era of Functional Imaging Biomarkers. Diagnostics. 2015 Dec;5(4):546–63.
Atassi B, Bangash AK, Lewandowski RJ, Ibrahim S, Kulik L, Mulcahy MF, et al. Biliary Sequelae following Radioembolization with Yttrium-90 Microspheres. J Vasc Interv Radiol. 2008 May 1;19(5):691–7.
Braat M, Erpecum K van, Zonnenberg B, Bosch M van den, Lam M. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol. 2017 Feb;29(2):144–52.
Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, et al. Radiotherapy and hepatocellular carcinoma: update and review of the literature. Eur Rev Med Pharmacol Sci. 2012 Oct;16(11):1599–604.
Kalogeridi M-A, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, et al. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015 Jan 27;7(1):101–12.
Ji R, Ng KK, Chen W, Yang W, Zhu H, Cheung T-T, et al. Comparison of clinical outcome between stereotactic body radiotherapy and radiofrequency ablation for unresectable hepatocellular carcinoma. Medicine (Baltimore). 2022 Jan 28;101(4):e28545.
Lee S, Jung J, Park J, Kim SY, Choi J, Lee D, et al. Stereotactic body radiation therapy as a salvage treatment for single viable hepatocellular carcinoma at the site of incomplete transarterial chemoembolization: a retrospective analysis of 302 patients. BMC Cancer. 2022 Feb 16;22(1):175.
Zeng Z-C, Seong J, Yoon SM, Cheng JC-H, Lam K-O, Lee A-S, et al. Consensus on Stereotactic Body Radiation Therapy for Small-Sized Hepatocellular Carcinoma at the 7th Asia-Pacific Primary Liver Cancer Expert Meeting. Liver Cancer. 2017 Nov;6(4):264–74.
Mastrocostas K, Jang H-J, Fischer S, Dawson LA, Munoz-Schuffenegger P, Sapisochin G, et al. Imaging post-stereotactic body radiation therapy responses for hepatocellular carcinoma: typical imaging patterns and pitfalls. Abdom Radiol. 2019 May 1;44(5):1795–807.
Haddad MM, Merrell KW, Hallemeier CL, Johnson GB, Mounajjed T, Olivier KR, et al. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol. 2016 Oct 1;41(10):2061–77.
Mendiratta-Lala M, Gu E, Owen D, Cuneo KC, Bazzi L, Lawrence TS, et al. Imaging Findings Within the First 12 Months of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018 15;102(4):1063–9.
Mendiratta-Lala M, Masch W, Shankar PR, Hartman HE, Davenport MS, Schipper MJ, et al. Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int J Radiat Oncol • Biol • Phys. 2019 Jan 1;103(1):169–79.
Oldrini G, Huertas A, Renard-Oldrini S, Taste-George H, Vogin G, Laurent V, et al. Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLOS ONE. 2017 Apr 25;12(4):e0176118.
Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother Oncol. 2019 Feb 1;131:135–44.
Park J, Park JW, Kang MK. Current status of stereotactic body radiotherapy for the treatment of hepatocellular carcinoma. Yeungnam Univ J Med. 2019 Aug 12;36(3):192–200.
Kellock T, Liang T, Harris A, Schellenberg D, Ma R, Ho S, et al. Stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma: imaging evaluation post treatment. Br J Radiol [Internet]. 2018 [cited 2020 Aug 10];91(1085). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6190776/
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alnammi, M., Wortman, J., Therrien, J. et al. MRI features of treated hepatocellular carcinoma following locoregional therapy: a pictorial review. Abdom Radiol 47, 2299–2313 (2022). https://doi.org/10.1007/s00261-022-03526-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03526-0