Abstract
Purpose
To update the oncologic outcomes and safety for microwave (MW) ablation of T1a (≤4.0 cm) and T1b (4.1–7.0 cm) renal cell carcinoma (RCC) with emphasis on tumor complexity and single session treatment.
Materials and Methods
Retrospective review of 29 consecutive patients (30 tumors) with localized (NOMO) RCC (23 T1a; 7 T1b) treated with percutaneous MW ablation between 3/2013 and 6/2014. Primary outcomes investigated were technical success, local tumor progression (LTP), and complications. Technical success was assessed with contrast-enhanced computed tomography (CECT) immediately after MW ablation. Presence of LTP was assessed with CECT or contrast-enhanced magnetic resonance at 6-month target intervals for the first two years and annually thereafter. Complications were categorized using the Clavien-Dindo classification system.
Results
Median tumor diameter was 2.8 cm [IQR 2.1–3.3] for T1a and 4.7 cm [IQR 4.1–5.7] for T1b tumors. Median RENAL nephrometry score was 7 [IQR 4–8] for T1a tumors and 9 [IQR 6.25–9.75] for T1b tumors. Technical success was achieved for 22 T1a (96%) and 7 T1b (100%) tumors. There were no LTP during a median imaging follow-up of 12.0 months [IQR 6–18] for the 23 patients (24 tumors) with greater than 6 months of follow-up. There were three Clavien-Dindo grade I–II complication (10%) and no Clavien-Dindo grade III–V complications (0%). All but two patients (93%) are alive without metastatic disease; two patients died after 12-month follow-up of causes unrelated to the MW ablation.
Conclusion
Percutaneous MW ablation appears to be a safe and effective treatment option for low, moderate, and highly complex T1a and T1b RCC in early follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
According to the American Urologic Association (AUA), thermal ablation of renal cell carcinoma (RCC) is an accepted treatment alternative for the suboptimal surgical candidate, including the elderly, those with significant comorbidities and those who have had prior surgery for RCC [1]. With proper patient selection, cryoablation and radiofrequency (RF) ablation are similarly effective for treating T1a (≤4.0 cm) RCC with local recurrence-free survival rates approaching 100% in long-term follow-up [2–8]. Even though the rate of comorbid disease is typically higher in thermal ablation cohorts, the rate of major complications compares favorably to laparoscopic partial nephrectomy (LPN) and other extirpative techniques [8–11]. There is less cumulative data for the use of microwave (MW) ablation in RCC and evidence for long-term oncologic efficacy is lacking. However, recent studies have shown MW to have local tumor control equivalent to RF and cryoablation in short- and intermediate-term follow-up for T1a RCC with a similar to lower rate of major complications [11–13].
The role of thermal ablation in T1b (4.1–7.0 cm) RCC is not as well defined. While AUA guidelines do cite thermal ablation as an option for T1b RCC, they also note a higher incidence of local tumor progression (LTP) [1]. However, those guidelines were based upon early experience that included RF ablation, which is now known to have limited efficacy for tumors larger than 3 cm or a significant endophytic component [3, 4, 14]. More recently, cryoablation was shown to have a local progression-free survival (PFS) comparable to LPN in intermediate- and long-term follow-up [5, 15]. This suggests that ablation may have a greater role in the management of localized T1b tumors than previously thought provided that adequate ablation zones can be created. Compared to RF electrical current, MWs can generate faster heating and produce higher tissue temperatures, resulting in more effective treatment of larger tissue volumes from a needle-like device [16–19]. However, clinical experience with MW ablation of T1b RCC has been limited to a single retrospective analysis of a small cohort using MW ablation technology that required overlapping and staged ablations to treat larger tumors, and techniques that may lead to suboptimal oncologic efficacy [12].
The purpose of this study was to evaluate the early oncologic outcomes and safety of MW ablation of T1a and T1b RCC with emphasis on tumor complexity and single ablation treatment sessions.
Methods
Patient selection
The institutional review board approved this retrospective review of a prospectively collected database of RCC treated with percutaneous ablation. The study was compliant with the Health Insurance Portability and Accountability Act. The requirement for informed consent was waived.
Consecutive patients with biopsy-proven localized (N0M0) T1 RCC treated with MW ablation between the inception of our MW experience, March 2013, and June 2014 were included. Tumors measuring up to 7.0 cm based upon maximum diameter obtained from cross-sectional imaging were included. Tumors measuring larger than 7.0 cm and patients receiving prior ipsilateral extirpative or ablative therapies were excluded from the study. Importantly, tumors near the ureter, usually those that are exophytic and arising from the medial lower pole, were treated with cryoablation rather than MW ablation to reduce the risk of ureteral injury. Percutaneous renal mass biopsy for the diagnosis of RCC was made at least two weeks prior to ablation. The decision to offer percutaneous MW ablation was made by consensus in a multidisciplinary team including a radiologist and urologist. Treatment intent was curative for all cases.
Procedure
The ablation team was composed of a radiologist with 2 years of experience with percutaneous MW and cryoablation and a urologist with 20 years of experience. All procedures were performed under general anesthesia in a computed tomography (CT) suite (HD750, GE Healthcare, Waukesha, Wisconsin) utilizing either US (iU22 xMATRIX, Philips, Eindhoven, Netherlands) or CT fluoroscopy (CTF) as guidance for antenna placement and CT to ensure precise antenna position.
A 2.45 GHz, gas-cooled MW ablation system with 17-gage antennas was used for all cases (Certus 140 and PR antennas; NeuWave Medical; Madison, Wisconsin). When placing multiple antennas and/or when CT was obtained to confirm antenna placement, each antenna was ‘fixed’ to the tissue approximately 2 cm proximal to the antenna tip using the cryogenic cooling system [20]. Up to three antennas were utilized depending upon the size and location of the tumor, with multiple antennas spaced approximately 1.5 cm apart and the array centered within the tumor.
Hydrodisplacement of non-target anatomy, including small bowel, colon, and pancreas or displacement of the kidney relative to the psoas muscle in order to protect the genitofemoral nerve/lateral femoral cutaneous nerve, was performed when their proximity was within 1 cm of the tumor or within the expected zone of ablation. For hydrodisplacement, faintly radiopaque normal saline (2% iohexol 350 mg/mL in saline) was infused through an 18-gage introducer situated between the tumor and non-target structure until an adequate margin of safety was achieved [21, 22].
As a routine, the MW generator was initially powered at 65 W per antenna for a prescribed time of 5 min. Ultrasound was used for continuous, real-time monitoring of the extent of ablation and proximity of the ablation to non-target anatomy (intermittent monitoring with CT fluoroscopy when utilized for antenna placement). The duration of the ablation cycle depended upon the time necessary for gas to cover the index tumor and circumferential 5 mm margin. If the index tumor and margin were covered prior to the prescribed 5 min, the ablation cycle was terminated and the antenna removed. However, if the index tumor and margin were not adequately covered with the initial ablation cycle, additional time was prescribed or the antennas repositioned and powered again. When the index tumor and margin were adequately covered, the ablation cycle was terminated and the antenna(s) removed. While the duration, antenna power or both can be modified during an ablation cycle, we limited variability by primarily adjusting ablation time. No staged ablations were performed.
While the patient was still under general anesthesia, contrast-enhanced CT (CECT) was performed immediately following ablation to evaluate technical success and to assess for complications. All patients were subsequently admitted for overnight observation. Follow-up imaging with contrast-enhanced CT or magnetic resonance (MR) at routine target intervals of 6, 12, 18, and 24 months and annually thereafter was recommended.
Data collection and statistical methods
Data were collected by one of six authors. Patient and disease characteristics including age, gender, body mass index (BMI), tumor size, location, histologic subtype, Fuhrman grade, and pre- and post-procedure creatinine were recorded. Charlson comorbidity index and RENAL nephrometry scores were calculated [23, 24]. Ablation protocol parameters including number of antennas, duration of ablation, generator output, and volume of hydrodisplacement fluid were recorded. Complications occurring during and within 30 days of ablation were classified according to the revised Clavien-Dindo System [25]. Duration of hospitalization was also recorded.
Continuous features were summarized as means and interquartile ranges (IQR); categorical features were summarized with frequency counts and percentages. PFS and cancer-specific survival (CSS) were estimated by the Kaplan–Meier method. The duration of follow-up for PFS was defined from the date of MW ablation to the date of last tumor imaging follow-up. The duration of follow-up for CSS was defined from the date of MW ablation to the date of death or the date the patient was last known to be alive. The paired t test was used to compare pre- and post-procedure creatinine. p values less than 0.05 were considered significant. Established criteria were used to define treatment outcomes [26].
Results
Patient and procedural data
Thirty biopsy-proven RCC were treated in 29 patients: 23 T1a and 7 T1b. Median age was 66 years [IQR 61–73]. The cohort was predominantly male (69%). Median BMI was 30 [IQR 25.9–33.6] and median Charlson comorbidity index (CCI) was 3.5 [IQR 3–5]. Patient and pathology characteristics are summarized in Table 1.
Median tumor diameter for the entire cohort was 3.1 cm [IQR 2.3–3.8 cm]. Median tumor diameter for T1a was 2.8 cm [IQR 2.1–3.3], and median tumor diameter for T1b was 4.7 cm [IQR 4.1–5.7]. Median RENAL nephrometry score was 7 [IQR 4–8] (anterior = 4; posterior = 25; X = 1). Anterior tumors were preferentially treated with partial nephrectomy due to the perceived increased risk of injury to non-target anatomy. Stratified by stage, median RENAL nephrometry score was 6.5 [IQR 4–8] for T1a tumors and 9 [IQR 6.25–9.75] for T1b tumors.
The median number of antennas used for the entire cohort was 2 [IQR 2.0–2.5]. Stratified by stage, the median number of antennas used for T1a tumors was 2 [IQR 2.0–2.0] and the median number of antennas used for T1b tumors was 3 [IQR 2.0–3.0]. The median generator output for the entire cohort, T1a and T1b subsets was 65 watts, full power for a PR antenna. The mean duration of treatment for the entire cohort, T1a and T1b subsets were 5 [IQR 4–6.25], 4.5 [IQR 4–5], and 7 min [IQR 5–11], respectively. Hydrodisplacement for non-target anatomy was performed in the treatment of eight tumors (26.6%) with a mean volume of 386 mL (range 200–900 mL). Stratified by stage, hydrodisplacement was performed for six T1a tumors (26%) with a mean volume of 373 mL and two T1b tumors (28.5%) with a mean volume of 425 mL. Following the post-treatment CECT, same-session repeat ablation was not performed for any patient. Median duration of hospitalization was 1 day (range 1 day). Mean pre- and post-procedure creatinine for the entire cohort was 1.09 mg/dL and 1.17 mg/dL (p = 0.41; 95% CI 0.20–0.08). Stratified by stage, pre- and post-procedure creatinine for the T1a and T1b cohorts were 1.04 and 1.14 mg/dL and 1.21 and 1.59 mg/dL. Ablation protocol and clinical results are summarized in Table 2.
Oncologic outcomes
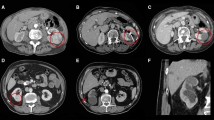
Technical success was achieved for 28 of 29 patients, 29 of 30 tumors (97%); stratified by stage, 22 T1a (96%) and 7 T1b (100%) were successful. The single primary failure was an oval-shaped T1a (2.9 cm) chromophobe subtype RCC, Fuhrman grade not specified, with a RENAL nephrometry score of 4p. Single corticomedullary phase, CECT, and pre- and peri-procedural US did not adequately characterize the true shape of the tumor and endophytic extent of the tumor. On immediate post-procedure axial CECT reviewed at the CT scanner following the procedure, the zone of ablation appeared to cover the index tumor including an adequate margin. Upon review of the coronal and sagittal reformatted images, available after the patient was awakened and transported to the post-anesthesia care unit, unabated tumor could be seen extending to the renal sinus and this was confirmed at MRI on six-month follow-up (Fig. 1). This patient was successfully retreated with salvage MW ablation conferring a primary effectiveness of 96% and secondary effectiveness of 100% for T1a RCC and a primary effectiveness of 100% for T1b RCC.
Percutaneous microwave ablation of a 2.9 cm chromophobe RCC partially endophytic T1a chromophobe RCC (arrow) on axial CECT (A). One microwave antenna (arrowhead) was placed into the mass, tangential to the renal sinus, with US guidance and position confirmed with CT (B). Lobular low-attenuation extending from the index ablation to the renal sinus (curved arrow) on the sagittal CECT (C) was discovered in retrospect, following the procedure after the patient was awakened. This primary failure (curved arrow) was confirmed at 6-month follow-up MRI (D)
Twenty-three of 28 patients (29 tumors) were followed for at least six months; LTP was assessed for this subset of patients. Mean and median duration of imaging follow-up was 11.1 months (SD 5.6) and 12.0 months [IQR 6–18], respectively. Stratified by stage, mean and median duration of follow-up for the 17 T1a tumors was 12 months (SD 6.2) and 12 months [IQR 6–18] and 6 months (SD 3.2) and 6 months [IQR 6–12] the 7 T1b tumors. There was no LTP (0%) in this group conferring a progression-free survival of 100%. The five patients without at least six months of imaging follow-up had T1a RCC and technically successful ablation procedures as determined on CECT performed immediately following the ablation procedure. The patient and pathology characteristics and ablation protocol for these patients were similar to the remainder of the T1a cohort. These patients have elected to receive their follow-up care locally.
Among the 29 patients with clinical and/or imaging follow-up, metastatic disease developed in one patient (3.3%). Prior to the MW ablation procedure, this patient had a partial nephrectomy in the contralateral kidney that was complicated by LTP. The patient died of metastatic disease 18 months following the MW ablation procedure. At 12 month surveillance imaging, there was no LTP at the index ablation. Another patient, a heart-transplant recipient, who had no LTP at 12 months, died of complications from pneumonia and sepsis. Cancer-specific and overall survival was 97% and 93%, respectively.
Complications
There was one Clavien-Dindo grade I complication (3.3%). A urine leak was present immediately following ablation of an entirely endophytic T1b RCC (RENAL score—10a). No intervention was required and the urine leak had resolved at 6-month follow-up. There were two Clavien-Dindo grade II complications (6.8%), both developed after discharge from the hospital. In detail, one patient developed pneumonia for which hospitalization and intravenous antibiotics were required. The other patient developed cystitis that was managed on an outpatient basis with oral antibiotics. All patients recovered and returned to their former state of function. There were no Clavien-Dindo grade III, IV or V complications.
Discussion
Despite significant comorbidities and obesity in our patient cohort, technically successful MW ablations were achieved for all but one T1a RCC and for all T1b RCC. The lone primary failure was successfully treated with salvage MW ablation conferring a secondary effectiveness of 100% for T1a RCC. Even though 16 of 23 tumors (including two hilar tumors) were moderate to high tumor complexity based upon the RENAL nephrometry score, there were only three low-grade (Clavien-Dindo grade I) complications, and no procedure related high-grade (Clavien-Dindo grade II–V) complications. All three patients returned to their baseline level of function. All but two patients are alive without evidence for LTP or metastatic disease over a median duration of imaging follow-up of 12.0 months. Based on our results, MW ablation with modern MW equipment appears to be a safe and effective treatment for T1a and T1b RCC.
We did not note a dependence of treatment outcome on tumor complexity. Of the five anatomic components of the RENAL nephrometry score, four are associated with treatment failure and three of the four correspond to tumor location: (1) exophytic/endophytic (E), (2) nearness to collecting system (N), and (3) location relative to polar lines (L) with the other factor being tumor diameter. [27] While RF ablation is effective for exophytic RCC smaller than 3 cm, the rate of treatment failure increases with increasing tumor size and complexity [7]. McClure et al. showed primary effectiveness of RF ablation for RCC was limited to small (2.1 cm vs 3.5 cm; p < 0.001), exophytic (62.8% vs 28.2%; p = 0.003) tumors with low (RENAL score 4–6) tumor complexity (56% vs 7.1%; p < 0.001) [28]. Schmit et al. showed that a higher RENAL score (7.6 ± 2.2 vs 6.7 ± 1.9, p = 0.001) was associated with treatment failure for both RF and cryoablation [27]. Further, Camacho et al. reported a LTP rate of 16.8% with 47% of recurrences occurring within the first 12 months following RF ablation and cryoablation of T1a tumors with a RENAL score over 8 (p = 0.0001) [29]. Our results suggest that MW ablation may be less vulnerable to tumor complexity. Specifically, there were no treatment failures and no LTP for the 16 (69.5%) tumors that were scored as moderate (RENAL score 7–9) or highly (RENAL score 10–12) complex.
Our results are additive and further validate prior high treatment success of MW ablation for biopsy-proven T1a RCC with low and moderate tumor complexity [13]. The lone treatment failure, a T1a chromophobe RCC with a RENAL nephrometry score of 4p, was the result of suboptimal pre-procedure imaging that failed to characterize the endophytic extent of the tumor. Based upon pre-procedure imaging findings, a tangential (to renal sinus), rather than perpendicular, approach was used for antenna placement. A single MW antenna was not able to overcome the robust perfusion-mediated tissue cooling intrinsic to the kidney with this antenna position (Fig. 1)
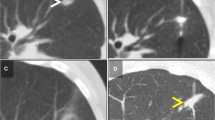
Our approaches to ablating T1a and T1b tumors were dissimilar, owing to the technical challenges larger tumors pose for complete ablation and risk of injury to non-target anatomy. The simultaneous use of multiple antennas and real-time monitoring of the ablation zone were important when treating larger tumors. The use of multiple applicators creates a “thermal synergy” between the growing ablations and, notably, MW energy can create an electromagnetic synergy when the antennas are controlled to have coherent phase [19, 30–32]. This synergy generates large, confluent ablations that are more likely to cover the tumor and margin without clefts and achieve temperature extremes that are less likely to be sublethal [5, 8, 15, 33–36]. Intra-procedural monitoring of the ablation zone can ensure adequate coverage of the tumor/margin and proximity to non-target anatomy. Gas generated during MW ablation is also readily visible at CT and US and has been shown to be an accurate biomarker for the zone of necrosis. Importantly and unlike cryoablation, the zone of tissue necrosis with MW is equal to or minimally larger than the gas cloud visualized with US [37] (Fig. 2).
Percutaneous microwave ablation of 2.7 cm clear cell RCC Partially endophytic T1a clear cell RCC (arrow) at US (A). Two microwave antennas (arrowhead) were placed into the mass with US guidance and position confirmed with CT. Continuous, real-time US performed during MW ablation (B). Notice the gas cloud (curved arrows) within the tumor (arrow) increases in size over time and eventually covers the entire tumor including a narrow margin. Immediate post-procedure axial CECT (C) confirms complete tumor ablation including a narrow margin (arrow). With US, the gas cloud formed during MW ablation has been shown to correlate closely with the zone of necrosis at pathology and is used as an imaging biomarker to determine adequacy of ablation
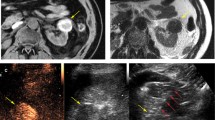
With a LTP rate of 0% at a mean follow-up of 8.6 months for T1b RCC, our results compare favorably with prior reported experiences. In a subset analysis of biopsy-proven T1b RCC, Yu et al. had a 50% rate of treatment failure (87% primary efficacy, 43% LTP) [12]. There are several potential explanations for our improved results. In our study, the endpoint of MW ablation sessions was based upon imaging findings rather than temperature feedback from a thermocouple or a strict power/time prescription. When gas covered the index tumor and a narrow margin, the ablation session was considered complete (Fig. 2). Staged ablations, repeat ablation sessions, and overlapping ablations used to treat T1b tumors in previous studies were not performed in our study. We used a 2.45 GHz MW device with up to three antennas whose output was synchronized to preclude destructive interference. The resulting large, confluent, spherical ablations were able to cover T1b tumors in a single treatment session without the need for overlapping ablations (Fig. 3). General anesthesia was used for every case in our study. The collaboration with anesthesiology allows the operators full attention to be directed toward the index tumor, placement of applicators, and monitoring the zone of ablation. Our anesthesia colleagues managed patient factors unrelated to the ablation including hemodynamic status and airway and also performed adjunctive maneuvers, such as Valsalva and apnea, which improve index tumor conspicuity and tumor targeting. Our results suggest that MW ablation may have a greater role in the management of T1b RCC than had been previously supported by the literature [1].
Percutaneous microwave ablation of a 4.8 cm pRCC endophytic T1b papillary RCC (arrow) on axial axial CECT (A). Three microwave antennas (arrowhead) were placed into the mass with US guidance and position confirmed with CT (B). Immediate post-procedure axial CECT (C) demonstrates complete tumor ablation including a narrow margin and no complication (arrow). There is no evidence of LTP at 12-month follow-up MRI (D). Notice the intrinsic T1W signal of the index ablation (arrows) at MRI (D) can be effectively subtracted (E) to improve diagnostic confidence
Despite a high comorbidity index, there were no high-grade complications directly related to the ablation procedure in our study. Our results confirm the previous results with MW ablation of T1a and T1b patient cohorts [11–13, 41]. Further, our results compare favorably with cryoablation of T1b RCC, which has been shown to be associated with an increased rate of high-grade procedure-related complications particularly for larger and more complex tumors [8, 10, 15, 27, 39]. Importantly, preablation renal artery embolization, which has been shown to decrease the rate of hemorrhage during cryoablation, was not performed for any case in our study because the risk for significant hemorrhage was expected to be very low given the cautery effect of MW [15, 40].
The most significant limitation of this study was length of follow-up. The retrospective single-center design is a limitation, but consistent with most prior studies on percutaneous ablation. The relatively short follow-up is an unavoidable limitation when evaluating new technology. However, follow-up times reported for early RF and cryoablation studies were similar. While growth rates of RCC can vary widely, low-grade RCC tend to grow slowly, at a rate of 2–4 mm/year with a mean doubling rate between 12–26 months [41–44]. Therefore, it is difficult to predict what length of follow-up would be necessary to increase our power for assessing LTP and CSS. With a larger cohort or longer-term follow-up, we may have encountered more treatment failures. Importantly, the duration of follow-up in our study is longer than the only two series in the literature using modern MW technology available in the US [13, 38]. While the T1b cohort size of seven is small, it is similar to the largest series of eight T1b RCC treated with MW ablation [12]. Lastly, this study is limited to a single MW ablation device and a single center. We emphasize caution in extrapolating these results to all devices or all centers.
In summary, percutaneous MW ablation appears to be an effective and safe treatment option for T1a and T1b RCC. Larger cohorts and longer-term follow-up is needed to establish durable oncologic efficacy and survival relative to competing ablation modalities and extirpative techniques.
References
Campbell SC, et al. (2009) Guideline for management of the clinical T1 renal mass. J Urol 182(4):1271–1279
Atwell TD, et al. (2013) Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol 200(2):461–466
Gervais DA, et al. (2005) Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185(1):64–71
Zagoria RJ, et al. (2007) Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol 189(2):429–436
Georgiades CS, Rodriguez R (2014) Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol 37(6):1494–1499
Goyal J, et al. (2012) Single-center comparative oncologic outcomes of surgical and percutaneous cryoablation for treatment of renal tumors. J Endourol 26(11):1413–1419
Psutka SP, et al. (2013) Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 63(3):486–492
Thompson RH, et al. (2015) Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 67(2):252–259
Klatte T, et al. (2011) Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol 60(3):435–443
Atwell TD, et al. (2012) Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 23(1):48–54
Yu J, et al. (2014) US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 270(3):880–887
Yu J, et al. (2012) US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263(3):900–908
Moreland AJ, et al. (2014) High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol 28(9):1046–1052
Varkarakis IM, et al. (2005) Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. J Urol 174(2):456–460 (discussion 460)
Atwell TD, et al. (2015) Percutaneous cryoablation of stage t1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol 26(6):792–799
Yu J, et al. (2011) A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 79(1):124–130
Andreano A, Brace CL (2013) A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 36(2):505–511
Laeseke PF, et al. (2009) Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol 20(9):1224–1229
Li X, et al. (2011) Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia 27(3):240–248
Knavel EM, et al. (2012) High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. Am J Roentgenol 198(3):W260–W265
Farrell MA, et al. (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. Am J Roentgenol 181(5):1315–1317
Campbell C, et al. (2012) Contrast media-doped hydrodissection during thermal ablation: optimizing contrast media concentration for improved visibility on CT images. Am J Roentgenol 199(3):677–682
Charlson ME, et al. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Ahmed M, et al. (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1):241–260
Schmit GD, et al. (2013) Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol 189(1):30–35
McClure TD, et al. (2014) Intermediate outcomes and predictors of efficacy in the radiofrequency ablation of 100 pathologically proven renal cell carcinomas. J Vasc Interv Radiol 25(11):1682–1688 (quiz 1689)
Camacho JC, et al. (2015) R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol 26(5):686–693
Brace CL, et al. (2007) Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 244(1):151–156
Brace CL (2009) Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol 38(2):61–67
Littrup PJ, et al. (2007) CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 18(3):383–392
Schmit GD, et al. (2010) Percutaneous cryoablation of renal masses >or= 3 cm: efficacy and safety in treatment of 108 patients. J Endourol 24(8):1255–1262
Lehman DS, et al. (2008) First prize (tie): laparoscopic renal cryoablation: efficacy and complications for larger renal masses. J Endourol 22(6):1123–1127
Hines-Peralta AU, et al. (2006) Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 239(1):94–102
Laeseke PF, et al. (2009) Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol 2(2):65–72
Ziemlewicz TJ, Brace CL, et al. Ultrasound predicts pathologic zone of necrosis better than non-contrast CT during RF and microwave ablation. World Conference on Interventional Oncology Annual Meeting. New York, NY. May 11–14, 2014.
Horn JC, et al. (2014) Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol 25(3):448–453
Schmit GD, et al. (2012) Percutaneous cryoablation of solitary sporadic renal cell carcinomas. BJU Int 110(11 Pt B):E526–E531
Woodrum DA, et al. (2010) Role of intraarterial embolization before cryoablation of large renal tumors: a pilot study. J Vasc Interv Radiol 21(6):930–936
Chawla SN, et al. (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 175(2):425–431
Nerli R, et al. (2014) Tumor doubling time of renal cell carcinoma measured by CT. Indian J Urol 30(2):153–157
Jilg CA, et al. (2012) Growth kinetics in von Hippel-Lindau-associated renal cell carcinoma. Urol Int 88(1):71–78
Jewett MA, et al. (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60(1):39–44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Neither internal nor external funding was provided for this research.
Conflict of interest
CL Brace is a shareholder and consultant for NeuWave Medical Inc. and Symple Surgical and receives grant support from the National Institute of Health. SA Wells, KM Wheeler, A Mithqal, MS Patel, and NS Schenkman have no potential conflict of interest.
Ethical approval
This study represents a retrospective review of patients who received tumor ablation as a part of clinical care. Tumor ablation is considered an alternative to surgery and is standard of care. Research was not performed on animals in this study.
Informed consent
The study is compliant with the Health Insurance Portability and Accountability Act. The requirement for informed consent was waived.
Rights and permissions
About this article
Cite this article
Wells, S.A., Wheeler, K.M., Mithqal, A. et al. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol 41, 1203–1211 (2016). https://doi.org/10.1007/s00261-016-0776-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0776-x