Abstract
A 41-year-old woman presented with atypical genital bleeding. Magnetic resonance imaging demonstrated a polypoid mass from the lower uterine segment to cervical canal, approximately 32 mm in size. Additionally, a thickened sigmoid colon wall showing a markedly high signal intensity on diffusion-weighted imaging was observed. Barium enema and colonoscopy revealed a type I sigmoid colon cancer. Since this patient was relatively young and had multiple relatives with colon cancer, Lynch syndrome was suspected and proved by an immunohistochemical survey. Uterine endometrial carcinoma related to Lynch syndrome tends to occur in the lower uterine segment. Radiologists should be aware of this syndrome so that the correct diagnosis can be suggested in the imaging report.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Case report
We presented the case of a 41-year-old woman (gravid 0, para 0) with atypical genital bleeding. She had complained of yellow vaginal discharge and genital bleeding for the past 2 months. She was referred to our hospital for genital bleeding and increased abdominal pressure. Laboratory data showed slight anemia. Transrectal ultrasound showed a large mass in the uterine cavity. The cytology was equivocal for malignancy.
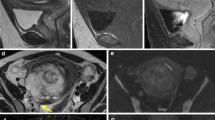
To examine the mass, contrast-enhanced magnetic resonance imaging (MRI) was performed. On MRI, a polypoid mass arising from the lower uterine segment (LUS) to cervical canal was observed, approximately 32 mm in size. The mass showed intermediate intensity on T2-weighted images (T2WI), relatively low intensity on post-contrast T1-weighted images (T1WI), and markedly high signal intensity on diffusion-weighted images (DWI) (Fig. 1A–D).
A, B, C, D Contrast-enhanced MRI. (A Sagittal T2-weighted image, B post-contrast fat-saturated T1-weighted image, C Axial T2-weighted image, D axial diffusion-weighted image) MRI showed a solid mass stretching from the lower uterine segment to cervical canal. The mass showed intermediate intensity on T2-weighted images and relatively low intensity on post-contrast T1-weighted images. A thickened wall of the sigmoid colon was observed and showed high intensity on diffusion-weighted image (arrows)
Based on the MR findings, a malignant polypoid mass stretching from the lower segment of the uterine endometrium to cervical canal was suspected. Pathologically, the endometrium of the uterine body is composed of two distinct regions: the uterine corpus proper (UC) and LUS. A uterine endometrial carcinoma (EC) arising from LUS was suspected, and in the differential diagnosis, adenosarcoma, atypical polypoid adenoma, and carcinosarcoma were also considered. Concurrently, a thickened sigmoid colon wall that showed markedly high signal intensity on DWI was observed. (Fig. 1C, D). Barium enema and colonoscopy then revealed a type 1 sigmoid colon cancer (Fig. 2).
Both radical hysterectomy and sigmoid colectomy were performed simultaneously. As this tumor was located mainly in the uterine cervix, the resected uterus revealed that the polypoid mass arising from LUS protruded into the cervical canal. The tumor was seen to involve the uterine endometrium at the base of the tumor stalk on microscopic examination and so was diagnosed as an endometrioid adenocarcinoma (G2) arising from the LUS. The sigmoid colon tumor was diagnosed as a tubular adenocarcinoma (Fig. 3A, B). Histologically, the tumor overgrew into the subserosal layer of the sigmoid colon.
Histopathological images of EC (A, B HE staining, C MSH2 staining, D MSH6 staining). The tumor showed granular architecture lined with columnar cells with atypia and was diagnosed as EC grade 2. The edge of the tumor continued to the epithelium of LUS (arrow) and was suspected to have arisen from the uterine endometrium. MSH2 and MSH6 were negative in EC
Since this patient developed double cancers concurrently in her 40s and her mother and uncle had been diagnosed with colon cancer, Lynch syndrome was suspected. An immunohistochemical survey of the resected tissues supported this diagnosis as MSH2 and MSH6 were negative in both EC and colorectal cancer (CRC) (Fig. 3C, D). The MSI (microsatellite instability) test and oncogenic screening to confirm Lynch syndrome were not undertaken as per the patient’s wish.
Discussion
Lynch syndrome is an autosomal dominant inherited disorder caused by germline mutations in one of the DNA mismatch repair genes (MMR): MLH1, MSH2, MSH6, or PMS2 [1]. This syndrome, also known as hereditary non-polyposis colon cancer (HNPCC), is associated with the development of multiple cancer types, especially colon and EC. 3%– 5% of all CRCs and 2%–3% of ECs are associated with Lynch syndrome [2–6]. The risk of EC in women with Lynch syndrome was reported to surpass that of CRC. In recent studies, the lifetime risk for developing EC in women with Lynch syndrome was estimated to be 40%–60% [7, 8]. Moreover, the risk of a second malignancy for patients with Lynch syndrome-related EC is estimated to be 25% within 10 years and 50% within 15 years after the initial diagnosis of EC [7].
Many CRCs associated with Lynch syndrome are reported as being poorly differentiated adenocarcinomas. They also tend to have the features of mucinous or signet ring cell differentiation, and infiltration of lymphocytes or Crohn’s-like lymphocytic reaction. On the other hand, the histologic features of Lynch syndrome-related EC remain unknown [3]. In some reports, EC in patients with Lynch syndrome has been described as being often aggressive histologically or poorly differentiated [9]. In our case, however, the tumor was a low-grade endometrioid adenocarcinoma. Meanwhile, the tumor of this case had continuity with the LUS epithelium on pathological findings. The endometrium of the LUS is generally thinner than that of UC, and glands and stroma in this region tend to be much less responsive to hormone stimulation. In some reports, the LUS epithelium is found to be more susceptible to mismatch repair errors [10]. Generally, EC arising from the LUS, which is almost the same region referred to as the isthmus, is rare, whereas EC most commonly arises from the UC endometrium (uterine body and fundus). The frequency of LUS tumors ranges from 3% to 8% in all EC cases [10]. 29% of women diagnosed with LUS tumors were found to have Lynch syndrome-related EC. This percentage is relatively high, since the frequency of Lynch syndrome-related EC among general ECs is only 1%–2% [3, 4, 10].
As before, revised Amsterdam and Bethesda criteria have been used to screen high-risk patients for Lynch syndrome. These criteria include patient age (below 50 years), family history, and patient’s medical history of malignancies. However, these conventional criteria have not been used properly for EC in Lynch syndrome as they lay the greatest emphasis on patients with CRC [1, 11, 12]. (Hampel et al. found that 61.5% of Lynch syndrome-related ECs did not meet the traditional criteria of Lynch syndrome [3, 4].)
So recently, when and how to screen high-risk patients for Lynch syndrome has become a pressing issue [1, 11–14]. Mills et al. found that using recently suggested histopathologic parameters (e.g., tumor infiltrating lymphocytes) besides traditional screening parameters, at least 41% of Lynch syndrome-related ECs were missed [12]. At present, more general criteria of Lynch syndrome-related EC are needed.
MRI has good resolution to clarify the EC location so that EC arising from LUS can be diagnosed objectively. The radiologist should recognize the higher potential of Lynch syndrome in this EC and can take on the role of alerting the clinician to this possibility.
In summary, we reported a case of Lynch syndrome-related EC associated with CRC concurrently. The Lynch syndrome-related EC tends to be located in LUS, and its characteristic findings have been clearly revealed by MRI in this study.
References
Umar A, Boland CR, Terdiman JP, et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Tafe LJ, Riggs ER, Tsongalis GJ (2014) Lynch syndrome presenting as endometrial cancer. Clin Chem 60(1):111–121. doi:10.1373/clinchem.2013.206888
Hampel H, Panescu J, Lockman J, et al. (2007) Comment on: screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 67(19):9603. doi:10.1158/0008-5472.CAN-07-2308
Hampel H, Frankel W, Panescu J, et al. (2006) Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66(15):7810–7817. doi:10.1158/0008-5472.CAN-06-1114
Meyer LA, Broaddus RR, Lu KH (2009) Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 16(1):14–22
Lu KH, Broaddus RR (2005) Gynecologic cancers in Lynch syndrome/HNPCC. Fam Cancer 4(3):249–254. doi:10.1007/s10689-005-1838-3
Wang Y, Wang Y, Li J, et al. (2013) Lynch syndrome related endometrial cancer: clinical significance beyond the endometrium. J Hematol Oncol 6:22. doi:10.1186/1756-8722-6-22
Lu KH, Dinh M, Kohlmann W, et al. (2005) Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol 105(3):569–574. doi:10.1097/01.AOG.0000154885.44002.ae
Broaddus RR, Lynch HT, Chen LM, et al. (2006) Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer 106(1):87–94. doi:10.1002/cncr.21560
Westin SN, Lacour RA, Urbauer DL, et al. (2008) Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol 26(36):5965–5971. doi:10.1200/JCO.2008.18.6296
Lu KH, Schorge JO, Rodabaugh KJ, et al. (2007) Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 25(33):5158–5164. doi:10.1200/JCO.2007.10.8597
Mills AM, Liou S, Ford JM, et al. (2014) Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol 38(11):1501–1509. doi:10.1097/PAS.0000000000000321
Resnick KE, Hampel H, Fishel R, Cohn DE (2009) Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecol Oncol 114(1):128–134. doi:10.1016/j.ygyno.2009.03.003
Brown GJ, St John DJ, Macrae FA, Aittomaki K (2001) Cancer risk in young women at risk of hereditary nonpolyposis colorectal cancer: implications for gynecologic surveillance. Gynecol Oncol 80(3):346–349. doi:10.1006/gyno.2000.6065
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Kiyoyuki Minamiguchi declares that he has no conflict of interest. Junko Takahama declares that she has no conflict of interest. Nagaaki Marugami declares that he has no conflict of interest. Aki Marugami declares that she has no conflict of interest. Masayo Haga declares that she has no conflict of interest. Megumi Takewa declares that she has no conflict of interest. Takahiro Itoh declares that he has no conflict of interest. Kimihiko Kichikawa declares that he has no conflict of interest. Tomoko Uchiyama declares that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Minamiguchi, K., Takahama, J., Marugami, N. et al. MR findings of Lynch syndrome-related uterine endometrial carcinoma: a case report. Abdom Radiol 41, 1703–1706 (2016). https://doi.org/10.1007/s00261-016-0721-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0721-z