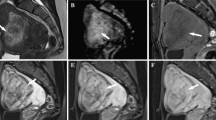
Abstract
Uterine sarcomas account for less than 1% of gynecological malignancies and 2–5% of all uterine malignancies. Such sarcomas mainly include leiomyosarcoma (LMS) and endometrial stromal sarcoma (ESS). Additionally, inflammatory myofibroblastic tumor (IMT) and endometrial carcinoma arising in adenomyosis can occur as uterine myometrial tumors. Their differentiation from leiomyoma (LM), particularly degenerated LM and the malignant tumors, is challenging, but preoperative diagnosis is very important for the patient’s management. We demonstrate the useful and compulsory findings to differentiate between uterine myometrial malignant tumors and degenerated LM with an unusual appearance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uterine sarcomas account for less than 1% of gynecological malignancies and 2–5% of all uterine malignancies. The most frequently occurring sarcomas type is leiomyomsarcoma (LMS), which accounts for approximately 50% of uterine sarcomas, followed by endometrial stromal sarcoma (ESS) [1]. Additionally, inflammatory myofibroblastic tumor (IMT), which is a mesenchymal neoplasm with an intermediate malignant potential, and endometrial carcinoma arising in adenomyosis, can occur as a uterine myometrial tumor. Their differentiation from leiomyoma (LM), particularly degenerated LM and malignant tumors, is challenging even when using MR imaging.
In the cases that are preoperatively considered as LM, approximately 0.3% end up being sarcomas [2]. As patient care and prognosis are significantly different from those for LM, preoperative diagnosis is very important for the management of the patients. The difficulty of the diagnosis is mainly because of the various degenerations in LM. MR imaging findings in the various degenerated LMs are sometimes quite similar to those of uterine malignant myometrial tumors. Therefore, we should precisely recognize several characteristic findings for the differentiation between LMs and malignant tumors.
In this review article, we aimed to show the useful and compulsory findings to differentiate between uterine myometrial malignant tumors and degenerated LM with an unusual appearance.
Uterine myometrial malignant and intermediate malignant tumors
Leiomyosarcoma (LMS)
The diagnosis of LMS is challenging. However, Lakhman Y et al. have reported the useful MR imaging findings suggesting leiomyosarcoma are nodular borders, hemorrhage (it is considered high intense foci on fat-saturated (fs) T1 weighted imaging (WI) in the figure), T2 dark areas, and central unenhanced areas (Figs. 1, 2). If the tumor shows more than or equal to three of these findings, they showed the sensitivity was 95–100% and the specificity was 95–100% [3]. According to a recent article by Ando et al. approximately 80% of LMSs show high intensity on T1WI [4]. We consider that especially high intense foci on fs-T1WI particularly matters for the differentiation. However, we should note that not all LMS contain high intensity lesions on T1WI (Fig. 3) Regarding contrast enhancement, Lin G et al. have reported that central nonenhancement on contrast enhanced imaging is the most characteristic imaging feature of LMS including smooth muscle tumor with uncertain malignant potential (STUMP), which yields a significantly higher diagnostic accuracy [5].
A 56-year-old woman with a histologically proven leiomyosarcoma. a Sagittal T2-weighted MR image shows a heterogeneous hyperintense tumor with hypointense areas. b Fat-saturated T1 weighted image shows irregular slight hyperintense areas. c Fat-saturated contrast enhanced T1 weighted image shows heterogeneous enhancement with small unenhancing areas. d, e Axial diffusion-weighted MR image (b = 1000 s/mm2) shows a heterogeneous hyperintense tumor with restricted diffusion areas on an apparent diffusion coefficient (ADC) map
A 61-year-old woman with a histologically proven leiomyosarcoma. a Sagittal T2-weighted MR image shows a heterogeneous hyperintense tumor with hypointense areas. b Fat-saturated T1 weighted image shows several tiny hyperintense areas (arrows). c Fat-saturated contrast enhanced T1 weighted image shows heterogeneous enhancement with irregular unenhancing areas
A 61-year-old woman with a histologically proven leiomyosarcoma. a Sagittal T2-weighted MR image shows an ill-defined and slightly heterogeneous isointense tumor (arrows). b Fat-saturated T1 weighted image shows no hyperintense areas. c Axial T2-weighted MR image shows an ill-defined heterogeneous isointense to hyperintense tumor. d Axial diffusion-weighted MR image (b = 1000 s/mm2) shows a heterogeneous hyperintense tumor with restricted diffusion areas on an apparent diffusion coefficient (ADC) map (not shown)
Meanwhile, several articles have reported that diffusion-weighted imaging (DWI) is a useful method for differentiation [6,7,8,9]. In fact, LMSs show high intensity with low apparent diffusion coefficient (ADC) values (Figs. 1, 3). Therefore, we definitely consider that high intensity on DWI is a very useful finding for spotting a tumor with the possibility of LMS. However, some LMs, including cellular LM (described below), have been reported to show high intensity on DWI and have broad ADC values and substantial overlaps [10]. Additionally, reported cut-off ADC values have been determined by post hoc analysis, and MR protocols including b value are different among reports. Thus, the optimized cut-off ADC value for differentiation is difficult to determine.
When necrosis or infarction occurs in almost the entire area of the LMS, the MR imaging findings can be very similar to those of red degenerated LM (detailed below) (Fig. 4), and differentiation is often difficult [11]. However, the possibility of LMS should be considered in the case with an enhancing small part of the tumor.
A 50-year-old woman with a histologically proven leiomyosarcoma. a–c: initial MR images, d–f: MR images after 1 year a Sagittal T2-weighted MR image shows a well-defined and slightly hyperintense tumor with a thin low intensity rim. b Fat-saturated T1 weighted image shows a thin discontinuous rim corresponding to low signal intensity on the T2-weighted image. c Fat-saturated contrast enhanced T1 weighted image shows that most of the tumor does not demonstrate contrast enhancement. However, the anterior of the tumor shows strong contrast enhancement (arrows). d Sagittal T2-weighted MR image shows a large slightly hyperintense tumor (arrows), which is assumed to be the area showing contrast enhancement in c. e Fat-saturated T1 weighted image shows irregular slight hyperintense areas (arrowheads). f Fat-saturated contrast enhanced T1 weighted image shows slightly heterogeneous contrast enhancement with small unenhancing areas in the anterior part of the tumor
Endometrial stromal sarcoma (ESS)
Endometrial stromal tumor is classified into five types: endometrial stromal nodule (benign), low grade ESS, high grade ESS, undifferentiated uterine sarcoma, and uterine tumor resembling ovarian sex cord tumor. Especially, low grade ESS matters for the differentiation from LM. Low grade ESS typically presents with extensive myometrial involvement, which is either sharply demarcated or diffusely infiltrative. Bands of low signal intensity within the areas of myometrial involvement on T2WI are important to note. They reflect pathologically the preserved bundles of myometrium (Fig. 5). As another characteristic finding, tumor extension along the vessels or ligaments is also observed [12].
A 35-year-old woman with a histologically proven low grade endometrial stromal sarcoma. a, b Sagittal and axial T2-weighted MR images show bands of low signal intensity in the tumor on T2WI (arrows). Additionally, a low intensity rim on T2WI is also observed in the peripheral area. c Axial diffusion-weighted MR image (b = 1000 s/mm2) shows a hyperintense tumor. The areas of the bands show low intensity compared to that of the tumor
A low intensity rim on T2WI (Fig. 5) has been reported to be a useful finding in ESS. It reflects fibrous tissue layers and/or a decrease in free water caused by distortion of the myometrial tissue following tumor expansion [13]. It should be recognized that the finding suggests ESS, but even though it is still controversial. In our experience, some LMs show a similar peripheral rim. Additionally, other myometrial tumors can also show this feature [14]. On the other hand, a recent report showed that ESSs do not have it [15].
There are other findings suggesting ESS. ESS shows high intensity on T2WI compared to LM and even LMS [16]. Additionally, more than half of ESS cases demonstrate degenerations such as cysts, necrosis (Fig. 5) and hemorrhage [17]. In general, most ESSs show hyperintensity on DWI (Fig. 5) [17]. The mean ADC value of the solid components in ESSs is reported to be 1.03–1.05 × 10−3 mm2/s [15, 17], and the value is shown to be lower than that of T2-hyperintense LM [15]. It has also been reported that DWI can help to detect a venous tumor thrombus of ESS [18].
Inflammatory myofibroblastic tumor (IMT)
IMT is a mesenchymal neoplasm with intermediate malignant potential, which was previously grouped in the category of inflammatory pseudotumors. Although IMTs have been found at every site in the body, such as in the soft tissue and lungs, uterine IMT has been considered extremely rare [19]. However, according to the report by Mohammad et al., there has been an increasing recognition of these neoplasms in recent years [20]. In fact, IMT has been included in tumors of the uterine corpus in the new 2020 WHO classification of Female Genital Tumors [21]. The prognosis is mostly benign. IMT rarely presents with metastases and/or recurrence [21]. In pathological specimens, IMT is usually positive for ALK (Anaplastic Lymphoma Kinase) by immunohistochemistry and is composed of myxoid vascular and compact spindle cell areas. On MR imaging, the tumor shows mixed signal intensity on T2WI. The part of the tumor with high intensity on T2WI shows mild contrast enhancement, which reflects the myxoid vascular area. On the other hand, a tumor with low intensity on T2WI and strong contrast enhancement reflects the areas of compact spindle cells (Fig. 6) [22].
A 35-year-old woman with a histologically proven inflammatory myofibroblastic tumor (IMT). a Sagittal T2-weighted MR image shows a heterogeneous hyperintense tumor with relatively hypointense irregular regions. b–d Dynamic contrast enhanced images before contrast (b), arterial (c), and late (d) phase show early heterogeneous prolonged contrast enhancement around the surrounding area of hypointense on T2WI
Endometrial carcinoma arising in adenomyosis
The incidence of endometrial carcinoma arising in adenomyosis is 0.7% of cases of endometrial carcinoma [23]. The diagnostic criteria are: (1) the carcinoma must not be situated in the endometrium or elsewhere in the pelvis, (2) the carcinoma must be seen to arise from the epithelium of adenomyosis and not to be invaded from another source, and (3) endometrial (adenomyotic) stromal cells should be surrounding the aberrant glands to support the diagnosis of adenomyosis [24]. The type of pathology is most commonly endometrioid carcinoma. Additionally, serous, clear, poorly differentiated carcinoma or endometrial stromal sarcoma and adenosarcoma [14] have been reported.
The lesion may manifest as a discrete tumor within, or adjacent to, the adenomyosis, which mimics degenerated LM or LMS on MR imaging (Fig. 7). Meanwhile, the lesion can exhibit an infiltrative form of invasion within the adenomyosis. MR imaging findings including signal intensities and enhancement patterns, can be similar to that of endometrial cancer. Therefore, most importantly, we should recognize the fact that an endometrial cancer can arise in the myometrium and appear as a myometrial tumor. Basically, the tumor does not continue to the endometrium. Thus, cytologic evidence is hard to obtain at an early stage, which leads to a diagnostic delay resulting in an unfavorable prognosis [25].
A 58-year-old woman with a histologically proven clear cell carcinoma arising from adenomyoma or adenomyosis. Her past history was hormonal therapy for breast cancer. a Sagittal T2-weighted MR image shows a heterogeneous hyperintense papillary tumor with a cystic component. The myometrium adjacent to the tumor shows low intensity with a clear margin, suggesting adenomyoma or posttherapeutic adenomyosis (arrows). b Diffusion-weighted MR image (b = 1000 s/mm2) shows a relatively heterogeneous hyperintense tumor with restricted diffusion on the ADC map (not shown). c Fat-saturated T1 weighted image shows a hypointense tumor with bloody fluid. d Fat-saturated contrast enhanced T1 weighted image shows heterogeneous mild enhancement in the tumor
Degenerated or unusual leiomyomas
Red degeneration
We must consider the possibility of red LM degeneration if the tumor shows high intensity on fs-T1WI. The causes of red degeneration include pregnancy, contraceptive drugs, and GnRH agonists [26]. Typical red degenerated LM shows a high intensity rim on T1WI and a low intensity rim on T2WI in the acute phase, which reflects the dilated thrombosed vessels filled with red blood cells. In the subacute-chronic phase, the entire tumor shows high intensity on T1WI (Fig. 8) [27], which reflects the cytoplasm of smooth muscle cells with coagulative necrosis [28]. A tumor suggested to be LM with ringed calcifications at the margins is sometimes observed on CT. This type of calcification appears to represent thrombosed veins from past red degeneration [29].
A 58-year-old woman with abdominal pain, and with a histologically proven leiomyoma. a Sagittal T1-weighted MR image shows a hypointense tumor with a high intensity rim. b T2-weighted MR image shows heterogeneous slight high intensity with a low intensity rim on T2WI corresponding to the high intensity rim on T1WI. These findings correspond to red degeneration
Additionally, in our experience, without a clinical history suggesting red degeneration, parts of the LM rarely show high intensity on T1WI, which is a similar finding to LMS (Fig. 9). According to a recent article [4], only 1.3% (15/1118) of LM, except red degenerated LM and lipoleiomyoma, showed high intensity on T1WI. Although they did not clearly explain their observations of high intensity, we consider that the high intensity they observed might be due to the same mechanism of red degeneration in a part of the tumor.
A 42-year-old woman with histologically proven leiomyoma with hyaline degeneration. a, b Sagittal and axial T2-weighted MR image shows a heterogeneous intensity tumor with a low intensity rim on T2WI. c Axial fat-saturated T1 weighted image shows slight hyperintense in the left area of the tumor (arrows)
Lipoleiomyoma
Lipoleiomyoma is a rare (0.03–2.1%) LM variant, composed of an intimate admixture of mature smooth muscle cells and adipocytes [30]. Fat in the well-demarcated tumor is easily recognized using fs-T1WI in most of the cases (Fig. 10). Consequently, the diagnosis is not generally difficult. However, the identification of the fat is sometimes difficult because of the small amount of fat in the tumor (Fig. 11) [31]. Chemical shift imaging can contribute to diagnose in such cases. Additionally, lipoleiomyomas are frequently seen in the postmenopausal ages, in which LMSs also frequently occur. Therefore, we should recognize lipoleiomyoma with a small amount of fat as a differential diagnosis.
A 57-year-old woman with a histologically proven lipoleiomyoma. a Sagittal T2-weighted MR image shows a heterogeneous hypointense tumor with slight high intensity. b T1-weighted MR image shows a heterogeneous hypointense tumor with admixed high intensity. c Fat-saturated T1 weighted image shows decreased signal intensity corresponding to high intensity on T1WI
A 51-year-old woman with a histologically proven lipoleiomyoma. a Axial T2-weighted MR image shows a hyperintense tumor with a tiny cystic area. b T1-weighted MR image (in phase) shows a hypointense tumor with slight high intensity in a part of the tumor (arrows). c Opposed phase MR image shows decreased signal intensity corresponding to the high intensity on T1WI (arrows). d Fat-saturated T1 weighted image shows decreased signal intensity corresponding to the high intensity on T1WI (arrows). However, the decreased intensity is unclear compared to the opposed phase MR image
Pyomyoma
Pyomyoma is clinically defined as a leiomyoma with suppurative inflammation, characterized by the production of pus or a purulent exudate that contains neutrophils and necrotic cells [2]. To our knowledge, only one case report regarding MR imaging has been reported [32]. According to that article, the cystic component of pyomyoma shows hypointense to isointense on T1WI and hypointense to hyperintense on T2WI with a peripheral rim with hyperintense on T1WI and hypointense on T2WI, which is assumed to be the same mechanism as in fibrous capsules of brain abscesses. In that article, the cause of this peripheral rim was suggested to be the presence of heterogeneously distributed free radicals, which were the products of respiratory bursts produced by actively phagocytosing macrophages in the capsular walls [33].
Myxoid leiomyoma
This degeneration is relatively rare and composed of abundant myxoid material between smooth muscle cells. Myxoid leiomyomas are considered to be difficult to precisely differentiate from myxoid leiomoyosarcomas, even in pathological specimens. The tumor shows high intensity with reticular low intensity on T2WI with delayed and prolonged contrast enhancement around the surrounding area of low signal intensity on T2WI (Fig. 12) [29].
A 23-year-old woman with a histologically proven myxoid leiomyoma. a Sagittal T2-weighted MR image shows a hyperintense lesion with relatively hypointense reticular areas in the pelvic cavity. A hypointense area with flow voids is observed at the uterine fundus, which is the so-called bridging vascular sign (arrow). b T1-weighted MR image shows a hypointense lesion with isointense reticular areas in the pelvic cavity. c Fat saturated contrast enhanced image shows heterogeneous contrast enhancement around the surrounding area of reticular hypointense areas on T2WI
Meanwhile, cystic degeneration of LM should be considered in case of the tumor accompanying well-circumscribed, round and homogeneous high intensity area on T2WI (Fig. 13), which is also important for the differentiation from ovarian tumors. Cystic degeneration is observed in approximately 4% of leiomyomas. This degeneration is caused by the development of cysts in the edematous, acellular areas [29].
A 34-year-old woman with a histologically proven leiomyoma with cystic degeneration. a Sagittal T2-weighted MR image shows a multi cystic hyperintense tumor with peripheral and internal septal hypointense in the posterior uterine body. b Fat saturated contrast enhanced image shows contrast enhancement at the hypointense areas of the tumor on T2WI
Cellular leiomyoma
Cellular leiomyomas are rare, comprising less than 5% of all LMs. They consist of compact smooth muscle cells with packed nuclei and scant intervening collagen. On MR imaging, cellular LMs are well circumscribed with relatively homogeneous, slightly high intensity on T2WI and homogenous contrast enhancement (Fig. 14) [34]. However, in our experience, cellular LM can show heterogeneous signal intensity and enhancement (Fig. 15). Increased cellularity also leads to restricted diffusion, a feature that overlaps with LMSs [6].
A 43-year-old woman with a histologically proven cellular leiomyoma. a Sagittal T2-weighted MR image shows a hyperintense tumor (arrow). b Contrast enhanced image shows relatively homogeneous contrast enhancement. c Axial diffusion weighted image shows hyperintensity with relatively restricted diffusion on the ADC map (not shown)
A 44-year-old woman with a histologically proven cellular leiomyoma. a Axial T2-weighted MR image shows a heterogeneous hyperintense tumor with multiple various sized cystic areas. b Fat-saturated contrast enhanced T1 weighted image shows heterogeneous enhancement with unenhancing areas. c, d Diffusion-weighted MR image (b = 1000 s/mm2) shows a hyperintense tumor with restricted diffusion on the ADC map, corresponding to the enhancing areas
How to differentiate leiomyomas from uterine myometrial malignant tumors
It is challenging to differentiate between LM and malignant tumors; however, many researchers have attempted to differentiate these tumors using MR imaging findings and by making decision trees [35, 36]. Although these contents are not uniform, myometrial tumors with an entirely low intensity both on T2WI and DWI compared to the myometrium are considered to be LMs. Myometrial tumors with high intensity on DWI as well as on T1WI have the possibility of being LMS. Additionally, the distribution of the high intensity on T1WI and signal suppression on fs-T1WI are important for differentiation. However, bands of low signal intensity and peripheral low intensity rim on T2WI are notable for the diagnosis of ESS. The mixed-signal tumor containing myxoid tissue can be an IMT. In the case of the tumor within or adjacent to the adenomyosis, endometrial carcinoma arising in the adenomyosis should be considered.
Conclusion
The differentiation from LM, particularly degenerated LM and the malignant tumors, is challenging due to quite a few overwrapped MR imaging findings. Therefore, precise knowledge about MR imaging findings for both degenerated LM and malignant myometrial tumors is mandatory for the correct diagnosis.
References
Oliva E, Zaloudek CJ, Soslow RA. Mesenchymal tumors of the uterus. In: Kurman RJ, Ronnett BM, Ellenson L, editors. Blaustein’s pathology of the female tract. New York: Springer; 2019. p. 535–647.
Paul PG, Rengaraj V, Das T, Garg R, Thomas M, Khurd AS. Uterine sarcomas in patients undergoing surgery for presumed leiomyomas: 10 years’ experience. J Minim Invasive Gynecol. 2016;23:384–9.
Lakhman Y, Veeraraghavan H, Chaim J, Feier D, Goldman DA, Moskowitz CS, et al. Differentiation of uterine leiomyosarcoma from atypical leiomyoma: diagnostic accuracy of qualitative mr imaging features and feasibility of texture analysis. Eur Radiol. 2017;27:2903–15.
Ando T, Kato H, Furui T, Morishige KI, Goshima S, Matsuo M. Uterine smooth muscle tumours with hyperintense area on T1 weighted images: differentiation between leiomyosarcomas and leiomyomas. Br J Radiol. 2018;91:20170767.
Lin G, Yang LY, Huang YT, Ng KK, Ng SH, Ueng SH, et al. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma /smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging. 2016;43:333–42.
Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723–30.
Takeuchi M, Matsuzaki K, Nishitani H. Hyperintense uterine myometrial masses on T2-weighted magnetic resonance imaging: differentiation with diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2009;33:834–7.
Namimoto T, Yamashita Y, Awai K, Nakaura T, Yanaga Y, Hirai T, et al. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2009;19:2756–64.
Tasaki A, Asatani MO, Umezu H, Kashima K, Enomoto T, Yoshimura N, et al. Differential diagnosis of uterine smooth muscle tumors using diffusion-weighted imaging: correlations with the apparent diffusion coefficient and cell density. Abdom Imaging. 2015;40:1742–52.
Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014;210(368):e1-8.
Ochiai R, Fujii S, Kudo A, Mukuda N, Murakami A, Fukunaga T, et al. A case of uterine leiomyosarcoma mimicking red degeneration. Rinshouhoushasen. 2019;64:1549–54 ((in Japanese)).
Koyama T, Togashi K, Konishi I, Kobayashi H, Ueda H, Kataoka ML, et al. MR imaging of endometrial stromal sarcoma: correlation with pathologic findings. AJR Am J Roentgenol. 1999;173:767–72.
Furukawa R, Akahane M, Yamada H, Kiryu S, Sato J, Komatsu S, et al. Endometrial stromal sarcoma located in the myometrium with a low-intensity rim on T2-weighted images: report of three cases and literature review. J Magn Reson Imaging. 2010;31:975–9.
Fujii S, Nosaka K, Mukuda N, Fukunaga T, Sato S, Ogawa T. MR Imaging of an intramural adenosarcoma with pathologic correlation. Magn Reson Med Sci. 2018;17:1–2.
Huang YL, Ueng SH, Chen K, Huang YT, Lu HY, Ng KK, et al. Utility of diffusion-weighted and contrast-enhanced magnetic resonance imaging in diagnosing and differentiating between high- and low-grade uterine endometrial stromal sarcoma. Cancer Imaging. 2019;19:63.
Kim TH, Kim JW, Kim SY, Kim SH, Cho JY. What MRI features suspect malignant pure mesenchymal uterine tumors rather than uterine leiomyoma with cystic degeneration? J Gynecol Oncol. 2018;29:e26.
Li HM, Liu J, Qiang JW, Gu WY, Zhang GF, Ma FH. Endometrial stromal sarcoma of the uterus: magnetic resonance imaging findings including apparent diffusion coefficient value and its correlation with Ki-67 Expression. Int J Gynecol Cancer. 2017;27:1877–87.
Fujii S, Kaneda S, Tsukamoto K, Kakite S, Kanasaki Y, Matsusue E, et al. Diffusion-weighted imaging of uterine endometrial stromal sarcoma: a report of 2 cases. J Comput Assist Tomogr. 2010;34:377–9.
Takahashi A, Kurosawa M, Uemura M, Kitazawa J, Hayashi Y. Anaplastic lymphoma kinase-negative uterine inflammatory myofibroblastic tumor containing the ETV6-NTRK3 fusion gene: a case report. J Int Med Res. 2018;46:3498–503.
Mohammad N, Haimes JD, Mishkin S, Kudlow BA, Leong MY, Chew SH, et al. ALK is a specific diagnostic marker for inflammatory myofibroblastic tumor of the uterus. Am J Surg Pathol. 2018;42:1353–9.
Parra-Herran C, Lee CH, Bennett JA, Rebban JT. Inflammatory myofibroblastic tumour WHO classification of tumours Editorial Board WHO classification of tumours of female reproductive organs. Lyon: International Agency for Research on Cancer; 2020.
Horger M, Pfannenberg C, Bitzer M, Wehrmann M, Claussen CD. Synchronous gastrointestinal and musculoskeletal manifestations of different subtypes of inflammatory myofibroblastic tumor: CT MRI and pathological features. Eur Radiol. 2005;15:1713–6.
Koshiyama M, Suzuki A, Ozawa M, Fujita K, Sakakibara A, Kawamura M, et al. Adenocarcinomas arising from uterine adenomyosis: a report of four cases. Int J Gynecol Pathol. 2002;21:239–45.
Colman HI, Rosenthal AH. Carcinoma developing in areas of adenomyosis. Obstet Gynecol. 1959;14:342–8.
Tamai K, Togashi K, Ito T, Morisawa N, Fujiwara T, Koyama T. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics. 2005;25:21–40.
Hachiya K, Kato H, Kawaguchi S, Kojima T, Nishikawa Y, Fujiwara S, et al. Red degeneration of a uterine fibroid following the administration of gonadotropin releasing hormone agonists. J Obstet Gynaecol. 2016;36:1018–9.
Kawakami S, Togashi K, Konishi I, Kimura I, Fukuoka M, Mori T, et al. Red degeneration of uterine leiomyoma: MR appearance. J Comput Assist Tomogr. 1994;18:925–8.
Nakai G, Yamada T, Hamada T, Atsukawa N, Tanaka Y, Yamamoto K, et al. Pathological findings of uterine tumors preoperatively diagnosed as red degeneration of leiomyoma by MRI. Abdom Radiol (NY). 2017;42:1825–31.
Ueda H, Togashi K, Konishi I, Kataoka ML, Koyama T, Fujiwara T, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics. 1999;19:131–45.
Akbulut M, Gündoğan M, Yörükoğlu A. Clinical and pathological features of lipoleiomyoma of the uterine corpus: a review of 76 cases. Balkan Med J. 2014;31:224–9.
Kitajima K, Kaji Y, Imanaka K, Sugihara R, Sugimura K. MRI findings of uterine lipoleiomyoma correlated with pathologic findings. AJR Am J Roentgenol. 2007;189:W100–4.
Ono H, Kanematsu M, Kato H, Toyoki H, Hayasaki Y, Furui T, et al. MR imaging findings of uterine pyomyoma: radiologic-pathologic correlation. Abdom Imaging. 2014;39:797–801.
Haimes AB, Zimmerman RD, Morgello S, Weingarten K, Becker RD, Jennis R, et al. MR imaging of brain abscesses. AJR Am J Roentgenol. 1989;152:1073–85.
Yamashita Y, Torashima M, Takahashi M, Tanaka N, Katabuchi H, Miyazaki K, et al. Hyperintense uterine leiomyoma at T2-weighted MR imaging: differentiation with dynamic enhanced MR imaging and clinical implications. Radiology. 1993;189:721–5.
Thomassin-Naggara I, Dechoux S, Bonneau C, Morel A, Rouzier R, Carette MF, et al. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol. 2013;23:2306–14.
Abdel Wahab C, Jannot AS, Bonaffini PA, Bourillon C, Cornou C, Lefrère-Belda MA, et al. Diagnostic algorithm to differentiate benign atypical leiomyomas from malignant uterine sarcomas with diffusion-weighted MRI. Radiology. 2020;297:361–71.
Acknowledgements
We thank Hiroaki Komatsu, Shinya Sato, Tetsuro Oishi, for their help in the tumor board, as well as Kanae Nosaka for her assistance with the pathological diagnosis and advices.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fujii, S., Mukuda, N., Ochiai, R. et al. MR imaging findings of unusual leiomyoma and malignant uterine myometrial tumors: what the radiologist should know. Jpn J Radiol 39, 527–539 (2021). https://doi.org/10.1007/s11604-021-01096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01096-7