Abstract
Renal and urinary tract imaging is commonly performed in the pediatric population, particularly in the setting of suspected or known congenital anomalies. In most cases, adequate anatomic assessment can be achieved using ultrasound and fluoroscopic techniques, and evaluation of differential renal function and urinary tract drainage can be accomplished with renal scintigraphy. However, in a subset of children, anatomic or functional questions may remain after this routine evaluation. In this setting, magnetic resonance imaging (MRI) tailored to evaluate the kidneys and urinary tract, known as MR urography (MRU), can be used to depict the kidneys, ureters, and urinary bladder in detail and to determine differential renal function and assess urinary tract drainage. The objectives of this review article are to (1) describe pediatric-specific MRI techniques for assessment of the kidneys and urinary tract and (2) present common clinical applications for pediatric MRU where imaging can “add value” in terms of diagnosis and patient management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Renal and urinary tract imaging is commonly performed in the pediatric population, particularly in the setting of suspected or known congenital anomalies. Congenital anomalies of the kidney and urinary tract, or CAKUT, represent 20–30% of developmental abnormalities detected by ultrasound and MRI during the antenatal period [1], and they occur in approximately 1 in 500 live births [2]. In most cases, adequate postnatal anatomic assessment can be accomplished using ultrasound and fluoroscopic techniques, whereas the evaluation of differential renal function and urinary tract drainage can be performed satisfactorily with renal scintigraphy. However, in certain children, anatomic or functional questions may remain after this routine radiologic evaluation. It is in this setting that magnetic resonance imaging (MRI) tailored to evaluate the kidneys and urinary tract, known as MR urography (MRU), can be used to evaluate the kidneys, ureters, and urinary bladder in detail as well as to accurately assess differential renal function and urinary tract drainage [3–6]. While intravenous urography has been used in the past to assess the pediatric kidneys and urinary tracts, MRU offers numerous advantages, including superior contrast resolution (allowing improved visualization of small urinary tract structures, obstructed urinary tracts, and subtle renal parenchymal changes) and the ability to perform dynamic postcontrast imaging with quantitative assessment of differential renal function and urinary tract drainage.
The primary objectives of this review article are to (1) describe pediatric-specific MRI techniques for anatomic and functional assessment of the kidneys and urinary tract and (2) present common clinical applications for pediatric MRU where imaging can “add value” in terms of diagnosis and patient management.
Pediatric MRU technique
Pediatric MRU can be performed successfully in children of all ages using both 1.5- and 3-Tesla (T) MR scanners [3]. The primary advantage of 3 T is superior spatial resolution with improved visualization of small urinary tract structures, particularly in young children, while the primary advantages of 1.5 T include more homogeneous fat saturation and decreased T2* effect of excreted contrast material in the urinary tracts. A multi-channel surface coil should be used to maximize image quality, including signal-to-noise ratio and spatial resolution, and to minimize examination length. Dedicated infant, knee, or head and neck coils can be used to acquire images in neonates and infants, while torso coils (and posterior spine coils, when available) should be used in older children and adolescents. In very young children, imaging may require general anesthesia or conscious sedation to prevent artifacts related to patient motion, while MRU examinations generally can be performed awake in older children and adolescents.
Most pediatric MRU protocols described in the literature are based on the combination of T2-weighted and gadolinium chelate-enhanced imaging [3–5]. T2-weighted imaging, or MR hydrography, allows assessment of fluid (urine)-filled urinary tract structures and the renal parenchyma, including evaluation for structural anomalies and dysplasia. Commonly employed T2-weighted pulse sequences include single-shot fast spin-echo (SSFSE), 2D fast spin-echo (FSE), and 3D FSE. SSFSE imaging allows for free-breathing, respiratory-triggered, or breath-held renal and urinary tract imaging that is rapid and relatively robust in terms of overcoming motion artifacts. 2D and 3D FSE imaging provides images with a better signal-to-noise ratio and improved spatial resolution compared to SSFSE, although these pulse sequences take longer to acquire and require respiratory-triggering or navigator gating to achieve suitable image quality. 3D FSE imaging allows for high-quality 2D reformations and 3D reconstructions if performed with isotropic or near-isotropic voxel size. Fast-recovery FSE (FRFSE) and fat-suppression techniques can be used to accentuate T2-weighted imaging by increasing image contrast resolution and likely improving the sensitivity of MRU for detecting subtle renal and urinary tract abnormalities. The primary drawback of T2-weighted imaging is that non-dilated/non-obstructed urinary tract structures may be difficult to visualize due to their small size.
Conversely, postcontrast imaging provides excellent depiction of non-dilated/non-obstructed kidneys and urinary tracts. There are two basic postcontrast techniques used in pediatric MRU protocols: (1) dynamic postcontrast imaging and (2) delayed postcontrast excretory phase imaging [3–5]. Dynamic postcontrast imaging involves the repeated acquisition of 3D spoiled gradient recalled echo images through the kidneys and ureters over a period of up to 10–15 min during and after the slow intravenous injection (0.1–0.3 mL/second) of contrast material [3–5]. This allows detailed assessment of the renal vasculature, renal parenchyma in multiple phases (e.g., corticomedullary and nephrographic phases), and contrast-opacified urinary tract. A typical dynamic postcontrast acquisition may obtain 50 or more 3D image volumes, which can be reviewed individually or as a concatenated maximum intensity projection (MIP) image series [3–5]. Dynamic postcontrast imaging (sometimes referred to as functional MRU, or fMRU) also can be used to assess differential renal function using the Patlak–Rutland method [7, 8] as well as to evaluate urinary tract drainage using the combination of subjective visual assessment, renal parenchymal time vs. signal intensity curves, and quantitative parameters (such as calyceal transit time and renal transit time) [5, 9]. Delayed postcontrast excretory phase imaging can be performed in the axial, sagittal, or coronal planes (commonly all three planes) and used to obtain high spatial resolution images of the kidneys and ureters, including the ureteropelvic and ureterovesical junctions (UVJ). Such images also can be used to create 2D reformations and 3D reconstructions.
Unlike other routine pediatric MRI examinations of the pediatric abdomen and pelvis, MRU is commonly optimized using several ancillary maneuvers [10]. Pre-procedural hydration (e.g., normal saline administered intravenously immediately prior to imaging) and diuretic administration (e.g., furosemide 0.5–1.0 mg/kg, up to 20 mg) increase urine production with resultant improved distention and visualization of the urinary tract [11]. These maneuvers also decrease the length of examination by expediting the passage of contrast material through the kidneys and ureters into the urinary bladder as well as minimize T2* effects related to excreted concentrated contrast material in the urinary tract, limiting signal decay and maintaining hyperintensity of fluid in the urinary tract [11]. A final benefit of pre-procedural hydration and diuretic administration is that the associated increased urine production serves as a “stress test” for the urinary tract, potentially revealing a mild or intermittent obstructing process (e.g., ureteropelvic junction obstruction due to a crossing vessel). When the primary reason for MRU is to diagnose or characterize urinary tract obstruction, similar to diuretic scintigraphy, placement of a Foley catheter in the urinary bladder should be considered to decompress the urinary bladder and minimize the back pressure on the kidneys and ureters that might spuriously delay the passage of contrast material through the kidneys and urinary tract.
Recently, based on a retrospective review of 99 MRU examinations, Delgado et al. [12] proposed an “optimized” pediatric MRU protocol that allows imaging to be completed in less than 20–30 min (vs. 40–60 min for usual MRU protocols). This protocol includes five pulse sequences, obtains only 8 min of dynamic postcontrast imaging, and images the child prone in order to promote urinary tract drainage. This represents a potential time savings of 20–30 min over the typical MRU protocol. While such a protocol is appealing in terms of workflow and limitation of sedation and scan time, further study is needed to confirm if this shortened protocol is sufficient when compared to the more standard comprehensive approach.
fMRU processing is most often performed using freeware software packages (e.g., www.chop-fmru.com) that aid with kidney segmentation, creation of time vs. signal intensity curves, and derivation of quantitative parameters, such as differential renal function and renal transit time (amount of time required for contrast material to pass from the renal cortex to the proximal ureter).
Common clinical applications for pediatric MRU
There are several applications for which MRU can add diagnostic value when ultrasound and scintigraphy fail to provide adequate assessment. Those clinical indications include, but are not limited to, the evaluation of complex anatomy, hydronephrosis, hydroureter, and ureteric ectopia.
Complex anatomy
The assessment of complex renal and/or urinary tract anatomy is an established clinical indication for MRU in the pediatric population. Commonly, this situation involves a very young child (e.g., neonate or infant) with a history of prenatal hydronephrosis and postnatal imaging showing marked hydroureteronephrosis and renal parenchymal abnormalities, such as severe thinning, altered echogenicity, and cysts (ultrasound findings indicative of renal dysplasia). Such severely dilated, often redundant, urinary systems can be difficult to fully assess with ultrasound and scintigraphy. MR hydrography, however, excellently depicts such dilated systems and allows for a variety of 2D reformations and 3D reconstructions which allow both a global and detailed assessment of anatomy. In our experience in this setting, MRU often confirms the presence of complex urinary tract duplication and can reveal the exact cause of upper (e.g., obstructing ectopic ureterocele or ectopic stenotic ureteral orifice) (Figs. 1 and 2) and lower (e.g., UPJ obstruction) (Fig. 3) moiety collecting system and ureter dilatation [6]. Based on urinary tract findings as well as detailed renal assessment (evaluating for parenchymal dysplasia, moiety size/volume, and differential function), the radiologist can provide information that assists the pediatric urologist in deciding which exact surgical intervention(s) is required in order to preserve functioning renal tissue and optimize patient outcomes.
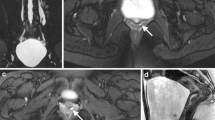
A 4-month-old girl with severe hydronephrosis on prenatal and postnatal ultrasound. MRU was performed to clarify the child’s complex urinary tract anatomy. A Coronal single-shot fast spin-echo image confirms that there is duplication of the left urinary tract with two separate ureters. There is both upper and lower moiety hydroureteronephrosis, and no ureterocele is seen. There is right pelvicaliectasis (arrow) due to ureteropelvic junction obstruction. B 3D T2-weighted fast spin-echo volume-rendered image (posterior view) confirms the above findings. The left kidney upper moiety ureteric insertion is ectopic (arrow), inserting into the urinary bladder neck
A 6-month-old boy with prenatal and postnatal hydronephrosis and solitary left kidney. Ultrasound suggested a single-system ureterocele. Coronal delayed postcontrast T1-weighted 3D spoiled gradient recalled echo image shows that there is a duplex left kidney. There is marked left upper moiety hydroureteronephrosis and severe overlying renal parenchymal thinning. The absence of contrast material in the left upper urinary tract and ureterocele (asterisk), which appears hypointense, at 20 min is consistent with severe obstruction. There is a small amount of normal-appearing left lower moiety renal parenchyma with contrast material seen in lower moiety calyces (arrow)
A 2-month-old girl with prenatal and postnatal hydronephrosis. MRU was performed to clarify the child’s complex urinary tract anatomy. Coronal single-shot fast spin-echo image confirms duplication of the right upper urinary tract, with two dilated right-sided ureters (medial to dilated lower moiety renal pelvis) and a large bilobed ureterocele (arrows). There is marked right lower moiety pelvicaliectasis due to ureteropelvic junction narrowing (not shown). The right upper moiety collecting system is not seen
Hydronephrosis
The majority of children with antenatal hydronephrosis have non-obstructive etiologies and pelvicaliectasis decreases over time, without intervention [13]. However, in some children, hydronephrosis may persist in the absence of vesicoureteral reflux. In the majority of these children, ultrasound and diuretic renal scintigraphy can adequately categorize hydronephrosis as obstructive or non-obstructive. MRU, however, can add value in atypical cases when there are anatomic findings that suggest diagnoses other than UPJ obstruction (e.g., could the child have a mid-ureter stricture, which requires a different surgical approach) (Fig. 4) or when renal scintigraphy results conflict with other radiologic or clinical data (e.g., significant hydronephrosis by ultrasound and symptoms consistent with UPJ obstruction, yet normal or equivocal urinary drainage by scintigraphy) (Fig. 5). MRU findings suggestive of significant UPJ obstruction on T2-weighted imaging include severe focal urinary tract narrowing, renal parenchymal signal hyperintensity, and hyperintense signal surrounding the kidney and/or renal collecting system due to transudation of fluid or forniceal rupture. Postcontrast imaging findings suggestive of significant UPJ obstruction include decreased perfusion on dynamic assessment, delayed passage of contrast material through the ipsilateral kidney and collecting system (delayed calyceal and renal transit times), and an obstructive pattern of the time vs. signal curve (increasing signal intensity over 15 min, consistent with hyperintense nephrogram) [14].
A 5-month-old girl with bilateral prenatal and postnatal hydronephrosis. A Coronal 3D T2-weighted fast spin-echo maximum intensity projection image shows moderate–severe dilatation of the proximal and mid-ureter bilaterally (arrows). The left renal collecting system is also dilated. Imaging findings are consistent with bilateral mid-ureteric strictures. B Antegrade nephrostogram (posterior view) confirms the presence of an obstructing left mid-ureteric stricture (arrow); right nephrostogram was not performed
A 15-year-old girl with history of bilateral hydronephrosis and diuretic renal scintigraphic findings equivocal for urinary tract obstruction. Coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image obtained 15 min after contrast material injection shows symmetric, normal-appearing kidneys, and timely excretion of contrast material. There is no focal urinary tract narrowing to indicate an area of obstruction
MRU also can be used to comprehensively evaluate older children with intermittent flank pain and suspected intermittent hydronephrosis/UPJ obstruction due to a crossing vessel [15, 16] (Fig. 6). Such patients may have a history of negative ultrasound examinations due to a lack of fluid stress at the time of imaging. While renal scintigraphy can provide a dynamic assessment under fluid stress similar to MRU in these patients, MRU has the added value of documenting and depicting the crossing vessel as causative of the intermittent obstruction. Recent studies by Parihk et al. [15] and Weiss et al. [16] both showed that MRU can document the presence of such vessels in the setting of pediatric UPJ obstruction, an observation that may be particularly important if a robot-based or laparoscopic surgical intervention is planned.
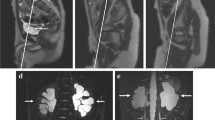
A 6-year-old boy with intermittent left flank pain due to left ureteropelvic junction obstruction related to a crossing vessel. A Coronal 3D T2-weighted fast spin-echo maximum intensity projection image shows severe left pelvicaliectasis. The left ureter is decompressed and not seen. B Arterial phase coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image shows a left lower pole accessory artery (arrow) that crosses the left ureteropelvic junction. C Coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image shows a small amount of excreted contrast material in the left renal collecting system 20 min after injection; no contrast material is present in the left ureter. Left kidney parenchyma also appears hyperintense compared to the right kidney. All of these findings confirm obstruction by the crossing vessel. The right urinary tract is normal
More isolated calyceal obstructions can be very difficult to adequately assess with ultrasound and renal scintigraphy. For example, in children with isolated hydrocalyx, MRU can be used to confirm the presence or absence of obstructive infundibular narrowing. Such narrowing may be acquired (e.g., post-traumatic) (Fig. 7) or developmental (e.g., due to a crossing accessory/polar renal artery) (Fig. 8). Finally, non-obstructive caliectasis due to congenital megacalyces can also be recognized and evaluated using MRU (Fig. 9).
A 13-year-old boy with right flank pain and history of prior right kidney laceration. A Longitudinal gray-scale ultrasound image shows a fluid-filled cystic structure (arrows) in the upper right kidney. B Excretory phase coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image shows contrast material filling right kidney lower pole calyces and the right ureter. No excreted contrast material is present in right kidney upper pole calyces. C More delayed excretory coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image shows excreted contrast material in dilated, obstructed right kidney upper pole calyces (arrows). Imaging findings are consistent with acquired infundibular stenosis
An 8-year-old boy with incidentally detected left kidney upper pole hydronephrosis on CT examination performed to evaluate right lower quadrant pain. A Sagittal single-shot fast spin-echo image shows left kidney upper pole hydronephrosis (arrows) with overlying parenchymal thinning. B Frontal projection 3D T2-weighted fast spin-echo volume-rendered image of the left upper pole renal collecting system shows substantial dilatation as well as abrupt infundibular narrowing with an apparent crossing vessel, presumably a vein due to slow flow (arrows). Imaging findings are consistent with congenital infundibular stenosis, possibly due to a crossing vessel. A vein as well as a large crossing artery were identified in this location at surgery
A 14-year-old boy with marked caliectasis on ultrasound due to presumed primary megacalyces. Coronal single-shot fast spin-echo image shows an increased number of crowded, dilated right-sided calyces with no significant pelviectasis; the right kidney is also enlarged, and postcontrast imaging (not presented) showed no evidence for right urinary tract obstruction. Right kidney parenchymal thinning is likely due to medullary pyramid hypoplasia
Hydroureter
Ureteric dilatation can be due to a variety of causes of the pediatric population, including vesicoureteral reflux, obstructing ureterocele, and ectopic insertion. Other common causes of ureteric dilatation are distal ureteric obstruction due to UVJ obstruction and congenital primary megaureter. UVJ obstructions occur at the level of the ureter–urinary bladder junction, while congenital primary megaureter usually presents as a relatively short segment of narrowed, aperistaltic distal ureter, although long-segment ureteric narrowing can be present in some children (Fig. 10) [17]. MRU adds value in these patients by providing a level of anatomic detail at the distal ureter and UVJ that is difficult to achieve with sonography and that does not exist with scintigraphy. In addition to providing anatomic detail, MRU can be used to simultaneously evaluate if surgical ureteric reimplantation is indicated. A variety of imaging findings that may indicate the need for surgical repair include the presence of acute (decompensated) obstruction after diuretic challenge and evidence of significant renal injury, including focal scarring and asymmetric renal function or decreasing ipsilateral renal function over time (Figs. 11 and 12) [18].
A 6-year-old boy with prenatal and postnatal left hydronephrosis. The left distal ureter was not seen at ultrasound. A Coronal-oblique 3D T2-weighted maximum intensity projection image shows dilatation of the left renal collecting system and proximal/mid-ureter. The left distal ureter appears narrowed over a several centimeter segment (arrow). B Retrograde pyelogram image shows a catheter in the left distal ureter. The left distal ureter is non-dilated and tortuous for several centimeters (arrows), while more proximal ureter is dilated and contains dilute contrast material. Long-segment congenital primary megaureter was confirmed at surgery
A 1-year-old girl with antenatal and postnatal right hydroureteronephrosis due to obstructing megaureter. A Coronal single-shot fast spin-echo image shows marked dilatation of the right distal ureter with abrupt distal narrowing (arrow). There is right pelvicaliectasis, and the right renal parenchyma appears abnormally hyperintense due to edema. B Excretory phase coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image obtained 15 min after contrast injection shows delayed passage of contrast material into the right renal collecting system and a right hyperintense nephrogram (arrows). There is normal contrast material excretion by the left kidney into a partially duplicated left upper urinary tract
A 3-year-old boy with left prenatal and postnatal hydroureteronephrosis. A Excretory phase coronal postcontrast T1-weighted 3D spoiled gradient recalled echo maximum intensity projection image obtained 15 min after contrast injection shows opacification of a dilated left renal collecting system and ureter; there is abrupt narrowing of the very distal left ureter, consistent with congenital primary megaureter. A focal parenchymal defect is present in the left kidney lower pole (arrow). B Axial delayed postcontrast T1-weighted 3D spoiled gradient recalled echo image confirms left kidney lower pole scarring (arrow)
Ureteric ectopia
Ectopic ureteric insertion may be suspected in a few specific clinical situations and is often a diagnostic challenge, with delayed diagnosis common [19, 20]. Much of this challenge reflects the small size of involved structures (decompressed ectopic ureter) and the myriad locations in which ectopia occurs. A common clinical presentation of ureteric ectopia is that of a successfully toilet trained pre-adolescent girl with persistent daytime (diurnal) and nighttime (nocturnal) enuresis [20, 21]. A second less common situation is that of a boy with recurrent genitourinary tract infections (e.g., epididymitis) or chronic pelvic pain [21]. Ectopic ureters most often arise from the upper moiety of a duplex kidney or from a small dysplastic kidney that may be ectopic in location. In the former case, the upper moiety collecting system may be rudimentary with the kidney appearing normal or only subtly abnormal at ultrasound (Fig. 13) [20]. In the latter case, the child may be thought to have a solitary kidney (Fig. 14) [22].
A 7-year-old girl with diurnal and nocturnal enuresis. Ultrasound imaging was normal. A Sagittal single-shot fast spin-echo image though the right kidney shows a very tiny upper pole cystic structure (arrow), proven to be a tiny solitary upper moiety calyx in the setting of a duplex kidney. B Axial delayed phase postcontrast 3D T1-weighted spoiled gradient recalled echo image shows contrast material within an ectopic right ureter (arrow) located between the infrasphincteric urethra and vagina
An 8-year-old girl with right “solitary” kidney and both diurnal and nocturnal enuresis. A Coronal single-shot fast spin-echo image shows a previously unidentified, small, likely dysplastic ectopic left kidney (arrow) located along the spine. The presumed “solitary” right kidney is partially seen. B Axial delayed phase postcontrast 3D T1-weighted spoiled gradient recalled echo image shows contrast material filling the vagina (arrows) due to ectopic insertion of the left ureter
MRU is likely the most sensitive and specific diagnostic test for confirming the presence of an ectopic ureter. As the renal parenchyma associated with ectopic ureters usually has poor function (due to dysplasia or severe long-standing obstruction) and may excrete only minimal contrast material into the urinary tract, T2-weighted imaging is critical to confirming this congenital anomaly [23]. On T2-weighted sequences, the ectopic ureter is usually contains hyperintense fluid and can be followed to its site of insertion. In girls, ectopic insertion sites below the external urethral sphincter are associated with enuresis; possible insertion sites include the distal (infrasphincteric) urethra, introitus, and vagina. Common symptomatic ectopic insertion sites in boys include the posterior urethra and the seminal vesicles (Fig. 15). On delayed postcontrast excretory phase imaging in children with functioning renal moieties associated with the ectopic ureter, contrast material may opacify the ureter and may be apparent within the vagina or on the introitus in girls (Fig. 14).
A 15-year-old boy with chronic pelvic pain due to ectopic ureter with seminal vesicle insertion. A Coronal 3D T2-weighted fast spin-echo maximum intensity projection image shows a duplex right kidney. There are multiple upper moiety cysts suggesting renal dysplasia. The right upper moiety collecting system and ureter are dilated, suggesting obstruction. B Coronal 3D T2-weighted fast spin-echo source image shows the dilated right distal ureter communicating with multiple cystic structures posterior to the urinary bladder (arrows), consistent with dilated seminal vesicles
Conclusion
Ultrasound and diuretic scintigraphy can frequently adequately characterize urinary tract abnormalities in children. There is a subset of patients and diagnoses, however, in which complex anatomy, conflicting imaging findings, and small anatomic structures are inadequately assessed with standard imaging. In these patients, MRU can add substantial diagnostic value as a “one-stop-shop” that allows comprehensive evaluation of the kidneys and urinary tract. MR provides a level of anatomic detail combined with dynamic functional assessment that can be critically important in a variety of congenital and acquired urinary tract abnormalities. Sensible use of MRU, as in the clinical settings described above, has the potential to expedite diagnoses, decrease the number of diagnostic tests performed in certain children, and improve patient outcomes.
References
Queisser-Luft A, Stolz G, Wiesel A, et al. (2002) Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990–1998). Arch Gynecol Obstet 266:163–167
http://www.omim.org/entry/610805. Accessed Nov 2015
Dickerson EC, Dillman JR, Smith EA, et al. (2015) Pediatric MR urography: indications, techniques, and approach to review. Radiographics 35:1208–1230
Grattan-Smith JD, Jones RA (2006) MR urography in children. Pediatr Radiol 36:1119–1132
Khrichenko D, Darge K (2010) Functional analysis in MR urography—made simple. Pediatr Radiol 40:182–199
Adeb M, Darge K, Dillman JR, Carr M, Epelman M (2013) Magnetic resonance urography in evaluation of duplicated renal collecting systems. Magn Reson Imaging Clin N Am 21:717–730
Jones RA, Easley K, Little SB, et al. (2005) Dynamic contrast-enhanced MR urography in the evaluation of pediatric hydronephrosis: part 1, functional assessment. AJR Am J Roentgenol 185:1598–1607
Jones RA, Votaw JR, Salman K, et al. (2011) Magnetic resonance imaging evaluation of renal structure and function related to disease: technical review of image acquisition, postprocessing, and mathematical modeling steps. J Magn Reson Imaging 33:1270–1283
Jones RA, Perez-Brayfield MR, Kirsch AJ, Grattan-Smith JD (2004) Renal transit time with MR urography in children. Radiology 233:41–50
Grattan-Smith JD, Little SB, Jones RA (2008) MR urography in children: how we do it. Pediatr Radiol 38(Suppl 1):S3–S17
Ergen FB, Hussain HK, Carlos RC, et al. (2007) 3D excretory MR urography: improved image quality with intravenous saline and diuretic administration. J Magn Reson Imaging 25:783–789
Delgado J, Bedoya MA, Adeb M, et al. (2015) Optimizing functional MR urography: prime time for a 30-minutes-or-less fMRU. Pediatr Radiol 45:1333–1343
Nguyen HT, Herndon CD, Cooper C, et al. (2010) The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 6:212–231
Grattan-Smith JD, Little SB, Jones RA (2008) MR urography evaluation of obstructive uropathy. Pediatr Radiol 38(Suppl 1):S49–S69
Parikh KR, Hammer MR, Kraft KH, et al. (2015) Pediatric ureteropelvic junction obstruction: can magnetic resonance urography identify crossing vessels? Pediatr Radiol 45:1788–1795
Weiss DA, Kadakia S, Kurzweil R, et al. (2015) Detection of crossing vessels in pediatric ureteropelvic junction obstruction: clinical patterns and imaging findings. J Pediatr Urol 11:173.e1–173.e5
Pfister RC, Hendren WH (1978) Primary megaureter in children and adults. Clinical and pathophysiologic features of 150 ureters. Urology 12:160–176
Farrugia MK, Hitchcock R, Radford A, et al. (2014) British Association of Paediatric Urologists consensus statement on the management of the primary obstructive megaureter. J Pediatr Urol 10:26–33
Heidemeier A, Kirchhoff-Moradpour A, Staatz G, Riedmiller H, Darge K (2007) Ectopic ureter with urinary dribbling in childhood—a diagnostic challenge: our own experience and review of the literature. Radiologe 47:411–420
Carrico C, Lebowitz RL (1998) Incontinence due to an infrasphincteric ectopic ureter: why the delay in diagnosis and what the radiologist can do about it. Pediatr Radiol 28:942–949
Mandell J, Bauer SB, Colodny AH, Lebowitz RL, Retik AB (1981) Ureteral ectopia in infants and children. J Urol 126:219–222
Gharagozloo AM, Lebowitz RL (1995) Detection of a poorly functioning malpositioned kidney with single ectopic ureter in girls with urinary dribbling: imaging evaluation in five patients. AJR Am J Roentgenol 164:957–961
Figueroa VH, Chavhan GB, Oudjhane K, Farhat W (2014) Utility of MR urography in children suspected of having ectopic ureter. Pediatr Radiol 44:956–962
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Dillman, J.R., Trout, A.T. & Smith, E.A. MR urography in children and adolescents: techniques and clinical applications. Abdom Radiol 41, 1007–1019 (2016). https://doi.org/10.1007/s00261-016-0669-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0669-z