Abstract
Purpose
To describe imaging findings in biphenotypic hepatic tumors (BPT) and a proposal for new imaging classification based on contrast-enhanced imaging.
Methods
Retrospective review of CT, MRI, PET/CT, and ultrasound findings in 39 patients with histologically confirmed BPT was performed. Tumor markers including AFP, L3 fraction, CA 19.9, CA 125, and CEA were recorded. Based on the dynamic enhancement features, BPT were categorized into 4 enhancement patterns (Types 1–4). Enhancement patterns were correlated with other imaging findings and tumor markers. Imaging features and tumor markers that were not consistent with diagnosis of hepatocellular carcinoma or intrahepatic cholangiocarcinoma based on enhancement pattern were considered discordant findings.
Results
Enhancement patterns in 29 patients (CT/MR) included 23 Type 2 (continuous peripheral rim of late arterial hyperenhancement with washout or fade in portal venous and/or delayed phases, ±delayed central enhancement) and 2 of each Types 1, 2, and 3. Discordant imaging findings were present in two patients with Type 2 pattern and in one patient with Type 1 pattern. Both AFP and CA 19.9 were elevated in 15 of 33 of patients. Tumor markers AFP and CA 19.9 were discordant in 17 of 21 patients with Type 2 pattern, two of two patients with Type 3 pattern. Most BPTs were markedly PET avid with average SUV max of 8.2. Most frequent ultrasound appearance is peripheral hypoechogenicity and central hyperechogenicity.
Conclusions
BPT most commonly present with imaging features similar to cholangiocarcinoma or metastases. BPT can be suggested when imaging findings or tumor markers are discordant with the most likely diagnosis based on enhancement pattern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The first case of a biphenotypic hepatic tumor (BPT) with combined histologic features of hepatocellular and cholangiocarcinoma was reported in 1903 [1]. Most recent estimates quote BPTs as accounting for roughly 1%–6% of primary liver tumors [2–7]. BPTs have been described in numerous reports representing a spectrum of neoplasia ranging from pathologic variants of hepatocellular carcinoma (HCC) to variants of intrahepatic cholangiocarcinoma (ICC). Related to its spectrum of pathologic findings, BPT has imaging appearances which overlap both HCC and ICC, therefore may be misdiagnosed as HCC or ICC and treated as such. Prior reports estimating radiologist accuracy for identifying BPT have proven the difficulty in identifying these tumors. Nishi et al. and Fowler et al. have both demonstrated poor sensitivity for diagnosing BPT which is frequently mistaken for HCC or ICC [8, 9]. Survival outcomes for BPT are consistently worse than HCC [7, 10]. Outcomes overall appear to be similar to those of ICC but this is controversial as some studies have shown variable outcomes [7, 10–12]. Accurate diagnosis of BPT is critical as misdiagnosis as HCC can lead to poor outcomes when treated by traditional HCC protocols such as liver transplant [13, 14]. In this paper, we retrospectively reviewed imaging and clinical findings in 39 patients with histologically proven BPTs and the findings are presented along with a review of English literature.
Review of the literature
Imaging descriptions of BPT on computed tomography (CT), magnetic resonance imaging (MRI), F-18 FDG positron emission tomography (PET), and ultrasound (US) have been published as case reports or case series over the last 20 years (Table 1) [3, 5, 8, 9, 11, 15–28]. Prior reports are difficult to compare for several important reasons including pathologic definitions of BPT, variable technology, imaging protocols, and variable descriptions of imaging characteristics such as enhancement.
The current pathologic definition of BPT is provided by the World Health Organization (WHO) [29]. The WHO definition states that a BPT must contain unequivocal elements of both hepatocellular and cholangiocarcinoma (Fig. 1), which are intimately admixed. Many prior reports describing BPT imaging characteristics were based on classifications systems proposed by Allen and Lisa in 1945 and Goodman et al. in 1985 [2, 4]. Allen and Goodman subtypes not compatible with the WHO system have been included in multiple prior imaging descriptions of BPT, limiting the usefulness of the described imaging features. More studies are needed utilizing a modern pathologic definition in order to identify clinically useful and predictive imaging characteristics.
Histology of biphenotypic tumor: hepatocellular carcinoma component showing a trabecular arrangement of the tumor cells (arrow A). Cholangiocarcinoma component within the same tumor as above showing a glandular architecture (arrow B). Polyclonal CAE stain showing a canalicular pattern of staining with absence of cytoplasmic staining within the HCC component (arrow C). Polyclonal CAE stain showing cytoplasmic staining within the tumor cells exhibiting a glandular architecture (arrow D). Cross sectional imaging of this tumor is provided in Fig. 5.
Some of the most interesting prior studies were able to obtain gross histologic specimens which were then compared directly to enhancement patterns found at CT and MRI. These studies found that patterns of enhancement and other imaging findings could be correlated directly with the gross histology of the specimen [15, 17, 18, 25, 27]. Areas of tumor that tend to have either a fibrous background or cholangiocarcinomatous predominance have less arterial enhancement and tend to be hyperattenuating on delayed images. Areas with a predominance of hepatocellular differentiation tend to enhance avidly on arterial phase imaging and washout in the delayed phases. Two representative reports include Aoki et al. and Sanada et al. and the main imaging findings are summarized in Table 2 [15, 25]. CT or MRI enhancement patterns mimicking metastases or ICC are the most common findings described in most studies including the recent study composed of 29 patients by Fowler et al. Mixed tumors with histologic predominance of the HCC type are the subtype most likely to demonstrate an imaging pattern like HCC [11, 15, 17, 25, 27]. These studies help explain the difficulty in identification of BPT by revealing the variability in histologic composition and corresponding enhancement patterns.
Limited literature has been published on the PET and US appearance of intrahepatic biphenotypic tumors. Prior descriptions including quantitative assessment of tumor FDG activity suggest that these tumors are often markedly hypermetabolic. A description of a single case from 1999 reported a large, centrally necrotic mass with markedly elevated FDG activity within the soft tissue components with standard uptake value (SUV) ranging from 9 to 11 [30]. A more recent report of three cases described markedly FDG avid masses with SUV max ranging 9.9–13.0 [31]. Prior reports describing the US appearance of BPT have reported variable echogenicity of these lesions.
In summary, previous reports have shown that the predominant histologic component of a mixed tumor is correlated with imaging findings such as pattern of enhancement. The variation of imaging findings makes prospective diagnosis of these tumors difficult. Clinically useful criteria are needed for when a radiologist should include a BPT in the differential diagnosis of an unknown mass. Knowledge of basic clinical management of HCC, ICC, and BPT is needed to understand the clinical implications of including or excluding BPT from the differential diagnosis. The purpose of this study is to describe the imaging findings of BPT. We also correlated clinical and imaging findings with enhancement patterns for identification of features helpful in their diagnosis.
Methods
Subjects
After obtaining institutional review board approval for retrospective analysis, cases were identified by electronically searching our institutional database of pathologic records from January 2000 to December 2013. Combinations of search terms including biphenotypic, combined, mixed, hepatocellular, and cholangiocarcinoma were used to identify potential patients. Patients with BPT pathologically confirmed at our institution and imaging available for review including CT, MRI, PET, or US were included in the study. A total 55 patients had a diagnosis of possible BPT. Seven cases not meeting WHO criteria after retrospective pathologic review (described below) were excluded. Nine cases with no imaging available for review were also excluded. The remaining 39 patients were included in the study.
Demographics and tumor biomarkers
Information recorded from chart review of each patient included age, sex, and serum tumor marker values including alfa fetoprotein (AFP), lens culinaris agglutinin bound fraction of AFP (L3%), carcinoembryonic antigen (CEA), carbohydrate antigen 19.9 (CA 19.9), and carbohydrate antigen 125 (CA 125).
Summary of imaging protocols
Standard MRI protocols performed at our institution included dynamic T1-weighted 3D gradient recalled echo fat suppressed unenhanced and contrast enhanced, axial fat saturated T2 weighted, coronal non-fat saturated T2 weighted, and axial in and opposed-phase series. MRI exams performed at other institutions included similar protocols with the exception of five exams which did not include in and opposed-phase images. Standard liver CT protocols performed at our institution included late arterial and portal with or without delayed-phase images. Available phases of post intravenous contrast enhancement on CT and MRI exams were categorized based on Liver Imaging Reporting and Data System (LIRADS) definitions including non-contrast, early arterial, late arterial, portal venous, and delayed phases [37]. Individual series of each contrast-enhanced MRI and CT exam were inspected for adequacy of bolus timing and excluded if they did not meet LIRADS criteria.
Image review
Imaging studies including US, PET, CT, and MRI were interpreted by two radiologists (abdominal imaging fellow and an attending abdominal radiologist with 10 years’ experience) over a 2-week period, and all findings were recorded by consensus. US, CT, and MRI were reviewed using a GE PACS workstation. The number of lesions in each patient was recorded. When multiple hepatic lesions were present, the mass which was biopsied or resected was chosen and imaging features reviewed. Tumor size was recorded. Enhancement was categorized into 4 types (Table 3) based on previously described patterns. The 4 types are also illustrated in Figs. 2, 3, 4, and 5. Type 1 was typical of an HCC and Type 2 is typical of ICC. Type 3 enhancement pattern is unusual for HCC but could be seen with a variety of masses including ICC, necrotic neoplasms, or infectious processes. Type 4 enhancement pattern suggests a tumor with components of different physiology or possibly two separate but directly adjacent neoplasms (collision tumor). Assignment of an enhancement category required a three-phase exam (late arterial, portal venous, and delayed phase) or an exam with late arterial phase and a delayed phase adequately demonstrating washout, delayed, or persistent enhancement. Additional enhancement characteristics were recorded including the presence of pseudocapsule. Additional imaging findings were evaluated including: the presence of tumoral fat, hemorrhage, necrosis, cystic regions, calcification, vascular invasion, biliary obstruction, and abdominal lymphadenopathy. Specific MRI findings that were recorded were T2 signal intensity, T1 signal intensity, and signal change on in and opposed-phase images.
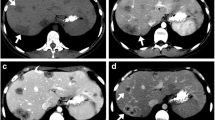
Type 2 enhancement pattern: continuous peripheral late arterial hyperenhancement with washout or fade (arrows B, C) with or without delayed central enhancement (arrowhead C). Pathology specimen (D) from another patient with correlation to the presurgical CT scan (arrow, E). Note the white, fibrous appearance of this infiltrative mass characteristic of ICC.
Type 4 enhancement pattern: focal region of late arterial hyperenhancement (arrows A–C) and separate area of delayed enhancement (arrowheads A–C). Histologic slides from this tumor representing the hepatocellular and cholangiocarcinoma components are provided in Fig. 1.
PET was reviewed using a workstation running OsiriX viewing software. PET characteristics recorded included SUV maximum (max) of lesion, SUV max of normal background liver, and the presence of FDG avid lymphadenopathy. Ultrasound images were reviewed for liver parenchymal echogenicity, lesion echogenicity, and the presence of posterior acoustic characteristics.
Pathologic analysis
All pathologic reports were manually reviewed. Initially the specimens were evaluated by the institution pathologist at the time of diagnosis. A second retrospective review of the cases was performed by an experienced pathologist (8 years post-board certification) with expertise in hepatobiliary pathology. A histological diagnosis of BPT was considered when cells showing features of HCC and ICC were present in the same tumor. Features of HCC included the presence of trabecular or pseudo acinar architecture with neoplastic cells resembling normal liver cells to a variable extent with or without bile production. Features of ICC included glandular features resembling glandular carcinomas or adenocarcinomas. Special stains such as hepatocyte paraffin 1, albumin in situ hybridization, polyclonal CEA, CK7, CK20, CK19, and mucicarmine were performed to confirm hepatocellular and cholangiocarcinoma components. Correlation with relative proportions of HCC and ICC could not be performed as entire resection specimens were not available for review and quantification in the microscopy slides was not possible.
Results
Patients (mean age 58.6 years, 25 males) with pathologically proven BPT formed the final study group (16 patients were excluded). 23 patients had a single hepatic mass. 15 patients with multiple masses had an average of 5.1 lesions (range 2–37); one patient with only ultrasound had numerous lesions reported which could not be adequately recounted. The mean size of the tumor was 7.0 cm (range 2.5–19.5 cm). The histological diagnosis was established with surgical resection in 19 patients and 20 by needle biopsy. 15 of 39 (38%) patients had known chronic liver disease, including 12 (31%) with cirrhosis. The etiologies for chronic liver disease were 9 (23%) hepatitis C, 3 (8%) alcoholic liver disease, and 2 (5%) steatohepatitis.
CT and MRI findings
Thirty patients had CT and 20 had MRI available for review. In total, 35 patients had CT and/or MRI available for assessment. 29 patients had multiphase CT or MRI adequate for determining a tumor enhancement pattern. Type 2 enhancement pattern was the most common pattern and found in 23 patients. Type 1, 3, and 4 enhancement patterns were found in two patients each. Capsular retraction was seen in nine; pseudocapsule, vascular invasion, and biliary obstruction in three patients each (Table 4). Nearly all lesions at MRI were T2 hyperintense (95%) and T1 hypointense (100%). Uncommon findings included lesional fat (3%), necrosis (11%), and cystic change (3%). Lesional calcification was not seen. Serum AFP and CA 19.9 were elevated in 76% and 59% of patients, respectively. Both AFP and CA 19.9 were elevated in only 45% (15/33) of patients. At least one tumor marker was elevated in 80% (33/39) patients. Correlation of enhancement pattern with serum markers are shown in Table 5. Nine of twelve patients with cirrhosis had adequate imaging to determine an enhancement pattern. There was no correlation with cirrhosis and enhancement pattern (6 Type 2, 1 of each Type 1, 3, and 4).
PET findings
Twelve patients had PET available for review. Ten patients had exams including original DICOM data available for calculation of SUV max values. The average SUV max for these patients was 8.2 (range 4.3–15.8). When SUV was normalized to background liver the average SUV ratio was 2.8 (range 1.0–7.5). In two patients, SUV value was similar to background liver with SUV ration of 1.0. Two patients had exams including only secondary capture images which allow generalized statements regarding FDG activity based on included color scales without the benefit of quantitative analysis. In these two patients, the max SUV values were clearly over 5 and 10, respectively.
Ultrasound findings
Thirteen patients had ultrasound images available for review. Background liver echogenicity was coarsened in 5 and there was good correlation with the presence of known cirrhosis or chronic liver disease. Masses were peripherally hypoechoic with central hyperechogenicity in 6, heterogenously hypoechoic in 4, homogenously hypoechoic in 1, and homogenously hyperechoic in 1. 1 mass was a large heterogenous mass with overall increased echogenicity.
Discussion
BPT are difficult tumors to prospectively diagnose because of the variety of imaging patterns displayed. By assigning an enhancement pattern, we could identify imaging findings which are discordant with the most likely diagnosis based on enhancement. For example, an enhancement pattern suggesting HCC with additional imaging findings characteristic of ICC, would therefore raise suspicion of BPT which should then be included in the differential diagnosis. We also found that the majority of the tumors with PET imaging demonstrated marked hypermetabolism. This finding could also be helpful for initial clinical evaluation as well as staging and evaluation of therapy. US was not found to be a useful modality for characterization.
The majority of patients in our study demonstrated a Type 2 enhancement pattern. The imaging pattern alone is suggestive of a hepatic metastasis or mass forming ICC. Within this group, two of the patients demonstrated gross venous vascular invasion which is more often associated with HCC [38, 39]. Two patients had tumors demonstrating a Type 1 imaging pattern, which is suggestive of HCC. One of these patients demonstrated biliary obstruction out of proportion to the tumor size, a feature typically associated with cholangiocarcinoma. These discordant imaging findings could have raised suspicion for an atypical neoplasm and prompted the inclusion of BPT in the differential diagnosis.
The Type 4 enhancement pattern is suspicious for BPT as the two different patterns of enhancement within the same tumor suggests components with different behavior. The pattern could also be seen with two separate tumors with different enhancement patterns which have grown into one another (collision tumor). One of the two patients with Type 4 imaging pattern had imaging findings of both gross vascular invasion and delayed enhancing pseudo capsule, suggestive of HCC. The other patient had imaging findings of both biliary obstruction and capsular retraction suggestive of ICC.
Discordance between the most likely diagnosis based on imaging findings and tumor marker profile should also raise suspicion of a BPT. Tumor markers in general have poor sensitivity and specificity but are commonly used and often clinically helpful when elevated [40, 41]. Serum markers including AFP, L3%, and des-gamma carboxyprothrombin are all suggestive of HCC, while markers such as CEA, CA 19.9, or CA 125 are associated with ICC [37, 41, 42]. Tumor markers representative of the individual HCC and ICC components can all be elevated in a biphenotypic tumor. Elevation of tumor markers suggesting both an HCC and ICC component should raise suspicion of a biphenotypic tumor. 15 of 33 (45%) patients in this study had elevation of both tumor markers. Of the patients with Type 2 enhancement pattern, 17 of 21 (81%) had elevation of AFP which could be interpreted as a discordant finding, and 10 of 19 (53%) had elevation of both AFP and CA 19.9. Two of two patients with Type 3 enhancement pattern both had elevation of AFP. Correlating these raised levels of serum tumor markers could have raised suspicion for BPT.
In our study, we found that enhancement pattern in combination with discordant imaging characteristics or discordant serum markers is perhaps the best way to suggest possibility of BPT. Similar to two recent reports in a US-based population by De Campos et al. and Fowler et al., we found the most common imaging pattern to be peripherally hyperenhancing in the arterial phase with slow central enhancement. Unlike De Campos, we did not observe a correlation between the presence of cirrhosis and the observed enhancement pattern. This could be related to the lower frequency of cirrhosis found in our series (33%) which is lower than that reported by De Campos (45%) and similar to other reports in the US-based population [8, 21]. Using the reported imaging features, two of the twelve patients reported by De Campos had a Type 2 enhancement pattern with venous invasion which we would consider a suspicious discordant finding, although this was not specifically suggested in that publication. Two reviewers attempting to prospectively diagnose BPT in a study by Fowler et al., reported vascular invasion in 13 of 29 and 15 of 29 patients. This is substantially higher than the number of cases we identified (3 of 32). In that study the criteria used by reviewers to prospectively identify BPT was not specifically illustrated. Our proposal of correlating imaging features and tumor markers with enhancement patterns may be a useful template for future prospective studies.
The FDG activity in the majority of tumors in this series was markedly elevated. The average SUV value of 8.2 is similar to the values reported by Ijichi et al. [31]. One unique case in our series demonstrated a Type 1 enhancement pattern and regions of both hemorrhage and fatty deposition. This constellation of imaging findings is suggestive of HCC. Notably, the regions of fatty infiltration were markedly FDG avid (Fig. 6). Fatty infiltration is typically a sign of well-differentiated histology in HCC which typically has FDG activity close to background liver. The marked FDG activity in this case could be viewed as a discordant finding and a diagnostically useful indicator of an atypical tumor.
PET has historically assumed a limited role in the diagnosis and management of both intrahepatic HCC and ICC. Low rates of detection are related to variable lesion tracer activity and relatively high background liver activity [43, 44]. The rate of detection of HCC overall is reported to be 50%–65% [44]. The presence or absence of FDG activity in HCC conveys clinically useful information. Increased level of FDG activity in HCC has been correlated with poorly differentiated histology, shorter doubling time, and worse patient outcomes [43–45]. Reported rates of ICC detection at PET are dependent on growth pattern. Generally, FDG PET is limited in detection of tumors with fibrous deposition, mucin production, or infiltrative growth patterns which decrease the spatial density of metabolically active tumor cells. PET detection of ICC is accordingly strongly influenced by mass forming vs. infiltrative growth patterns.
The marked elevation in FDG activity of BPT could be related to poor differentiation of the HCC and ICC subtypes or may be related to the suspected origin of these tumors from a primitive precursor cell line [46]. The elevated level of activity may also have clinical significance as it could be an indicator of the known clinical aggressiveness of these neoplasms. PET was also able to identify lymphadenopathy in four out of the eleven patients, including one patient in which the primary hepatic lesion had activity similar to background liver (Fig. 7). PET has shown extremely good results in detecting extrahepatic metastases from both HCC and ICC [44, 47]. Despite the limited data available, similar results would be expected in the FDG avid BPT tumor population. The elevated FDG activity in BPT could have important implications for diagnosis, staging, and possibly follow-up of these tumors after treatment. As combined PET–MR becomes implemented more widely in clinical practice, it may have an increasing role in characterization of these tumors.
Correlation between CT and PET with lymphadenopathy. Contrast enhanced CT demonstrating a right hepatic mass with a Type 2 enhancement pattern (arrow A). The primary lesion is iso-intense to background liver on PET (arrow B), while a large necrotic metastatic portahepatis lymph node is FDG avid (arrows C, D). Both sites were surgically biopsied confirming BPT.
Ultrasound appearances in thirteen patients were heterogenous. The most common appearance of BPT on US resembled a metastasis with a typical “target” lesion appearance (Fig. 8). While ultrasound may be useful for detection and biopsy of BPT, it is probably not useful for characterization. These findings are concordant with prior ultrasound descriptions [16, 23].
Clinically it may be much more important to differentiate a BPT from HCC than from ICC. BPTs tend to present at more advanced stage with venous invasion, lymph node, and distant metastases with a frequency much higher than HCC and more like ICC. Not only has it been found that these tumors recur after treatment in a pattern similar to ICC but also it has been reported that the metastases can contain the ICC elements [48]. A single case in our series did have biopsy proven metastasis containing only the cholangiocarcinoma component of the tumor. The presence of the ICC component in a BPT appears to be the significant factor for patient outcome and therefore for its presence should influence management and treatment decisions.
Staging and treatment guidelines for HCC differ markedly from those of ICC. The management of HCC has been well outlined by the AASLD guidelines, including the BCLC staging system [40, 49]. The guidelines used for management of HCC are likely not applicable in BPT due to the behavior of the ICC component. The principle treatment approach for BPT is attempted curative resection [10]. Reports suggest that local therapies (RFA, TACE) may also be useful [10]. TACE in particular may have benefit although suspicion has been raised that it might not be as effective in less vascular tumors [7, 10, 50, 33].
Curative transplant is not currently recommended for BPT due to poor 5-year outcomes, although there may be a future role of transplant when combined with neoadjuvant therapy [13]. One report including 10 unsuspected BPT and ICC tumors detected after transplant for suspected HCC found postoperative rates of recurrence and survival much worse than the reference HCC population and comparable to transplant for ICC [14]. In retrospect all eight patients with tumors >1 cm demonstrated early enhancement with progressive-delayed enhancement and lack of washout characteristics on their CT exams, a finding which may have made HCC a less likely imaging diagnosis depending on the associated findings. In our series five patients had received liver transplant for suspected HCC. In retrospective review, three of these patients had a Type 2 imaging pattern, one patient had a Type 1 imaging pattern, and one did not have contrast-enhanced imaging available for review. Limited follow-up is available on these five patients; two patients died 14 and 33 months after transplant due to recurrent metastatic disease, three patients did not have evidence of recurrence at 11, 24, and 38 months of follow-up. These findings highlight the need for accurate diagnosis prior to treatment planning.
Accurate diagnosis prior to treatment such as planned curative liver transplant is essential for patients with BPT. The clinical and radiologic suspicion for BPT needs to be balanced with the risk of pretreatment percutaneous biopsy. These risks include tumor seeding along the biopsy tract which is uncommon but does occur and can result in a patient becoming unresectable. The rates of tumor seeding for HCC biopsy are controversial. Most reports cite the rate of biopsy tract seeding between 0% and 3% and biopsy may also result in increased risk of extrahepatic recurrence after transplant [51, 52]. The decision to biopsy a potential transplant candidate is best made in a multidisciplinary fashion involving surgeons, oncologists, and hepatologists taking into account the differences in treatments based on potential results of the biopsy.
Our study has some limitations. First, the study group is small although it is probably the largest series based on our literature search. We excluded several patients who did not meet our strict inclusion criteria. Second, there were small numbers of patients in Type 1, 3, and 4. Our institution has a small local population; most cases are referred from outside institutions often due to patient self-referral, difficulty with making a diagnosis, or situations requiring complex management. It is important to highlight that only 2 of 28 patients had a Type 1 enhancement pattern suggestive of HCC. This is in contrast to some older reports (Aoki 4 of 14, Lin 16 of 30) [11, 15]. This discrepancy does raise the possibility that BPT tumors with Type 1 enhancement patterns are being given an imaging diagnosis of HCC and treated as such, while tumors imaging like ICC with worse prognosis and relatively less well-defined options for management are referred to specialized institute like ours. Third, the scanning techniques were not uniform which may introduce overlapping contrast-enhancement patterns. This was inevitable as this is a retrospective study and some studies were performed when advanced MRI techniques were not available. Fourth, histological specimens were limited. However, an experienced pathologist performed a second review confirming the diagnosis of BPT. We could not perform correlation of enhancement pattern to the amount or proportion of the HCC and ICC component which may have further supported our classification.
Conclusions
BPT are uncommon tumors whose frequency may be under reported due to missed diagnosis on imaging and inadequate sampling at biopsy or gross pathology. Prospective imaging diagnosis is difficult but inclusion in the differential diagnosis can be facilitated by categorizing enhancement patterns and comparing with other imaging features and tumor markers. FDG PET is markedly avid in the majority of these tumors suggesting a role for diagnosis, staging and follow-up after therapy. Ultrasound findings are not specific. Given the frequently similar imaging appearances of BPTs and ICC and the clear differences in management of HCC, exclusion of a monophenotypic HCC is a reasonable goal of imaging. Raising suspicion of a non-hepatocellular component should prompt evaluation with tumor markers or biopsy and could subsequently change decisions regarding staging and treatment.
References
Wells ML (1903) Primary carcinoma of the liver. Am J Med Sci 126(3):403–417
Allen RA, Lisa JR (1949) Combined liver cell and bile duct carcinoma. Am J Pathol 25(4):647–655
de Campos ROP, Semelka RC, Azevedo RM, et al. (2012) Combined hepatocellular carcinoma-cholangiocarcinoma: report of MR appearance in eleven patients. J Magn Reson Imaging 36(5):1139–1147
Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L (1985) Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 55(1):124–135
Hwang J, Kim YK, Park MJ, et al. (2012) Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging 36(4):881–889
Jarnagin WR, Wever S, Tickoo SK, et al. (2002) Combined hepatocellular and cholangiocarcinoma. Cancer 94(7):2040–2046
Lee JH, Chung GE, Yu SJ, et al. (2011) Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol 45(1):69–75
Fowler KJ, Sheybani A, Parker RA, et al. (2013) Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. Am J Roentgenol 201(2):332–339
Nishie A, Yoshimitsu K, Asayama Y, et al. (2005) Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. AJR Am J Roentgenol 184(4):1157–1162
Yin X, Zhang B-H, Qju S-J, et al. (2012) Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol 19(9):2869–2876
Lin G, Toh CH, Wu RC, et al. (2007) Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract 62(8):1199–1205
Zuo HQ, Yan LN, Zeng Y, et al. (2007) Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 6(2):161–165
Razumilava N, Gores GJ (2014) Liver transplantation for intrahepatic cholangiocarcinoma—authors’ reply. Lancet 384(9949):1182–1183
Sapisochin G, Fidelman N, Roberts JP, Yao FY (2011) Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 17(8):934–942
Aoki K, Takayasu K, Kawano T, et al. (1993) Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology 18(5):1090–1095
Choi BI, Han JK, Kim YI, et al. (1994) Combined hepatocellular and cholangiocarcinoma of the liver: sonography, CT, angiography, and iodized-oil CT with pathologic correlation. Abdom Imaging 19(1):43–46
Fukukura Y, Taguchi J, Nakashima O, Wada Y, Kojiro M (1997) Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr 21(1):52–58
Hashimoto T, Nakamura H, Hori S, et al. (1994) MR imaging of mixed hepatocellular and cholangiocellular carcinoma. Abdom Imaging 19(5):430–432
Bhagat V, Javle M, Yu J, et al. (2006) Combined hepatocholangiocarcinoma: case-series and review of literature. Int J Gastrointest Cancer 37(1):27–34
Danet IM, Semelka RC, Leonardou P, et al. (2003) Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol 181(3):809–817
Ebied O, Federle MP, Blachar A, et al. (2003) Hepatocellular-cholangiocarcinoma: helical computed tomography findings in 30 patients. J Comput Assist Tomogr 27(2):117–124
Jeon TY, Kim SH, Lee WJ, Lim HK (2009) The value of gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging for the differentiation of scirrhous hepatocellular carcinoma and cholangiocarcinoma with or without hepatocellular carcinoma. Abdom Imaging 35(3):337–345
Panjala C, Senecal DL, Bridges MD, et al. (2010) The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant 10(5):1263–1267
Saboo SS, Krajewski KM, Jagannathan JP, et al. (2011) Rapid progression of combined hepatocellular carcinoma and cholangiocarcinoma. Cancer Imaging 11(1):37–41
Sanada Y, Shiozaki S, Aoki H, et al. (2005) A clinical study of 11 cases of combined hepatocellular–cholangiocarcinoma. Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res 32(3):185–195
Shetty AS, Fowler KJ, Brunt EM, et al. (2014) Combined hepatocellular-cholangiocarcinoma: what the radiologist needs to know about biphenotypic liver carcinoma. Abdom Imaging 39(2):310–322
Shin CI, Lee JM, Kim SH, et al. (2007) Recurrence patterns of combined hepatocellular-cholangiocarcinoma on enhanced computed tomography. J Comput Assist Tomogr 31(1):109–115
Toh CH, Cheung YC, Ng SH, et al. (2004) Combined hepatocellular-cholangiocarcinoma: a case report. Int J Clin Pract 58(12):1170–1173
Theise ND, Nakashima O, Park Y, Nakanuma Y (2010) Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system, 4th edn. Lyon: IARC Press, pp 225–227
Shiomi S, Sasaki N, Kawashima D, et al. (1999) Combined hepatocellular carcinoma and cholangiocarcinoma with high F-18 fluorodeoxyglucose positron emission tomographic uptake. Clin Nucl Med 24(5):370–371
Ijichi H, Shirabe K, Taketomi A, et al. (2013) Clinical usefulness of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for patients with primary liver cancer with special reference to rare histological types, hepatocellular carcinoma with sarcomatous change and combined hepatocellular and cholangiocarcinoma. Hepatol Res 43(5):481–487
Murakami T, Kim T, Tomoda K, et al. (1997) Combined hepatocellular and cholangiocellular carcinoma. Radiat Med 15(4):243–246
Nagaoka S, Itano K, Ishibashi M, et al. (2006) Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int 26(7):781–788
Phongkitkarun S, Srisuwan T, Sornmayura P, Jatchavala J (2007) Combined hepatocellular and cholangiocarcinoma: CT findings with emphasis on multiphasic helical CT. J Med Assoc Thai 90(1):113–120
Schiettecatte A, Dujardin M, Beeck BOd, de Mey J (2008) Combined hepatocellular-cholangiocarcinoma: MR findings. Eur J Radiol Extra 67:75–77
Willekens I, Hoorens A, Geers C, et al. (2009) Combined hepatocellular and cholangiocellular carcinoma presenting with radiological characteristics of focal nodular hyperplasia. World J Gastroenterol 15(31):3940–3943
American College of Radiology (2013) Liver Imaging Reporting and Data System in version 2013.1. Reston: American College of Radiology
Chung YE, Kim MJ, Park YN, et al. (2009) Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 29(3):683–700
Hussain SM, Semelka RC, Mitchell DG (2002) MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am 10(1):31–52, v
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Page AJ, Cosgrove DC, Philosophe B, Pawlik TM (2014) Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am 23(2):289–311
Khan SA, Davidson BR, Goldin RD, et al. (2012) Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 61(12):1657–1669
Cheung TT, Ho CL, Lo CM, et al. (2013) 11C-Acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of milan criteria: surgeon’s perspective. J Nucl Med 54(2):192–200
Sacks A, Peller PJ, Surasi DS, et al. (2011) Value of PET/CT in the management of primary hepatobiliary tumors, Part 2. Am J Roentgenol 197(2):W260–W265
Lam MGEH, Kwee TC, Basu S, Alavi A (2013) Underestimated role of 18F-FDG PET for HCC evaluation and promise of 18F-FDG PET/MR imaging in this setting. J Nucl Med 54(8):1510–1511
Yeh MM (2010) Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol 25(9):1485–1492
Ayuso J-R, Pagés M, Darnell A (2013) Imaging bile duct tumors: staging. Abdom Imaging 38(5):1071–1081
Lee CH, Hsieh SY, Chang CJ, Lin YJ (2013) Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol 28(1):122–127
Forner A, Rodríguez-Lopez C, Reig M (2012) Natural history and staging for hepatocellular carcinoma. Clin Liver Dis 1(6):183–185
Kassahun WT, Hauss J (2008) Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract 62(8):1271–1278
Brown DB, Gonsalves CF (2008) Percutaneous biopsy before interventional oncologic therapy: current status. J Vasc Interv Radiol 19(7):973–979
Robertson EG, Baxter G (2011) Tumour seeding following percutaneous needle biopsy: the real story!. Clin Radiol 66(11):1007–1014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wells, M.L., Venkatesh, S.K., Chandan, V.S. et al. Biphenotypic hepatic tumors: imaging findings and review of literature. Abdom Imaging 40, 2293–2305 (2015). https://doi.org/10.1007/s00261-015-0433-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0433-9