Abstract
Objectives
To explain the new changes in pathologic diagnoses of biphenotypic primary liver cancer (PLC) according to the updated 2019 World Health Organization (WHO) classification and how it impacts Liver Imaging Reporting and Data System (LI-RADS) classification using gadoxetic acid–enhanced MRI (Gd-EOB-MRI).
Methods
We retrospectively included 209 patients with pathologically proven biphenotypic PLCs according to the 2010 WHO classification who had undergone preoperative Gd-EOB-MRI between January 2009 and December 2018. Imaging analysis including LI-RADS classification and pathologic review including the proportion of tumor components were performed. Frequencies of each diagnosis and subtype according to the 2010 and 2019 WHO classifications were compared, and changes in LI-RADS classification were evaluated. Univariable and multivariable analysis were performed to determine significant tumor component for LI-RADS classification.
Results
Of the 209 biphenotypic PLCs of the 2010 WHO classification, 177 (84.7%) were diagnosed as bipheonotypic PLCs, 25 (12.0%) as hepatocellular carcinomas (HCCs), and 7 (3.3%) as cholangiocarcinomas (CCAs) using the 2019 WHO classification. Of the 177 biphenotypic PLCs, LR-M, LR-4, and LR-5 were assigned in 77 (43.5%), 21 (11.9%), and 63 (35.5%), respectively. There were no significant differences in the proportion of LR-5 and LR-M categories between the WHO 2010 and 2019 classifications (p = 0.941). Proportion of HCC component was the only independent factor for LI-RADS classification (adjusted odds ratio, 1.02; p < 0.001).
Conclusion
According to the 2019 WHO classification, 15% of biphenotypic PLCs from the 2010 WHO classification were re-diagnosed as HCCs or CCAs, and a substantial proportion of biphenotypic PLCs of the 2019 WHO classification could be categorized as LR-4 or LR-5 on Gd-EOB-MRI.
Key Points
• Among 209 diagnosed biphenotypic PLCs according to the 2010 WHO classification, 177 (84.7%) lesions were reclassified as bipheonotypic PLCs, 25 (12.0%) as HCCs, and 7 (3.3%) as CCAs using the 2019 WHO classification.
• Of the 177 biphenotypic PLCs at the 2019 WHO classification, LR-M, LR-4, and LR-5 were assigned in 77 (43.5%), 21 (11.9%), and 63 (35.5%), respectively.
• LI-RADS classification relied on the proportion of HCC component (adjusted odds ratio,1.02; p < 0.001).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biphenotypic primary liver cancer (PLC), also referred to as combined hepatocellular cholangiocarcinoma (cHCC-CCA), is an uncommon PLC containing mixed elements of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) [1, 2]. Since its first description in 1903 [3], the diagnostic criteria for biphenotypic PLC have continued to evolve. Adopting the concept of hepatic stem/progenitor cells (HPC) as the potential origin of biphenotypic PLCs [1, 4, 5], the 2010 World Health Organization (WHO) classification categorized biphenotypic PLCs into the classical type and subtypes with stem cell features [6]. However, the 2010 WHO classification has more recently been challenged particularly in regards to subtypes with stem cell features owing to controversy on the role of HPC features in biphenotypic PLCs and ambiguity and difficulty in subtype classification [7, 8]. To address these criticisms [2, 9], the 2019 WHO classification [10] revised the definition of biphenotypic PLCs by excluding typical HCCs or CCAs expressing immunohistochemical stem cell markers and cholangiolocarcinomas (CLC) without an HCC component. It also abandoned subtype classifications and changed the diagnostic terminology of biphenotypic PLCs by including a list of all tumor components [9].
Biphenotypic PLCs predominantly occur in patients with liver cirrhosis or chronic viral hepatitis [11,12,13]. The imaging findings of biphenotypic PLCs have been variably reported, but the prevalence of HCC-like imaging features is known to be as high as 70% [13,14,15]. This large overlap in risk factors and clinical and imaging features of biphenotypic PLCs and HCCs has challenged the non-invasive imaging diagnosis of HCC in at-risk patients. Yet, given that surgical resection is the current treatment standard for biphenotypic PLC, imaging misdiagnosis of biphenotypic PLC as HCC can induce non-standard treatment for biphenotypic PLC, such as liver transplantation or systemic chemotherapy including Sorafenib [16]. Moreover, pre-treatment liver biopsy does not solve this problem, as only a small sample can be obtained from a portion of tumor which consists of heterogeneous tumor component. Therefore, given the different treatment options and prognoses for biphenotypic PLCs as opposed to HCCs and limitation of biopsy, accurate imaging differentiation between these two entities is of critical importance [17, 18].
Liver Imaging Reporting and Data System (LI-RADS) was developed in 2011 to standardize the imaging diagnosis of HCCs in at-risk patients, and was recently updated in 2018 [19]. Although LI-RADS category-5 is considered to be highly specific for the diagnosis of HCC, prior studies have demonstrated that biphenotypic PLC is one of the main causes for false positives [20, 21]. We thus postulated that the recent revision in the pathologic diagnosis of biphenotypic PLC in the 2019 WHO classification may result in significant changes in the LI-RADS categorization of biphenotypic PLCs compared with previous classification systems. Given the widespread use of LI-RADS in clinical practice, awareness of the changes in LI-RADS classification results of biphenotypic PLCs would have meaningful clinical implications for LI-RADS classification.
Therefore, we aimed to investigate the changes in pathologic tumor diagnoses according to the 2019 WHO classification compared to the 2010 WHO classification, as well as the corresponding LI-RADS classification with gadoxetic acid–enhanced MRI (Gd-EOB-MRI), in patients with pathologically proven biphenotypic PLCs.
Materials and methods
This retrospective study was approved by the Institutional Review Board of two tertiary university hospitals, and the requirement for written informed consent was waived.
Study population
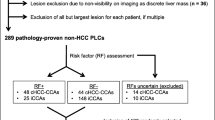
Using a computerized search of pathology database, our study included consecutive patients with treatment-naïve biphenotypic PLCs (PLCs with mixed hepatobiliary differentiation) who underwent surgical resection between January 2009 and December 2018 at two tertiary hospitals (Seoul National University Hospital (SNUH), Asan Medical Center (AMC)). Inclusion criteria were (a) pathologically proven biphenotypic PLCs according to the 2010 World Health Organization (WHO) classification [6], (b) patients at high risk of HCC according to the 2018 practice guidance of the American Association for the Study of Liver Disease (AASLD) [22], (c) gadoxetic acid–enhanced MRI performed within 2 months prior to liver surgery, (d) no preoperative treatment for biphenotypic PLCs, and (e) available surgical specimen for pathologic review. We opted to include only surgically confirmed tumors so as to avoid the potential risk of biopsy sampling errors. For patients with multiple biphenotypic PLCs, the largest mass was selected as the index lesion for further analysis. A flow diagram for our study population is presented in Fig. 1. Ninety-nine of the 209 patients have been previously reported [13, 20, 23]. The prior articles evaluated biphenotypic PLCs diagnosed only by the 2010 WHO classification, whereas this study reevaluated the pathologic and radiologic findings of the tumors with emphasis on the changes in pathologic diagnoses and LI-RADS classification results based on the 2010 WHO and 2019 WHO classifications.
Histopathological analysis
Histopathologic specimens were reviewed by one of two experienced liver pathologists (H.K. and Y.P.). Each pathologist from two hospitals reviewed histopathologic specimens of each hospital, and the histopathologic diagnoses of hepatic nodules were determined according to the 2010 WHO classification [6] as well as the recently updated 2019 WHO classification [10] at the same review session, based on morphologic features on hematoxylin-eosin-stained slices with the assistance of immunohistochemistry analysis, such as hepatocyte paraffin 1, alpha-fetoprotein, polyclonal carcinoembryonic antigen, cytokeratin 7, cytokeratin 19, CD 56, and CD 117. In addition, tumor components comprising each hepatic nodule and the proportion of each tumor component were recorded, including HCC, CCA, intermediate cell carcinoma (IC), and CLC. For the 2010 WHO classification, biphenotypic PLCs were subclassified into the classical type or subtypes with stem cell features (typical, intermediate, cholangiolocellular subtypes) based on their predominant histologic feature. When tumor components with stem cell features did not meet any of the subtypes mentioned above, the tumor was considered as “unclassified.” Details regarding the definitions and nomenclature of biphenotypic PLCs in the 2019 WHO classification have been presented in previous publications [2, 9]. In brief, in the 2019 WHO classification, subtype classification is no longer recommended. Instead, the diagnostic terminology of biphenotypic PLCs includes a list of all of the combined tumor components (e.g., cHCC-CCA, CCA-IC, HCC-CLC, IC-CLC). In addition, morphologically typical HCCs or CCAs with only immunohistochemical expression of stem cell markers are not considered as a biphenotypic PLC, but rather as HCCs or CCAs. Furthermore, a CLC not combined with HCC is to be diagnosed as a CCA, not a biphenotypic PLC. In this study, we divided biphenotypic PLCs diagnosed by the 2019 WHO classification into cHCC-CCA which consists of only HCC and CCA components and other types which include tumors with various combinations of IC, CLC, HCC, and/or CCA components.
Liver MRI
Gadoxetic acid–enhanced liver MRI was performed with various 1.5T or 3.0T scanners. Detailed imaging protocols are summarized in Supplementay A.
MR image analysis
For the MR image review, a study coordinator at each hospital (S.H.C. at AMC and S.K.J. at SNUH) annotated each tumor on the best visible sequence with reference to pathologic tumor location. MR images were reviewed by four board-certified abdominal radiologists (S.S.L, S.H.C., B.Y.H, and H.K., with over 8 years of experience in liver imaging in all reviewers) who were blinded to the results of pathologic examinations. After two radiologists of each hospital-reviewed image findings in consensus, cross-validation was performed between two hospitals. Any discrepancies between readers were resolved by a fifth radiologist (J.M.L., with 25 years of experience in liver imaging).
The reviewers evaluated the size of the observation, the presence or absence of major features, ancillary features, and imaging features favoring non-HCC malignancies according to LI-RADS v2018 [24]. Detailed imaging features are summarized in Supplementary B. Thereafter, the reviewers assigned a LI-RADS category for each hepatic observation: LR-TIV (tumor in vein), LR-M (definitely or probably malignant, not HCC-specific), and LR 1-5 (1, definitely benign; 2, probably benign; 3, indeterminate probability of HCC; 4, probably HCC; 5, definitely HCC). As this study evaluated only a single MR examination, threshold growth was not used to assign a LI-RADS category.
Statistical analysis
Continuous variables were reported as mean values with SD or median values with interquartile range (IQR). Categorical variables were presented as number and percentages. Differences in the proportions of LI-RADS categories between the biphenotypic PLCs diagnosed by the 2010 WHO classification and the 2019 WHO classification were compared using the chi-squared test. Frequencies of ancillary featyres were compared among biphenotypic PLC, HCC, and CCA by the 2019 WHO classification using the chi-squared test or Fisher’s exact test, as appropriate. Inter-reader agreement for LI-RADS categorization of biphenotypic PLC of 2010 and 2019 WHO classification were analyzed using κ statistics and were interpreted as follows: poor, less than 0.20; fair, 0.20–0.39; moderate, 0.40–0.59; substantial, 0.60–0.79; and almost perfect, 0.80 or greater. For biphenotypic PLCs diagnosed according to the 2019 WHO classification, pathologic tumor components (HCC, CCA, IC, and CLC) associated with radiologic LI-RADS categorization (LR-4 and LR-5 vs. LR-M) were evaluated. Differences in the amount of tumor components between LR-4 and LR-5 vs. LR-M were compared using the independent t-test at univariable analysis. Thereafter, multivariable logistic regression analysis was conducted to determine independent pathologic factors associated with LR-4 or LR-5 categories using the significant variables from univariable analysis. All statistical analysis was performed using SPSS, version 25.0 (IBM), and a p value of less than 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Clinical characteristics of the 209 study patients (162 men [mean age ± standard deviation, 54.0 years ± 9.7; range 30–78 years] and 47 women [mean age ± standard deviation, 55.5 years ± 10.3; range 35–80 years]) are summarized in Table 1. Hepatitis B was the most common cause of chronic liver disease. Mean diameter of tumors was 41.4 ± 27.3 mm (range, 10–170).
Pathologic diagnoses of biphenotypic PLCs in the updated 2019 WHO classification
Pathologic tumor diagnoses according to the 2010 WHO classification and the 2019 WHO classification are summarized in Table 2. According to the 2010 WHO classification, a total of 209 biphenotypic PLCs were classified as the classical type (n = 114) or subtypes with stem cell features (n = 95) including 42 typical, 24 intermediate, 25 cholangiolocellular, and four unclassified subtypes. Of the 209 biphenotypic PLCs diagnosed by the 2010 WHO classification, 32 (15.3%) tumors, 25 typical subtype and 7 cholangiolocellular subtype, were no longer considered as biphenotypic PLCs according to the 2019 WHO classification and were reclassified as HCCs (n = 25) and CCAs (n = 7). The remaining 177 lesions were diagnosed as biphenotypic PLCs according to the 2019 WHO classification, including 114 (64.4%) cHCC-CCAs and 63 (35.6%) tumors of other types. According to the diagnostic terminology defined by the 2019 WHO classification, nine different types other than cHCC-CCAs were noted, including cHCC-CCA-IC (n = 11), cHCC-CCA-CLC (n = 5), cHCC-CCA-IC-CLC (n = 3), cHCC-IC (n = 13), cHCC-CLC (n = 19), cHCC-IC-CLC (n = 7), cIC-CLC (n = 3), cIC-CCA (n = 1), and cIC-CCA-CLC (n = 1).
LI-RADS classification of biphenotypic PLCs with the updated 2019 WHO classification
Table 3 summarizes the LI-RADS categories of the 209 and 177 tumors which were diagnosed as biphenotypic PLCs according to the 2010 and 2019 WHO classifications, respectively. Among the 209 biphenotypic PLCs diagnosed by the 2010 WHO classification, there were 92 LR-Ms (44.0%), 21 LR-4s (10.0%), 78 LR-5s (37.3%), and 18 LR-TIVs (8.6%). Among the 177 biphenotypic PLCs diagnosed according to the 2019 WHO classification, there were 77 LR-Ms (43.5%), 21 LR-4s (11.9%), 63 LR-5s (35.5%), and 16 LR-TIVs (9.0%). There were no significant differences between the 2010 and 2019 WHO classifications in the proportions of LI-RADS categories of biphenotypic PLCs (p = 0.941). As for the 32 tumor diagnoses which were revised as HCC or CCA according to the 2019 WHO classification, 25 HCCs were categorized as LR-5, LR-M, and LR-TIV in 15 (60.0%), 8 (32.0%), and 2 (8.0%), respectively, and all seven CCAs were categorized as LR-M (Fig. 2). In the 177 biphenotypic PLCs according to the 2019 WHO classification, overall proportions of LI-RADS categories were not significantly different between cHCC-CCAs and other types of biphenotypic PLC (p = 0.224), although the frequency of LR-TIV was higher in cHCC-CCAs than in other types of biphenotypic PLC (12.3% [14/114] vs. 3.2% [2/63]). LI-RADS categories of the 177 biphenotypic PLCs according to each diagnostic terminology defined by the 2019 WHO classification are presented in the Supplementary Table 1. Among ancillary features, fat in mass were more frequent in HCCs than in non-HCC malignancies including biphenotypic PLC or CCA (p = 0.029), whereas other ancillary features did not show significant differences (Supplementary Table 3).
MR images of an HCC assigned as LR-5 in a 63-year-old female with chronic hepatitis B, which was previously classified as a biphenotypic PLC with stem cell features, typical subtype according to the 2010 WHO classification. A 2.4-cm observation can be seen in segment VI of the liver showing moderate hyperintensity on T2-weighted imaging (a), and non-rim arterial hyperenhancement (b) and washout appearance on the portal venous phase (c) and transitional phase (d). Homogeneous hypointensity on the hepatobiliary phase (e) and nodular hyperintensity on diffusion-weighted imaging (b = 800 s/mm2) (f) can also be observed
Inter-reader agreement for LI-RADS categorization for biphenotypic PLC of 2010 and 2019 WHO classiciation showed substantial agreement (κ = 0.697 and 0.696; 95% confidence interval (CI), 0.613–0.780 and 0.606–0.786; respectively).
Correlation of the tumor components of biphenotypic PLCs with LI-RADS classification
For the biphenotypic PLCs diagnosed according to the 2019 WHO classification, the pathologic tumor components, i.e., HCC, CCA, IC, and CLC, associated with radiologic LI-RADS categorization were evaluated. On univariate analysis, tumor of LR-4 or LR-5 tumors had a larger proportion of the HCC component (71.8% ± 27.9 vs. 54.5% ± 31.7, p < 0.001) and a smaller proportion of the CCA component (17.5% ± 22.2 vs. 29.7% ± 31.5, p = 0.005) than those of the LR-M category (Table 4). However, multivariate analysis revealed that the proportion of the HCC component (adjusted odds ratio, 1.02 (95%CI, 1.011.03; p < 0.001) was the only independent factor associated with the LI-RADS category, indicating that a larger proportion of the HCC component is associated with LR-4 or LR-5 categories. When the biphenotypic PLCs were divided into two groups based on the amount of the HCC component, tumors with ≥ 50% of the HCC component were more frequently assigned as LR-4 or LR-5 than those with < 50% of the HCC component (60.9% [78/128] vs. 28.6% [16/56]; p = 0.003) (Supplementary Table 2, Figs. 3 and 4).
MR images of a biphenotypic PLC (cHCC-IC subtype with an 80% HCC component and 20% intermediate cell component) assigned as LR-5, in a 52-year-old male with chronic hepatitis B. There is a 3.5-cm observation showing moderate hyperintensity on T2-weighted imaging (a), and non-rim arterial hyperenhancement (b) and washout appearance on the portal venous phase (c) and transitional phase (d). Hepatobiliary phase image shows homogeneous hypointensity (e), while nodular hyperintensity can be observed on diffusion-weighted imaging (b = 800 s/mm2) (f)
MR images of a biphenotypic PLC (cHCC-IC-CLC subtype with a 10% HCC component, 80% IC component, and 10% CLC component), assigned as LR-M, in a 62-year-old male with chronic hepatitis B. There is a 4-cm observation showing moderate hyperintensity on the T2-weighted image (a), rim hyperenhancement on the arterial phase (b), and peripheral washout with centripetal enhancement on the portal venous phase (c) and transitional phase (d). Hepatobiliary phase image (e) and diffusion-weighted imaging (b = 800 s/mm2) (f) both show the targetoid appearance of the lesion
Discussion
Our study demonstrated that approximately 15% of biphenotypic PLCs diagnosed by the 2010 WHO classification were pathogically reclassified as either HCCs or CCAs using the updated 2019 WHO classification. Specifically, biphenotypic PLCs with stem cell features of the typical subtype and those of the cholangiolocellular subtype were pathologically reclassified as HCCs and CCAs, respectively. The remaining 85% of biphenotypic PLCs in the 2010 WHO classification remained as biphenotypic PLCs by the 2019 WHO classification.
However, the changes in pathologic diagnoses 2010 and 2019 classifications did not significantly affect overall radiologic tumor categorization using LI-RADS at gadoxetic acid–enhanced MRI, even though gadoxetic acid–enhanced MRI provides different ancillary features using hepatobiliary phase and diffusion-weighted imaging. Although all CCAs in the 2019 WHO classification (n = 7) were categorized as LR-M, we found that the proportions of biphenotypic PLCs categorized as LR-M, LR-4, LR-5, and LR-TIV were similar between the two classifications. This finding may be attributable to the fact that only a small proportion of biphenotypic PLCs in the 2010 WHO classification were excluded from the diagnosis of biphenotypic PLCs with the revised 2019 WHO classification.
Our results also suggested that the amount of the HCC component may be the main determinant of radiologic LI-RADS categories of biphenotypic PLCs. Indeed, we found that tumors of LR-4 or LR-5 categories were associated with a larger proportion of the HCC component and a smaller proportion of the CCA component. At multivariable analysis, the amount of the HCC component was the only independent factor associated with the LI-RADS categories of biphenotypic PLCs. In line with our results, previous studies based on the 2010 WHO classification [13, 25, 26] also reported that biphenotypic PLCs showing HCC-like arterial phase hyperenhancement were associated with a larger HCC component.
As non-HCC malignancies including CCA and biphenotypic PLC have different treatment options and worse prognosis compared with HCCs, accurate differentiation could be important. However, we found that despite the revised histological diagnosis of biphenotypic PLCs using the updated 2019 WHO classification, approximately half of the biphenotypic PLCs were still categorized as probable or definite HCCs (i.e., LR-4 or LR-5) with LI-RADS, raising concern over the possibility of a false positive diagnosis of HCC with the current non-invasive diagnosis approaches in patients at risk of HCC. This finding is also consistent with the results of a previous study which reported that 64.4% of biphenotypic PLCs were misclassified as HCCs using LI-RADS [20].
However, it remains undetermined as to whether such misclassification of the LI-RADS category would cause any real clinical problems in the management of patients at risk of HCC. Firstly, the incidence of biphenotypic PLCs is much lower than that of HCCs, occupying 1.0 to 6.5% of all primary liver cancers in the cirrhotic liver [20, 27]. Thus, in actual clinical practice, even though a false positive diagnosis of HCC caused by a misclassified biphenotypic PLC occurs, it may not considerably affect the overall performance of non-invasive diagnosis methods in at-risk patients. Secondly, previous studies have demonstrated that biphenotypic PLCs showing HCC-like imaging features had better survival outcomes after surgical resection than those showing CCA-like imaging features [20, 23]. Furthermore, biphenotypic PLCs categorized as LR-4 or LR-5 exhibited similar post-surgical outcomes to HCCs [20]. Considering that LI-RADS classification is associated with the HCC component of the tumor and that prognosis is associated with the HCC component and corresponding LI-RADS categories, stratification of cHCC-CCA into two groups according to LI-RADS classification (LR-5 or 4 vs. LR-M) would be clinically meaningful in treatment planning and in the prediction of prognosis. However, as the results of previous studies were obtained mainly after liver resection, outcomes of biphenotypic PLCs categorized as LR-4 or LR-5 after liver transplantation have not yet been studied.
Our study had several limitations. First, our study has the inherent limitations of a retrospective study design. Our study included only surgically resected biphenotypic PLCs. Although, this approach provided a more reliable pathologic diagnosis, avoiding the sampling error associated with biopsy; it is subject to selection bias. Second, although our study was based on a large study population from two tertiary institutions, the number of tumors in each tumor type classified based on the diagnostic terminology of the 2019 WHO classification was too small to evaluate differences in LI-RADS categorization results according to specific tumor types. Third, we did not evaluate the prognostic implications of LI-RADS categories of biphenotypic PLCs in our study. Fourth, inter-reader agreement for pathologic diagnosis was not performed as each pathologist from two hospitals reviewed histopathologic specimens of each hospital according to the 2019 WHO classification.
In conclusion, 15% of biphenotypic PLCs diagnosed by the 2010 WHO classification were reclassified as HCCs or CCAs using the updated 2019 WHO classification. However, the changes in the pathologic diagnoses of biphenotypic PLCs did not affect overall radiologic tumor categorization using LI-RADS, with a substantial proportion of biphenotypic PLCs categorized as LR-4 or LR-5.
Abbreviations
- CCA:
-
Cholangiocarcinoma
- cHCC-CCA:
-
Combined hepatocellular cholangiocarcinoma
- CLC:
-
Cholangiolocarcinoma
- HCC:
-
Hepatocellular carcinoma
- HPC:
-
Hepatic stem/progenitor cells
- IC:
-
Intermediate cell carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- PLC:
-
Primary liver cancer
- WHO:
-
World Health Organization
References
Joo I, Kim H, Lee JM (2015) Cancer stem cells in primary liver cancers: pathological concepts and imaging findings. Korean J Radiol 16:50–68
Sciarra A, Park YN, Sempoux C (2020) Updates in the diagnosis of combined hepatocellular-cholangiocarcinoma. Hum Pathol 96:48–55
Wells HG (1903) Primary carcinoma of the liver. The Am J of the Med Sci 126:403–417
Libbrecht L (2006) Hepatic progenitor cells in human liver tumor development. World J Gastroenterol 12:6261–6265
Kim H, Choi GH, Na DC et al (2011) Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 54:1707–1717
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. World Health Organization
Akiba J, Nakashima O, Hattori S et al (2013) Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 37:496–505
Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y (2015) Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int 35:1024–1035
Brunt E, Aishima S, Clavien PA et al (2018) cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 68:113–126
Nagtegaal ID, Odze RD, Klimstra D et al (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76:182–188
Potretzke TA, Tan BR, Doyle MB, Brunt EM, Heiken JP, Fowler KJ (2016) Imaging features of biphenotypic primary liver carcinoma (hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LI-RADS analysis of CT and MRI features in 61 cases. AJR Am J Roentgenol 207:25–31
Jung DH, Hwang S, Song GW et al (2017) Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl 23:330–341
Park SH, Lee SS, Yu E et al (2017) Combined hepatocellular-cholangiocarcinoma: gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging 46:267–280
Fowler KJ, Sheybani A, Parker RA 3rd et al (2013) Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol 201:332–339
Li R, Yang D, Tang CL et al (2016) Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer 16:158
Leoni S, Sansone V, Lorenzo S et al (2020) Treatment of combined hepatocellular and cholangiocarcinoma. Cancers (Basel):12
Yin X, Zhang BH, Qiu SJ et al (2012) Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol 19:2869–2876
Gera S, Ettel M, Acosta-Gonzalez G, Xu R (2017) Clinical features, histology, and histogenesis of combined hepatocellular-cholangiocarcinoma. World J Hepatol 9:300–309
Elsayes KM, Kielar AZ, Chernyak V et al (2019) LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 6:49–69
Choi SH, Lee SS, Park SH et al (2019) LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology 290:388–397
Fraum TJ, Tsai R, Rohe E et al (2018) Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver imaging Reporting and Data System version 2014. Radiology 286:158–172
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Jeon SK, Joo I, Lee DH et al (2019) Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 29:373–382
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830
Stavraka C, Rush H, Ross P (2019) Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma 6:11–21
Maximin S, Ganeshan DM, Shanbhogue AK et al (2014) Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open 1:40–48
Lin G, Toh CH, Wu RC et al (2008) Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract 62:1199–1205
Acknowledgements
We thank Chris Woo for his assistance in English editing of this manuscript.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jeong Min Lee.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
No complex statistical methods were necessary for this paper.
Informed Consent
Written informed consent was waived by the Institutional Review Board.
Ethical Approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects (99 of 209 patients) have been previously reported [1,2,3]. The prior articles evaluated biphenotypic PLCs diagnosed only by the 2010 WHO classification, whereas this study reevaluated the pathologic and radiologic findings of the tumors with emphasis on the changes in pathologic diagnoses and LI-RADS classification results based on the 2010 WHO and 2019 WHO classifications.
References
1Jeon SK, Joo I, Lee DH et al (2019) Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 29:373-382
2Choi SH, Lee SS, Park SH et al (2019) LI-RADS Classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology 290:388-397
3Park SH, Lee SS, Yu E et al (2017) Combined hepatocellular-cholangiocarcinoma: gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging 46:267-280
Methodology
• retrospective
• cross-sectional study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sang Hyun Choi and Sun Kyung Jeon are co-first authors.
Supplementary information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Choi, S.H., Jeon, S.K., Lee, S.S. et al. Radio-pathologic correlation of biphenotypic primary liver cancer (combined hepatocellular cholangiocarcinoma): changes in the 2019 WHO classification and impact on LI-RADS classification at liver MRI. Eur Radiol 31, 9479–9488 (2021). https://doi.org/10.1007/s00330-021-07984-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07984-w