Abstract
Purpose
To explore associations of whole-lesion histogram diffusion metrics with pathologic findings and subsequent metastatic disease in bladder cancer patients undergoing radical cystectomy.
Methods
Twenty-three bladder cancer patients (21M, 2F; mean 70 ± 11 years) underwent MRI before cystectomy. A volume-of-interest was placed on all slices on the ADC map encompassing each lesion. Whole-lesion mean, kurtosis, and skewness of ADC were calculated and compared with T stage and pelvic nodal status at cystectomy and with subsequent metastasis in 20/25 patients with available follow-up.
Results
At cystectomy, 39 % (9/23) were stage T2, 61 % (14/23) ≥T3, and 28 % (5/23) exhibited positive nodes; 35 % (7/20) developed later metastases. Mean ADC was significantly lower in stage ≥T3 than in lower stage tumors (1.20 ± 0.36 × 10−3 vs. 1.55 ± 0.36 × 10−3 mm2/s; p = 0.044), but showed no association with nodal or metastatic disease (p = 0.362–0.709). Kurtosis was significantly lower in tumors with, compared to without, nodal disease (−0.05 ± 0.29 vs. 0.91 ± 1.16; p = 0.037), and showed a non-significant decrease in tumors with, compared to without, later metastases (0.23 ± 0.63 vs. 0.83 ± 0.89; p = 0.088). Kurtosis was not associated with T stage (p = 0.811), and skew was not associated with any outcome (p = 0.516–0.643). Mean ADC achieved highest AUC for identification of stage ≥T3 (AUC = 0.754 vs. 0.516–0.643 for other metrics). Kurtosis achieved highest AUC for nodal disease (AUC = 0.811 vs. 0.522–0.556 for other metrics) and metastases (AUC = 0.736 vs. 0.516–0.626 for other metrics). Only difference in AUC between skewness and kurtosis for nodal disease was significant (p = 0.031).
Conclusion
While requiring larger studies, kurtosis has potential to complement mean ADC in bladder cancer prognosis using whole-lesion histogram analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Bladder cancer is common, with an estimated 72,570 new cases and 15,210 deaths in the United States in 2013 [1]. However, bladder cancer is a heterogeneous disease, with substantial variability in prognosis between cases. Critical determinants of outcome, and thus treatment selection, include disease stage and grade [2, 3]. While there is an excellent 5-year survival of 96 % for non-muscle invasive tumors [1], this rate drops to 70 % for muscle-invasive tumors [1], 33 % for regionally advanced disease [1], and 6 % for metastatic lesions [4]. While radical cystectomy with pelvic lymphadenectomy is the standard of care for muscle-invasive tumors, there is an approximately 50 % risk for the development of metastatic disease despite this invasive treatment [5]. Cisplatin-based neo-adjuvant chemotherapy added to radical cystectomy reduces the risk of metastatic recurrence and improves survival, being supported by level I evidence [6, 7]. However, a significant proportion of patients are ineligible due to advanced age or coexistent medial conditions such as renal impairment. Furthermore, there remains a lack of an effective means of predicting those patients with muscle-invasive tumor that will progress to metastatic disease and to thus tailor therapy accordingly [6, 8].
The apparent diffusion coefficient (ADC) value of bladder cancer, derived from diffusion-weighted imaging (DWI) acquired during MRI, reflects random molecular motion of water, which in turn is influenced by cellular proliferation and other structural changes occurring in tumors. ADC of bladder tumors exhibits statistically significant inverse correlations with tumor stage and grade [9]. In addition, ADC has been shown to be associated with development of recurrent bladder tumors following transurethral resection [10] as well as with cancer-specific survival for upper tract lesions [11]. Thus, ADC has strong potential to serve as a non-invasive biomarker of bladder cancer aggressiveness, with a possible role in guiding prognostic assessment and treatment selection.
One limitation of ADC evaluation is that this metric is frequently measured by a single ROI placed in a non-systematic within the tumor on a single slice, thus being prone to sampling error and an incomplete evaluation of the lesion [12]. Alternatively, whole-lesion ADC histogram metrics entail placement of a 3-dimensional (3D) volume-of-interest (VOI) encompassing the tumor on multiple slices and can provide a more sophisticated and comprehensive assessment including metrics reflecting lesion texture [12]. This approach may further improve the prognostic performance of ADC and enhance the potential value of ADC metrics in clinical practice. For instance, ADC histograms metrics have shown improved performance compared with standard mean ADC alone in predicting chemotherapy response of metastatic ovarian and primary peritoneal cancer [13], aggressive subtypes of cervical cancer [14], and high Gleason grade of prostate cancer [15].
Given the value of whole-lesion ADC metrics in characterizing other pelvic malignancies, we hypothesize that this approach may also have value for characterizing bladder cancer aggressiveness. Therefore, our purpose was to explore associations between whole-lesion histogram diffusion metrics with pathologic findings and development of metastatic disease in bladder cancer patients undergoing radical cystectomy.
Methods
Patients
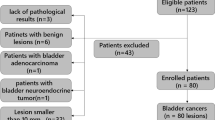
This retrospective study was HIPAA-compliant and approved by our institutional review board with a waiver of written informed consent. We searched institutional databases to identify 51 consecutive patients who underwent radical cystectomy for bladder cancer between June 2006 and December 2013, as well as pre-operative bladder MRI including DWI at our institution. Cases were then excluded for the following reasons: non-standard DWI parameters (n = 3); previous neo-adjuvant chemotherapy (n = 14); only carcinoma in situ identified in cystectomy specimen (n = 2). These exclusions left a final cohort of 23 patients (21 men, 2 women; mean age 70 ± 11, range 49–88 years). None of the included patients underwent treatment between MRI and surgery. All tumors were high-grade based on the 2004 WHO criteria for bladder cancer grading.
MRI
Patients underwent MRI of the bladder using a 1.5T (n = 22; MAGNETOM Avanto, Sonata, or Symphony; Siemens Healthcare) or 3T (n = 1; MAGNETOM Trio, Siemens Healthcare) system and anterior and posterior coil arrays. In addition to standard T1- and T2-weighted imaging, axial DWI of the bladder was performed using a single-shot fat-suppressed echo-planar imaging (EPI) technique that employed tridirectional motion-probing gradients and the following pulse parameters: TR/TE 2000–2800/76–83 ms; field-of-view (FOV) 350–400 mm with 70–80 % rectangular FOV; matrix 264 − 384 × 384; slice thickness 6–8 mm; parallel imaging factor of 2; b-values 0, 400, and 800 s/mm2). From these images, the ADC map was computed on a voxel-by-voxel basis using a standard mono-exponential approach based on the equation S = S 0 · exp(−b · ADC), in which S refers to signal intensity and b to b-value. Pulse parameters varied slightly between patients given differences in body habitus as well as the use of different scanners for the study.
Image assessment
A radiologist with 1 year of experience in abdominopelvic MRI assessed cases blinded to clinical and pathologic details, aside from knowledge that patients had undergone cystectomy. This radiologist reviewed the ADC maps using in-house developed software (Firevoxel, https://files.nyu.edu/hr18/public/projects.html) and placed a 3D VOI on the tumor (Fig. 1). The VOI was traced just inside the outer edge of the lesion on all slices on the ADC map on which the tumor was visible; VOI editing was performed using an interactive, mouse-driven paintbrush/eraser tool. All ADC values within the VOI were used to compute the average ADC within the lesion. The ADC values were then binned to construct the histogram. Using the histogram, kurtosis and skewness of ADC of the entire lesion were computed [16].
52-year-old male with bladder cancer. A Axial turbo spin-echo T2-weighted image shows tumor along anterior and lateral aspects of urinary bladder (arrow). B Axial apparent diffusion coefficient (ADC) map shows decreased ADC in tumor (arrow). C Sample of several slices of ADC map form whole-lesion volume-of-interest assessment show placement of VOI encompassing voxels on multiple slices across area of tumor. D Corresponding ADC histogram from VOI placement. Whole-lesion mean ADC was 1.70 × 10−3 mm2/s; whole-lesion kurtosis was 2.63; whole-lesion skewness was 0.84. Cystectomy demonstrated tumor to be stage T2 and all sampled pelvic lymph nodes negative; patient did not develop metastatic disease at approximately a one-year follow-up interval.
Reference standard
Three endpoints were recorded for each patient. First, the tumor stage, based on histopathologic assessment of the cystectomy specimen, was recorded in a binary fashion as ≤T2 or ≥T3. In addition, the presence or absence of nodal metastatic disease was recorded; this was achievable given the performance of pelvic lymphadenectomy in all patients. Finally, the presence or absence of subsequent development of metastatic disease was recorded. This determination was made on the basis of follow-up CT and MRI examinations of the abdomen and pelvis, in combination with the results of any performed chest CT, nuclear medicine bone scan, and biopsy. Four patients with no follow-up imaging following cystectomy were excluded from the evaluation of metastatic disease.
Statistical assessment
The whole-lesion ADC metrics were compared between lesion groups (stage ≤T2 vs. ≥T3; presence vs. absence of nodal disease; presence vs. absence of metastasis on follow-up evaluation) using exact Mann–Whitney tests. The area-under-the-curve (AUC) from receiver-operating characteristic (ROC) curve analysis was computed and compared between the metrics in these determinations. Analyses were two-sided and considered significant at p < 0.05. Statistical assessment was performed using software (SAS, version 9.0, SAS Institute, Cary, North Carolina; and MedCalc for Windows, version 12.7, MedCalc Software, Ostend, Belgium).
Results
Patients
At radical cystectomy, 39 % (9/23) of lesions were stage T2 and 61 % (14/23) were stage ≥T3. 28 % (5/23) exhibited nodal metastatic disease at cystectomy. Among 20 patients with available post-operative imaging, 35 % (7/20) developed metastatic disease. The distribution of location of metastatic disease at the time of recurrence was as follows (values total greater than 10 given presentation with metastatic disease in multiple sites in some patients): bone (n = 3); lung (n = 2); node (n = 3); liver (n = 1); pelvic implant (n = 5). Mean time to recurrence was 8.0 ± 6.0 months (median 5.6 months). The remaining 65 % (13/20) of these patients did not exhibit metastatic disease (average follow-up 32.0 ± 25.8 months; median 30.9 month).
ADC histogram metrics
Tables 1, 2, and 3 show the results of the whole-lesion ADC metrics in the cohort subsets. Mean ADC was significantly lower in stage ≥T3 tumors than in lower stage tumors (1.20 ± 0.36 × 10−3 vs. 1.55 ± 0.36 × 10−3mm2/s; p = 0.044), but did not show any association with nodal disease or later development of metastasis (p = 0.362–0.709). On the other hand, kurtosis was significantly lower in tumors with nodal disease compared to those without nodal disease (−0.05 ± 0.29 vs. 0.92 ± 1.16; p = 0.037), and showed a non-significant decrease in those with later development of metastases in comparison with those without later metastases (0.23 ± 0.63 vs. 0.83 ± 0.89; p = 0.088). Kurtosis did not show any association with T stage (p = 0.811), and skew did not show any association with any of the outcome measures (p = 0.516-0.643).
At ROC analysis, mean ADC achieved the highest area-under-the-curve (AUC) for identification of stage ≥T3 (AUC = 0.754 vs. 0.516–0.643 for other metrics). However, kurtosis achieved the highest AUC both for nodal disease (AUC = 0.811 vs. 0.522–0.556 for the other metrics) and for later metastases (AUC = 0.736 vs. 0.516–0.626 for other metrics). Aside for a significantly higher AUC in prediction of nodal disease for kurtosis in comparison with skewness (p = 0.031), these differences in AUC between metrics were not statistically significant (p ≥ 0.153). Figure 2 shows the ROC curves for the whole-lesion ADC metrics.
Discussion
Several past studies have established a significant association between standard ADC values of bladder cancer and tumor stage [9, 17, 18]. This relationship was confirmed in our study when using whole-lesion evaluation. Mean ADC was the one metric to exhibit significant differences between tumors of different stage and had the highest AUC in prediction of higher-stage lesions, although this was not statistically significant. However, mean ADC did not demonstrate an association with nodal disease at the time of cystectomy or with subsequent metastases.
We were able to improve upon this limitation of standard mean ADC by utilization of a more sophisticated histogram assessment of lesion ADC that reflects lesion texture and heterogeneity. We evaluated two metrics that are central in assessing the shape of the histogram, namely kurtosis, that reflects the peakedness of the histogram distribution, and skewness, that reflects the shift of the median of the distribution from the mean value. In this assessment, kurtosis was significantly lower in tumors with nodal disease at cystectomy and tended to be lower in tumors with subsequent metastases. Kurtosis also had the highest AUC in these assessments, although the difference in AUC was only significant in comparison with skewness for assessment of nodal disease. Our preliminary investigation thus suggests that kurtosis has potential to complement mean ADC in providing a more complete evaluation of the aggressiveness of bladder cancer and suggesting worse prognostic behavior. While the histogram kurtosis reflects heterogeneity of tissue, the exact basis for a potential association between kurtosis and bladder cancer aggressiveness is unknown from our study [19].
Mean ADC has previously been explored for predicting response of bladder cancer to neo-adjuvant chemotherapy and transurethral resection [10, 20]. In addition, one past study of 17 patients suggested an association between mean ADC of bladder cancer and metastatic disease [21]. However, that study had a highly heterogeneous population in which not all subjects underwent cystectomy; rather the cohort included some bulky tumors that had metastatic disease present at baseline [21], such that the association between ADC and future development of metastatic disease was not evaluated. In our current study, we have demonstrated a possible role for histogram assessment to expand the role of ADC as a prognostic marker in comparison with this past literature. In particular, by using a whole-lesion assessment, unlike the subjective single ROI approach of these past studies, our current methodology may provide a more objective and comprehensive approach to interrogating diffusion behavior of bladder cancer. The value of this approach in comparison with mean ADC measurement has likewise been previously demonstrated in the cervix [22], ovary [23], and prostate [15].
Although requiring further investigation in larger studies, we intend for our findings to ultimately be useful clinically for improving determination of patient prognosis and treatment selection in bladder cancer. The treatment options for bladder cancer continue to evolve and increase in complexity, as neo-adjuvant chemotherapy before cystectomy for muscle-invasive tumor [8], early radical cystectomy for refractory high-grade non muscle-invasive tumor [24], and partial cystectomy for a select subset of tumors [25], all receive increasing attention and clinical adoption. Thus, there could be a critical role for non-invasive imaging markers, such as we have explored in our study, to help guide these decisions.
This study has a number of limitations. First, the sample size was small, limiting our power, and possibly accounting for the lack of statistically significant results for some of the assessments. In addition, by requiring that all patients had undergone cystectomy for study inclusion, we did not evaluate the role of ADC metrics in patients with advanced tumors who were not surgical candidates. Also, patients were imaged using a variety of clinical MR systems. Finally, given that this was a retrospective study, the exact role of the ADC metrics in guiding treatment decisions in a prospective setting remains unknown.
In conclusion, we explored whole-lesion ADC histogram metrics in patients undergoing radical cystectomy for bladder cancer. Whole-lesion ADC metrics have the potential of less operator-dependence than traditional single-slice partial-lesion ROIs, and can provide a more comprehensive lesion assessment reflecting texture and heterogeneity. Mean ADC was significantly lower in tumors with higher T stage. On the other hand, kurtosis tended to be lower in tumors with nodal disease at cystectomy as well as in those with later development of metastases, although small sample size limited the power to detect significant differences in these comparisons. While such histogram metrics may have a role in improving prognostic assessment and treatment selection in bladder cancer, further studies with larger patient cohorts are required.
References
American Cancer Society (2013) Cancer facts and figures 2013. Atlanta: American Cancer Society
Verma S, Rajesh A, Prasad SR, et al. (2012) Urinary bladder cancer: role of MR imaging. Radiographics 32(2):371–387. doi:10.1148/rg.322115125
Vikram R, Sandler CM, Ng CS (2009) Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. Am J Roentgenol 192(6):1481–1487. doi:10.2214/AJR.08.1318
Gschwend JE, Dahm P, Fair WR (2002) Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur Urol 41(4):440–448
Ghoneim MA, Abdel-Latif M, el-Mekresh M, et al. (2008) Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol 180(1):121–127. doi:10.1016/j.juro.2008.03.024
Grossman HB, Natale RB, Tangen CM, et al. (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349(9):859–866. doi:10.1056/NEJMoa022148
International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, et al. (2011) International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clinical Oncol 29(16):2171–2177. doi:10.1200/JCO.2010.32.3139
Raj GV, Karavadia S, Schlomer B, et al. (2011) Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 117(2):276–282. doi:10.1002/cncr.25429
Kobayashi S, Koga F, Yoshida S, et al. (2011) Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. doi:10.1007/s00330-011-2174-7
Funatsu H, Imamura A, Takano H, Ueda T, Uno T (2012) Can pretreatment ADC values predict recurrence of bladder cancer after transurethral resection? Eur J Radiol 81(11):3115–3119. doi:10.1016/j.ejrad.2012.06.009
Yoshida S, Kobayashi S, Koga F, et al. (2013) Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol 23(8):2206–2214. doi:10.1007/s00330-013-2805-2
Rosenkrantz AB (2013) Histogram-based apparent diffusion coefficient analysis: an emerging tool for cervical cancer characterization? Am J Roentgenol 200(2):311–313. doi:10.2214/AJR.12.9926
Kyriazi S, Collins DJ, Messiou C, et al. (2011) Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging-value of histogram analysis of apparent diffusion coefficients. Radiology 261(1):182–192. doi:10.1148/radiol.11110577
Lista F, Andres G, Caceres F, et al. (2013) Evaluation of risk of muscle invasion, perivesical and/or lymph node affectation by diffusion-weighted magnetic nuclear resonance in the patient who is a candidate for radical cystectomy. Actas Urol Esp 37(7):419–424. doi:10.1016/j.acuro.2013.04.003
Donati OF, Mazaheri Y, Afaq A, et al. (2014) Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 271(1):143–152. doi:10.1148/radiol.13130973
Wheeler D (2004) Advanced topics in statistical process control, 2nd edn. Knoxville: SPC Press
Rosenkrantz AB, Haghighi M, Horn J, et al. (2013) Utility of quantitative MRI metrics for assessment of stage and grade of urothelial carcinoma of the bladder: preliminary results. Am J Roentgenol 201(6):1254–1259. doi:10.2214/AJR.12.10348
Kobayashi S, Koga F, Kajino K, et al. (2014) Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging 39(1):172–178. doi:10.1002/jmri.24148
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440. doi:10.1002/mrm.20508
Yoshida S, Koga F, Kobayashi S, et al. (2012) Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 83(1):e21–e27. doi:10.1016/j.ijrobp.2011.11.065
Rosenkrantz AB, Mussi TC, Spieler B, et al. (2012) High-grade bladder cancer: association of the apparent diffusion coefficient with metastatic disease: preliminary results. J Magn Reson Imaging 35(6):1478–1483. doi:10.1002/jmri.23590
Downey K, Riches SF, Morgan VA, et al. (2013) Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. Am J Roentgenol 200(2):314–320. doi:10.2214/AJR.12.9545
Kierans AS, Bennett GL, Mussi TC, et al. (2013) Characterization of malignancy of adnexal lesions using ADC entropy: comparison with mean ADC and qualitative DWI assessment. J Magn Reson Imaging 37(1):164–171. doi:10.1002/jmri.23794
Raj GV, Herr H, Serio AM, et al. (2007) Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol 177(4):1283–1286 (discussion 1286). doi:10.1016/j.juro.2006.11.090
Knoedler JJ, Boorjian SA, Kim SP, et al. (2012) Does partial cystectomy compromise oncologic outcomes for patients with bladder cancer compared to radical cystectomy? A matched case-control analysis. J Urol 188(4):1115–1119. doi:10.1016/j.juro.2012.06.029
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenkrantz, A.B., Obele, C., Rusinek, H. et al. Whole-lesion diffusion metrics for assessment of bladder cancer aggressiveness. Abdom Imaging 40, 327–332 (2015). https://doi.org/10.1007/s00261-014-0213-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0213-y