Abstract
Purpose
To identify the MRI sequences producing the greatest pancreatic adenocarcinoma conspicuity and to assess correlations linking MRI signal intensity and apparent diffusion coefficient to histopathological findings.
Methods
We retrospectively included 22 patients with pancreatic adenocarcinoma who underwent MRI (1.5 or 3 T) before surgical resection. Fat-suppressed (FS) T1- and T2-weighted sequences; 3D FS dynamic T1-weighted gadolinium-enhanced gradient-echo (GRE) imaging at the arterial, portal, and delayed phases; and diffusion-weighted imaging (DWI) with b values of 600–800 s/mm2 were reviewed. On each sequence, we assessed tumor conspicuity both qualitatively (3-point scale) and quantitatively (tumor-to-proximal and -distal pancreas contrast ratios), and we performed paired Wilcoxon tests to compare these data across sequences. We evaluated correlations between histopathological characteristics and MRI features.
Results
21/22 (95%) tumors were hypointense by 3D FS T1 GRE arterial phase imaging, which produced the greatest tumor conspicuity (p ≤ 0.02). By DWI, 5/20 (25%) of tumors were isointense. The correlation between size by histology and MRI was strongest with DWI. A progressive enhancement pattern was associated with extensive and dense fibrous stroma (p ≤ 0.03).
Conclusions
3D FS T1 GRE arterial phase imaging produces greater pancreatic adenocarcinoma conspicuity compared to DWI but underestimates tumor size. DWI provides the best size evaluation but fails to delineate the tumor in one-fourth of cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pancreatic adenocarcinoma carries a grim prognosis, with an overall 5-year survival rate of less than 5%, chiefly because the diagnosis is made late. Computed tomography (CT) is the reference-standard imaging technique for diagnosing pancreatic adenocarcinoma but exhibits several limitations, most notably isoattenuation in 5% to 20% of all pancreatic adenocarcinomas, a pattern possibly associated with better survival [1, 2]. Magnetic resonance imaging (MRI) provides excellent contrast resolution and has benefited over the last few years from technical advances associated with marked improvements in performance. Diffusion-weighted MR imaging (DWI), in particular, has been garnering considerable interest and has produced encouraging preliminary sensitivity and specificity results [3, 4]. Nevertheless, in a recent study, DWI failed to delineate 47% of pancreatic adenocarcinomas, because of hyperintensity of the pancreatic parenchyma distal to the tumor [5], related to upstream obstructive pancreatitis. Most of the studies comparing tumor detectability across MRI sequences were performed several years ago [6–8], and involved 2D imaging after gadolinium injection [6, 7]. We are not aware of published studies comparing tumor detectability between DWI and conventional MRI sequences. Furthermore, although diffusion abnormalities have been ascribed to fibrosis and tumor differentiation [9, 10], studies of correlations between MRI and histopathological findings have produced variable and at times contradictory results [9–12].
The primary objective of this study was to compare DWI and conventional MRI sequences in terms of pancreatic adenocarcinoma detection. Our secondary objective was to evaluate potential correlations between tumor apparent diffusion coefficient (ADC) values and histopathological findings.
Materials and methods
Patients
We conducted a single-center retrospective study by searching our institution’s histopathological database for patients who had had pancreatic resection between May 2006 and July 2012. Among these patients, we identified those with a diagnosis of pancreatic adenocarcinoma and a preoperative MRI evaluation performed within 2 months before surgery. At our institution, CT is obtained routinely in patients with suspected pancreatic adenocarcinoma. Since 2009, MRI is performed in all patients whose tumor is considered resectable because of its higher conspicuity for detection of pancreatic adenocarcinoma [13] and its higher sensitivity for detection of liver metastases [14].
MRI technique
MRI was performed using a 1.5-Tesla General Electric machine with a 16-channel phased-array coil or a 3-Tesla General Electric machine with a 32-channel phased-array coil. The patients were imaged after an overnight fast. The following sequences were obtained: 2D T2 fat-saturation (FS) fast-recovery fast spin echo (FRFSE); 3D T1 FS dynamic gradient echo (GRE) at the arterial phase (25 s), portal phase (70 s), and delayed phase (3 and 5 min) after gadolinium injection; and DWI sequences (three directions, b0 and varying b values among the following: 50, 200, 400, 600, and 800 s/mm2). The contrast agent was either gadoteric acid (Dotarem®, Guerbet, Villepinte, France) in a dose of 0.2 mL/kg or gadobutrol (Gadovist®, Bayer Schering Pharma, Berlin-Wedding, Germany) in a dose of 0.1 mL/kg, injected at a flow rate of 2 mL/s and followed by a saline flush (40 mL at 2 mL/s). ADC maps were obtained using a post-processing console (Advantage Windows) with a monoexponential decay model.
Given the 6-year inclusion period and use of two different MRI machines, the sequence parameters varied across investigations. Table 1 lists representative parameters. All sequences were acquired in the axial plane.
Image analysis
Qualitative analysis
Consensus review of the MRIs was performed by two radiologists (LL and MZ) who knew the patients had been diagnosed with pancreatic adenocarcinoma but were blinded to the histopathological data. The following were analyzed on each sequence: size (largest diameter in the axial plane, measured when the conspicuity on a given sequence was sufficient to correctly delineate the tumor), signal intensity (SI; hypointense, isointense, or hyperintense) of the tumor compared to the adjacent healthy pancreas (separately for the tumor center and periphery), and tumor detectability on a subjective 3-point scale (1, not visible; 2, low to fair visibility, i.e., subtle to moderate signal abnormalities without clearly visible tumor contours; and 3, good to excellent visibility, i.e., major signal abnormalities with clearly visible tumor contours). The sequence considered to best visualize the tumor was recorded.
Quantitative analysis
Mean SI was computed for the full set of sequences by selecting three round or oval regions of interest (ROIs), each measuring at least 20 mm2: one within the tumor (and as large as possible), one in the proximal pancreas (downstream of the tumor), and one in the distal pancreas (upstream of the tumor). ROI positions were chosen carefully to avoid including blood vessels, pancreatic ducts, or artifacts.
The pancreatic time-SI curve was generated as a percentage increase in the SI, according to the following enhancement formula:
where SIpre and SIpost represent the pre- and post-contrast SIs, respectively [15].
To compute the contrast ratios between the tumor and proximal pancreas (C tumor/proximal pancreas) and between the tumor and distal pancreas (Ctumor/distal pancreas), we used the following formulas [16]:
C tumor/proximal pancreas and C tumor/distal pancreas were computed on each sequence. These formulas obviate the need for measuring noise, which can be difficult with parallel imaging given the noise variations within the field of view [17, 18]. They provide a fraction between 0 and 1, with higher values indicating better relative contrast.
ADC values were determined in the same three regions (tumor, proximal pancreas, and distal pancreas). The ROIs were first positioned on the conventional sequences that best visualized the tumor (usually the arterial sequence) then projected onto the ADC map after automatic and manual image realignment.
Histopathology
The pancreatectomy specimens were processed using standard procedures, fixed in formalin, and embedded in paraffin. Slices 4 μm in thickness were stained using hematoxylin–eosin–saffron combined with Astra blue to detect mucus secretion. For each tumor, we extracted the following data from the histopathology report: location, size, T and N stages in the TNM classification, and whether resection (R) was complete (R0) or incomplete (R1 or R2). A pathologist (VD) reviewed the slides to determine the tumor differentiation stage. Well-differentiated adenocarcinomas were defined as exhibiting well-developed infiltrating glands with minimal to moderate cell atypias, abundant mucus secretion, and a low mitotic index. Poorly differentiated adenocarcinomas had a solid trabecular architecture, with isolated tumor cells and a few glandular structures in some cases and with marked cell atypias, no mucus secretion, and a high mitotic index. Moderately differentiated adenocarcinomas had features that were intermediate between those of well-differentiated and poorly differentiated adenocarcinomas [11]. The tumor grade determined upon slide revision was compared to the tumor grade in the histopathology report. In addition, the following information was recorded: proportion of fibrous stroma (≥50% or <50%) comparatively to the proportion of tumor cells, type of fibrosis (dense vs loose, using a semi-quantitative scale), amount of mucin (none, scant, or abundant), amount of necrosis (none, limited, or extensive), and fibrosis in the tumor-free pancreatic tissue (none, limited, or marked).
Statistical analysis
For each tumor, the subjective tumor detectability score (on a 3-point scale) and contrast ratios were recorded on each sequence. Pairwise Wilcoxon tests were performed to compare tumor detectability scores and contrast ratios across sequences; as well as to compare ADC values of the tumor, proximal pancreas, and distal pancreas. Correlations between tumor size measured on the operative specimen and on the various MRI sequences were evaluated by computing Spearman’s correlation coefficients. To assess correlations between histological features (differentiation, fibrosis, mucin, and necrosis) and MRI data (SI and ADC), we compared continuous variables using the Mann–Whitney test and categorical values using Fisher’s exact test. p values <0.05 were considered significant. All statistical tests were performed using SPSS software for Windows (version 19.0, IBM SPSS Statistics, IBM, Armonk, NY, USA).
Results
Patients
We identified 84 patients who had had pancreatic resection between May 2006 and July 2012 (Fig. 1). Among them, 43 had a diagnosis of pancreatic adenocarcinoma, including 23 who underwent MRI of the pancreas within 2 months before surgery. We excluded 1 patient with major MRI artifacts and fragmentation of the operative specimen noted at arrival at the histopathology laboratory. This left 22 patients for the study, 10 males and 12 females with an age range of 45–81 years (mean ± SD, 64 ± 10 years).
Histopathology
Of the 22 operative specimens, 19 (86%) showed adenocarcinomas located in the head and/or isthmus. Mean tumor size was 33 ± 10 mm (range 18–60 mm). In the 2009 pTNM classification [19], the tumor stages were T2: n = 3; T3: n = 17; and T4: n = 2; node status was N0 in 6 cases and N1 in 16 cases. The resection was R0 in 13 cases, R1 in 8 cases, and R2 in 1 case. Slide revision showed good differentiation in 8 (36%) cases, moderate differentiation in 10 (46%) cases, and poor differentiation in 4 (18%) cases. In 13 (59%) cases, a discrepancy was noted between the differentiation grades described in the histopathology report and determined upon slide revision. The overall proportion of fibrous stroma was ≥50% in 17 (77%) cases and <50% in 5 (23%) cases. This proportion differed between the center and periphery of the tumor in 5 patients, among whom 4 had a higher percentage of fibrosis in the periphery. Dense fibrosis was noted in 16 (73%) tumors (center: n = 9; periphery: n = 2; and both: n = 5). Mucin was recorded as absent in 8 cases, scant in 12 cases, and abundant in 2 cases. Necrosis was considered absent in 15 cases, limited in 6 cases, and extensive in 1 case. Fibrosis in tumor-free pancreas was absent in 6 cases and limited in 16 cases.
MRI
A 3-Tesla machine was used in 8 patients and a 1.5-Tesla machine in 14 patients. Mean time from MRI to surgery was 19 days (range 1–56 days). The reason for performing MRI was failure of CT to visualize the tumor in 4 cases, doubt about the presence of liver metastases in 1 case, and routine evaluation to look for liver metastases not visualized by CT in 17 cases. DWI was performed in 20 patients. Each patient had at least one high b value (600 or 800 s/mm2).
Signal
Qualitative analysis
Table 2 lists the tumor signal data; the signals generated by the center and periphery of the tumor are reported separately only for the portal and delayed sequences, as they were identical in 100% of cases on T2 and DWI sequences, as well as in >80% of cases on T1 FS and arterial sequences. Hypointensity was noted for 77% of tumors on the T1 FS sequence before gadolinium injection and 95% of tumors on the arterial sequence. At the portal phase, 82% of tumors remained hypointense centrally, whereas enhancement was often already visible at the periphery (isointense in 27% and hyperintense in 46% of cases). At the delayed phase, all tumors were isointense (27%) or hyperintense (73%) peripherally; whereas the center was hypointense, isointense, or hyperintense. Hyperintensity was noted for 68% of tumors on T2 images and 75% by DWI. Isointense DWI tumors were more frequent among b values of 600 s/mm2 (3/7 = 43%) than 800 s/mm2 (2/13 = 15%), but these results were not significant (p = 0.19).
Quantitative analysis
Mean SI was computed for the tumors in the 22 patients, for the proximal pancreas in 10 patients and for the distal pancreas in 19 patients.
A progressive enhancement pattern (greater SI on the delayed sequence than on the portal sequence) was seen for 14/22 (64%) tumors. Figure 2 depicts the mean time-SI curves obtained using the 9 MRI sets in which enhancement was measurable in the tumor, proximal pancreas, and distal pancreas.
Tumor detectability
Table 3 recapitulates the qualitative (subjective) and quantitative (contrast ratios) detectability data for each sequence. The numbers of cases for which each sequence provided the best detectability are shown also.
By qualitative analysis, detectability was significantly better on the arterial sequence than on the T1 FS, T2, portal, and delayed sequences (p < 0.03 for all comparisons). No significant difference was found between the arterial sequence and DWI (p = 0.16).
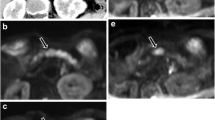
By quantitative analysis, Ctumor/proximal pancreas values were significantly higher with the arterial sequence than with the portal sequence (p < 0.05) and delayed sequence (p < 0.04); DWI produced significantly higher Ctumor/proximal pancreas values compared to the delayed sequence (p < 0.03). Ctumor/distal pancreas values were significantly higher with the arterial sequence compared to the T1 FS, portal, delayed, and T2 sequences (p ≤ 0.003) and to DWI (p = 0.02). None of the other differences was statistically significant. Figure 3 illustrates the superior tumor detectability with the arterial sequence compared to DWI.
Apparent diffusion coefficients (ADCs)
Mean ADC values were 1.76 ± 0.55 × 10−3 mm2/s (range 1.02–2.75) in the tumor (n = 20), 2.25 ± 1.08 × 10−3 mm2/s (0.96–3.94) in the proximal pancreas (n = 8), and 2.21 ± 0.67 × 10−3 mm2/s (1.22–3.84) in the distal pancreas (n = 17). ADC values were significantly lower in the tumors than in the distal pancreas (p = 0.001), whereas the difference was not significant between the tumors and proximal pancreas (p = 0.15).
Correlation between MRI and histopathology findings
Fibrosis
Tumor fibrosis.
Of the 14 (64%) tumors exhibiting a progressive enhancement pattern, 11 (11/14, 79%) had both ≥50% of fibrotic stroma and dense fibrosis. In contrast, of the 8 tumors without a progressive enhancement pattern, only 2 (2/8, 25%) had both ≥50% of fibrotic stroma and dense fibrosis (p = 0.03). Mean ADC values did not differ significantly between tumors having <50% of fibrotic stroma (n = 3) and those having ≥50% of fibrotic stroma (n = 16) (p = 0.94) or between tumors containing dense fibrosis (n = 15) and those containing loose fibrosis (n = 4) (p = 0.81). Percentages of DWI isointense tumors were similar when dense fibrosis was present or absent (20 vs. 27%, respectively).
Fibrosis of tumor-free pancreas.
Mean ADC for the distal pancreas was lower in patients with fibrosis of tumor-free pancreatic tissue (2.05 ± 0.59 × 10−3 mm2/s) than in the other patients (2.60 ± 0.77 × 10−3 mm2/s), although the difference was not statistically significant (p = 0.20). C tumor/distal pancreas values were not different in patients with and without fibrosis of tumor-free pancreatic tissue, most notably by DWI (p = 0.40). All 6 patients without peritumoral fibrosis had tumor hyperintensity (at least peripherally) on the delayed sequence compared to only 63% (10/16) of patients with peritumoral fibrosis (p = 0.13).
Differentiation
Mean ADC of highly differentiated adenocarcinomas was 1.43 ± 0.12 × 10−3 mm2/s, i.e., lower than that of moderately/poorly differentiated adenocarcinomas (1.94 ± 0.62 × 10−3 mm2/s, p = 0.05).
Necrosis and mucin
The ADC value was not significantly associated with the presence of mucin or of necrosis by histopathology. Of the 22 tumors, 5 (23%) had central hypointensity on the delayed sequence. Mean size of these 5 tumors (43 ± 11 mm) was significantly greater than that of the other tumors (p = 0.03). Central hypointensity at the delayed phase did not correlate significantly with the presence of central fibrosis, necrosis, or mucin (p = 1.00, p = 0.27, and p = 0.62, respectively).
Size
Tumor sizes measured on the operative specimen and various MRI sequences are reported in Fig. 4. DWI had the highest correlation coefficient (0.84, p < 0.001), followed, in decreasing order, by the T2, delayed, arterial, T1 FS, and portal sequences (0.82, 0.74, 0.72, 0.68, and 0.48, respectively; p < 0.04). Among tumors for which DWI was performed, only 75% (n = 15), that were hyperintense, were considered measurable (and were therefore measured on the highest b value obtained); size correlated best with pathological tumor size in these 15 DWI hyperintense tumors. Correlation coefficient was higher in cases of peritumoral fibrosis (0.84, p = 0.002; n = 10) than in cases of no peritumoral fibrosis (0.67, p = 0.22; n = 5). On the arterial sequence, tumor size was consistently underestimated compared to tumor size measured on the operative specimen (mean underestimation 10 mm), as shown in Fig. 5.
54-year-old man with adenocarcinoma of the head of the pancreas. A MRI, axial view, arterial sequence. B MRI, axial view, diffusion-weighted imaging with b = 800 s/mm2. C Operative specimen. Tumor is 28 mm on the arterial phase, 39 mm on the diffusion-weighted sequence, and 40 mm on the operative specimen.
Discussion
The 3D T1 FS GRE arterial sequence provided the best detectability of pancreatic adenocarcinoma but consistently underestimated tumor size. Tumor size was best evaluated by DWI, which failed to correctly delineate the tumor in 25% of cases.
Detectability
The arterial sequence has been reported to provide the best contrast between pancreatic tumors and the rest of the pancreatic parenchyma [6–8, 11, 20–23]. Thus, adenocarcinomas are seen as a hypointense zone that is visible clearly within the strongly enhanced pancreas. Our study confirms this point. Many investigators have evaluated DWI for investigating the abdomen in general and the pancreas in particular [5, 24]. DWI has generated considerable hope as a means of early detection or even screening of pancreatic cancer. High sensitivity values have been reported (92% to 96%) [3, 4]. To the best of our knowledge, none of the available studies of pancreatic adenocarcinoma detectability (with comparisons across sequences) included DWI. Our study shows that DWI is not optimal for detecting pancreatic adenocarcinoma. Fibrotic remodeling of the pancreatic tissue adjacent to the tumor generates a high SI that blurs the contours of the lesion. In a study of 80 patients with pancreatic adenocarcinoma [5], DWI failed to delineate the tumor in 47% of cases. In keeping with this result, we found that the contrast ratios for the adenocarcinoma and proximal pancreas were not significantly different between the arterial sequence and DWI, whereas the contrast ratios for the adenocarcinoma and distal pancreas were significantly higher on the arterial sequence than by DWI.
Apparent diffusion coefficient (ADC)
ADC values vary across studies. Pancreatic adenocarcinomas usually have significantly lower ADC values compared to the adjacent pancreatic parenchyma [5, 9, 25, 26] and to pancreases of healthy volunteers [25–27]. However, considerable overlap occurs [28], and in some studies ADC values were not significantly different between the adenocarcinoma and adjacent healthy pancreatic tissue [10] or between adenocarcinomas and pancreases of healthy volunteers [29]. Many factors can influence ADC values, which vary widely across institutions [28]. Comparisons of ADC values across studies are often impossible due to differences in the patient populations, acquisition parameters (sequence types and b values), and post-processing parameters (positioning and size of the ROI). These differences result in nonstandardized results that are not applicable to everyday clinical practice. Atrophy, fatty infiltration, and pancreatic fibrosis, which increase with age, may also influence the ADC values [30]. In our study, the ADC was significantly lower for the tumor than for the distal pancreas. The absence of a significant difference between the tumor and proximal pancreas can be ascribed to the small number of patients (n = 8) in whom ADC was measurable in the proximal pancreas.
Enhancement
In our study, 73% of the tumors were hyperintense peripherally at the delayed phase, in keeping with earlier data. Among studies that investigated the delayed enhancement of pancreatic adenocarcinomas by MRI [6–8, 20, 22, 31–34], most showed isointensity or hyperintensity at the delayed phase. In two studies [8, 34], however, 68% and 57% of tumors, respectively, were hypointense at the delayed phase. There is some evidence to suggest that the variability in the appearance of pancreatic adenocarcinoma at the delayed phase may depend chiefly on the size of the interstitial space and on the venous drainage of the tumor [22, 23]. Large tumors may tend to remain hypointense on delayed images, whereas small tumors may be hypointense, isointense, or hyperintense, in agreement with the findings from our study. One group reported that the most common enhancement pattern in small (<3 cm) pancreatic adenocarcinoma was early heterogeneous and progressive enhancement (73% of cases) [33]. In our study, SI usually differed between the center and the periphery of the tumor at the portal and delayed phases. The peripheral SI was consistently equal to or greater than the central SI, supporting centripetal enhancement of some pancreatic adenocarcinomas. At the delayed phase, the periphery was clearly enhanced (usually hyperintense), whereas the center was hypointense, isointense, or hyperintense. Although the delayed sequence provided optimal tumor detection in 4/22 (18%) of cases, its performance seemed limited in the quantitative analysis.
MRI–histopathology correlations
We are aware of a single study [35] of correlations between tumor size measured on the operative specimen and tumor size on postgadolinium MRI sequences. However, no study sought to determine which sequence provided the best estimate of tumor size. In our work, the correlation coefficient with tumor size measured on the operative specimen was best for DWI. However, the tumor was not measurable by DWI in 25% of the patients for whom this sequence was available. Moreover, peritumoral fibrosis, which appears hyperintense on DWI, likely increases apparent size tumor on this sequence. It is therefore probably contributing to better size correlation of pancreatic adenocarcinoma on DWI sequence. Tumor size underestimation on the arterial sequence (which occurred in all our patients) might be related to the centripetal enhancement of the tumor demonstrated in our study. This point is particularly important, as the arterial sequence is considered optimal for evaluating pancreatic adenocarcinoma. The better tumor size estimation by DWI suggests that this sequence may be useful for assessing local tumor spread and predicting whether resection will be complete or incomplete. This possibility requires confirmation by prospective studies in larger numbers of patients.
The histopathology of pancreatic adenocarcinoma is characterized by a marked desmoplastic response (fibrous stroma), which is widely believed to explain the progressive enhancement pattern and hypersignal by DWI of these tumors. That marked fibrosis is also a feature of chronic pancreatitis, which probably explains the similar imaging findings in this disease and in pancreatic adenocarcinoma [32]. Significant correlations have been reported between MRI quantitative perfusion parameters and both fibrosis and microvascular density [36]. We found a significant association between the combination of ≥50% of fibrous stroma with dense stroma by histopathological analysis and a progressive enhancement pattern by MRI (characterized by a higher delayed SI compared to the portal SI, which was found in 64% of cases). Similarly, in another study, a progressive enhancement pattern was noted in 61% of cases of pancreatic adenocarcinoma [15]. However, another important point is the possible presence of peritumoral fibrosis, which is common in the distal pancreas in the event of pancreatic duct obstruction and upstream pancreatitis. Delayed tumor enhancement may be less clearly visible in patients with delayed enhancement of the distal pancreas, as occurs in chronic pancreatitis. Thus, the delayed hyperintensity of pancreatic adenocarcinoma may be related not only to the fibrous tumor component, but also to the absence of peritumoral fibrosis.
A study designed to determine which tumor component (fibrosis, cellularity, necrosis, or mucin) was responsible for diffusion abnormalities in pancreatic adenocarcinoma pointed to the collagen fibers [9]. In our study, mean ADC values were not significantly different between patients with dense vs loose fibrosis. Neither did the ADC values vary significantly with the degree of tumor differentiation, whereas another group found lower ADCs in poorly differentiated tumors than in moderately/well-differentiated tumors [10]. However, interobserver reproducibility of the differentiation grade was poor, in keeping with previously published results [37]. Finally, a recent study found no correlations between ADC values and histopathological features [12].
Study limitations
The main limitations of our study are the small number of patients and retrospective design. Selection bias may have occurred, as we needed to confine our study to surgically treated patients in order to investigate correlations between MRI and histopathological findings. Other possible sources of bias are the use of two MRI machines (1.5 and 3 T, respectively), with different protocols, particularly for DWI (differences in b values, fat-suppression technique, and breathing during acquisition). It is worth noting, however, that the pancreatic ADC values computed in healthy volunteers were similar with 1.5- and 3-T imaging in a recent study [38]. For qualitative analysis, MRIs were reviewed by two radiologists in consensus. Therefore, interobserver agreement for signal intensity or detectability could not be assessed. We did not assess tumor detectability by DWI with low or intermediate b values; and neither did we use the IntraVoxel Incoherent Motion (IVIM) technique, which involves multi-b acquisition, thus providing an estimate of the effect of perfusion on DWI. SI and ADC values were computed based on a single ROI positioned at each site, namely, the tumor, proximal pancreas, and distal pancreas. As most tumors exhibit a heterogeneous appearance by MRI, this technique may have influenced the findings. However, each ROI had a surface area of at least 20 mm2 and was positioned on each sequence based on the full set of other sequences to ensure optimal tumor coverage. The quantitative assessment of tumor detectability relied on the contrast ratio, as opposed to signal-to-noise ratios, as noise is difficult to compute during parallel imaging. No quantitative histopathological assessments were performed, although visual assessments, for instance of the amount of fibrosis, may be challenging. We did not assess microvascular density. Tumor spread to the vessels and nodes was not studied, as this characteristic was outside the scope of our study.
Conclusion
The arterial 3D T1 FS GRE sequence is optimal for detecting pancreatic adenocarcinoma but consistently underestimates tumor size. When the tumor is delineated by DWI, this sequence provides the best estimate of tumor size. A prospective study in a large number of patients would be helpful in assessing the performance of DWI for evaluating the local spread of pancreatic adenocarcinoma.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- FRFSE:
-
Fast-recovery fast spin echo
- FS:
-
Fat-suppressed
- GRE:
-
Gradient echo
- ROI:
-
Region of interest
- SI:
-
Signal intensity
References
Kim JH, Park SH, Yu ES, et al. (2010) Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 257:87–96
Yoon SH, Lee JM, Cho JY, et al. (2011) Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 259:442–452
Ichikawa T, Erturk SM, Motosugi U, et al. (2007) High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR 188:409–414
Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N (2009) Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol 19:1981–1990
Fukukura Y, Takumi K, Kamimura K, et al. (2012) Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology 2012:732–740
Gabata T, Matsui O, Kadoya M, et al. (1994) Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology 193:683–688
Obuz F, Dicle O, Coker A, Sagol O, Karademir S (2001) Pancreatic adenocarcinoma: detection and staging with dynamic MR imaging. Eur J Radiol 38:146–150
Chandarana H, Babb J, Macari M (2007) Signal characteristic and enhancement patterns of pancreatic adenocarcinoma: evaluation with dynamic gadolinium enhanced MRI. Clin Radiol 62:876–883
Muraoka N, Uematsu H, Kimura H, et al. (2008) Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. JMRI 27:1302–1308
Wang Y, Chen ZE, Nikolaidis P, et al. (2011) Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. JMRI 33:136–142
Elias J Jr, Semelka RC, Altun E, et al. (2007) Pancreatic cancer: correlation of MR findings, clinical features, and tumor grade. JMRI 26:1556–1563
Rosenkrantz AB, Matza BW, Sabach A, Hajdu CH, Hindman N (2013) Pancreatic cancer: lack of association between apparent diffusion coefficient values and adverse pathological features. Clin Radiol 68:e191–e197
Park HS, Lee JM, Choi HK, et al. (2009) Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. JMRI 30:586–595
Motosugi U, Ichikawa T, Morisaka H, et al. (2011) Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 260:446–453
Tajima Y, Kuroki T, Tsutsumi R, et al. (2007) Pancreatic carcinoma coexisting with chronic pancreatitis versus tumor-forming pancreatitis: diagnostic utility of the time-signal intensity curve from dynamic contrast-enhanced MR imaging. World J Gastroenterol 13:858–865
Rosenkrantz AB, Patel JM, Babb JS, Storey P, Hecht EM (2010) Liver MRI at 3 T using a respiratory-triggered time-efficient 3D T2-weighted technique: impact on artifacts and image quality. AJR 194:634–641
Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO (2007) Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. JMRI 26:375–385
Heverhagen JT (2007) Noise measurement and estimation in MR imaging experiments. Radiology 245:638–639
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. New York: Springer
Semelka RC, Kroeker MA, Shoenut JP, et al. (1991) Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology 181:785–791
Engelhard K, Hollenbach HP (1997) High-resolution MRI of pancreatic masses with a new circularly polarized body phased-array coil. Eur Radiol 7:643–648
Kelekis NL, Semelka RC (1997) MRI of pancreatic tumors. Eur Radiol 7:875–886
Vachiranubhap B, Kim YH, Balci NC, Semelka RC (2009) Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging 20:3–9
Kartalis N, Loizou L, Edsborg N, Segersvard R, Albiin N (2012) Optimising diffusion-weighted MR imaging for demonstrating pancreatic cancer: a comparison of respiratory-triggered, free-breathing and breath-hold techniques. Eur Radiol 2012:2186–2192
Matsuki M, Inada Y, Nakai G, et al. (2007) Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging 32:481–483
Fattahi R, Balci NC, Perman WH, et al. (2009) Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. JMRI 29:350–356
Lee SS, Byun JH, Park BJ, et al. (2008) Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. JMRI 28:928–936
Wang Y, Miller FH, Chen ZE, et al. (2011) Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 31:E47–E64
Re TJ, Lemke A, Klauss M, et al. (2011) Enhancing pancreatic adenocarcinoma delineation in diffusion derived intravoxel incoherent motion f-maps through automatic vessel and duct segmentation. Magn Reson Med 66:1327–1332
Yoshikawa T, Kawamitsu H, Mitchell DG, et al. (2006) ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR 187:1521–1530
Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR (1997) Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics 17:281–301
Johnson PT, Outwater EK (1999) Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology 212:213–218
Yu MH, Lee JY, Kim MA, et al. (2010) MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR 195:1324–1332
Hur BY, Lee JM, Lee JE, et al. (2012) Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. JMRI 36:188–197
Hall WA, Mikell JL, Mittal P, et al. (2013) Tumor size on abdominal MRI versus pathologic specimen in resected pancreatic adenocarcinoma: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 86:102–107
Bali MA, Metens T, Denolin V, et al. (2011) Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology 261:456–466
Luttges J, Schemm S, Vogel I, et al. (2000) The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol 191:154–161
Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B (2011) Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. JMRI 33:128–135
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Legrand, L., Duchatelle, V., Molinié, V. et al. Pancreatic adenocarcinoma: MRI conspicuity and pathologic correlations. Abdom Imaging 40, 85–94 (2015). https://doi.org/10.1007/s00261-014-0196-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0196-8