Abstract
The duodenum is a unique segment of intestine, occupying both intra and extra-peritoneal locations. There is a wide spectrum of abnormalities of the duodenum that range from congenital anomalies to traumatic, inflammatory, and neoplastic entities. The duodenum may be overlooked on cross-sectional imaging due to its location and small size. Duodenal pathologies may, therefore, be missed or wrongly diagnosed. Knowledge about duodenal pathologies and optimal imaging techniques can increase diagnostic yield and permit optimal patient management. Conventionally, the duodenum was evaluated with upper GI studies on fluoroscopy; however, endoluminal evaluation is better performed with endoscopy. Additionally, a broad array of cross-sectional imaging modalities permits comprehensive assessment of the duodenum and surrounding viscera. While endoscopic sonography is increasingly used to locally stage duodenal malignancies, MDCT remains the primary modality widely used in the detection and characterization of duodenal abnormalities. MRI is used as a "problem solving" modality in select conditions. We present a comprehensive review of duodenal abnormalities with an emphasis on accurate diagnosis and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The duodenum, by virtue of its complex anatomical position, small size, and important visceral relationships poses specific challenges to the clinical imager and the duodenal pathologies may, therefore, be missed or wrongly diagnosed. A clear and comprehensive understanding of the normal duodenum and its myriad abnormalities will enable the radiologist to make the correct diagnosis.
The normal duodenum measures approximately 25 cm in length and 2.5 cm in transverse diameter, with mucosal folds measuring roughly 2 mm in thickness [1, 2]. Traditionally, the duodenum is divided into four discrete segments: the first portion, commonly known as the duodenal bulb, is suspended intraperitoneally and extends from the gastric pylorus to the gallbladder neck; the second portion includes an upper and lower flexure, extends retroperitoneally from the gallbladder neck to the level of the lower lumbar spine, and is referred to as the descending duodenum; the third portion extends retroperitoneally from right to left and traverses the inferior vena cava and aorta; the fourth portion ascends briefly to the ligament of Treitz. The serosal surface of the descending duodenum is closely related to the pancreatic head, forming the pancreaticoduodenal groove, an anatomic space that contains pancreaticoduodenal arterial arcades, mesenteric veins, and lymphatics.
Both bile and pancreatic fluids drain into the duodenum, via ductal insertions that may be anatomically diverse. Most commonly, the common bile duct and main pancreatic duct drain into the major duodenal papilla to form the ampulla of Vater, which is surrounded by the sphincter of Oddi. The major duodenal papilla is located in the descending duodenum in 75% of cases and in the horizontal segment in 25% of cases [3]. In approximately slightly more than half of all patients, additional pancreatic drainage occurs via an accessory duct (of Santorini) that inserts just proximally at the minor duodenal papilla; uncommonly, the main pancreatic duct makes its insertion at the minor, rather than the major, duodenal papilla [4].
Overview of imaging techniques
Upper endoscopy allows for direct visualization, endoluminal sonography, and biopsy. However, diagnostic imaging remains a crucial element in the diagnostic workup of several duodenal diseases. Given the diverse pathology that may involve the duodenum, the ideal method of imaging depends on the clinical circumstances.
Upper GI studies can be used to evaluate the duodenal lumen and mucosa in real-time, but provides limited extra-luminal information. Double-contrast studies can provide detailed mucosal assessment in suspected peptic ulcer disease or neoplastic process. However, in the modern scenario, its primary uses are for visualizing motility, assessing for pathologic or iatrogenic perforation, and characterizing postoperative anatomy following bariatric surgery. Low-osmolar iodinated contrast should be used when a leak is suspected.
Cross-sectional techniques can be used to evaluate both extra-luminal extents of disease and as an adjunct to endoscopy for delineating endoluminal disease. Computed tomography (CT) is a rapid and widely-accessible means of evaluating the bowel wall and surrounding viscera, but its use is limited, while scanning children and pregnant women due to its inherent utilization of ionizing radiation. Magnetic resonance (MR) imaging is becoming increasingly popular for evaluating the small bowel due to its favorable tissue contrast, lack of ionizing radiation, and capacity for dynamic functional imaging of intestinal motility [5].
Imaging of the small intestine requires luminal distention with an enteric contrast agent because luminal collapse may obscure mucosal disease or mimic abnormal wall thickening. Optimal distention of the duodenum occurs immediately after the ingestion of roughly 900 mL of liquid contrast [6]; when the entire distal small bowel is also of interest, 1350 mL or more of enteric contrast may be administered over several minutes, with the last few cups consumed immediately prior to imaging [7, 8].
Although considerable variability exists, most protocols for cross-sectional imaging of the small bowel employ neutral contrast agents for CT and biphasic media (T1-hypointense and T2-hyperintense) for MR. Examples of neutral CT contrast agents include water, polyethylene glycol, methylcellulose mixed in water, and 0.1% wt/vol barium sulfate suspension (VoLumen; Bracco Diagnostics, Princeton, NJ, USA). The low photoattenuation of these agents and their intrinsic T1-hypointensity improves the conspicuity of surface lesions and inflammatory mucosal hyperenhancement on CT and MR, respectively. Intravenous administration of scopolamine butylbromide (Buscopan; Boehringer Ingelheim, Germany) [9] or glucagon [7] prior to imaging may improves imaging of the duodenum by slowing transit of enteric contrast, limiting peristaltic motion artifact, and prolonging distention [10]. Positive oral contrast should be avoided for CT enterography, as beam hardening artifact can limit assessment of the bowel wall and complicate volumetric post-processing [8].
Patients undergoing cross-sectional imaging of the alimentary tract often will be advised to avoid eating for at least 4–6 h prior to the study [5, 8], in order to minimize any intraluminal debris that could be mistaken for tumor.
CT techniques
Following oral contrast ingestion, the intravenous (IV) administration of an iodinated contrast agent (typically 50–100 mL at 3–5 mL/s) is a standard feature of CT enterography techniques. Protocols may call for imaging during both arterial and venous phases [7, 8] or may omit the arterial phase acquisition if acute inflammation or bleeding is not suspected [7].
Imaging data from the helical acquisition will generally be reformatted in 3–5 mm axial slices for workstation review. Multiplanar reconstructions [1, 8, 11] and maximum intensity projections [8] can provide useful information regarding both the duodenum itself and extraenteric structures such as the mesenteric vasculature and neighboring viscera.
MR techniques
MR duodenography/enterography protocols vary widely, but often are performed at 1.5 T (less commonly, at 3 T) with a torso coil [5]. They include balanced gradient-echo and fat-suppressed T2-weighted imaging to evaluate for abnormal wall thickening and/or masses. Balanced gradient-echo pulse sequences—termed by various manufacturers as true FISP, balanced steady-state free precession, or free induction echo stimulated acquisition (FIESTA)—are useful for obtaining an anatomic overview and identifying suspicious segments of bowel [5, 12]. Fat-suppressed T2-weighted sequences such as single-shot fast spin-echo (SSFE)—also known as single-shot spin-echo (HASTE)—are useful for identifying perienteric edema/inflammation; because of the rapidity with which these sequences are obtained, peristaltic artifacts are minimized [7, 12]. Although the primary role for diffusion-weighted imaging (DWI) in body imaging has been for evaluating malignancy [13], DWI may also have applications in evaluating inflammatory bowel disease [14, 15].
Gadolinium-enhanced fat-suppressed T1-weighted sequences can be used to identify acute inflammation, characterize stigmata of chronic inflammation, evaluate mass lesions, and determine vascular relationships. A typical enterography protocol might include coronal precontrast and arterial phase enhanced sequences followed by a venous phase acquisition in axial plane. Delayed phase imaging can help to distinguish peristalsis from fibrotic or malignant stricture [5, 7, 12].
Congenital anomalies affecting the duodenum
Annular pancreas
Annular pancreas is a rare congenital anomaly caused by an incomplete rotation of the ventral pancreatic anlage during embryological development. Annular encasement of the descending duodenum by the aberrant pancreatic tissue results in varying degrees of stenosis. Severe cases may present in infancy with symptoms of gastrointestinal (GI) and/or biliary obstruction; other congenital anomalies may coexist. However, nearly half of all cases present in adulthood [16], often as peptic ulcer disease or pancreatitis [17, 18].
Annular pancreas may be detected on either CT or MR as a ring of pancreatic tissue encasing the descending portion of the duodenum [19] (Fig. 1A, B). Complete circumferential encasement is not necessary to make the diagnosis; up to one-third of patients may have an incomplete annulus [20]. Cholangiopancreatographic MR protocols (MRCP) often will depict an annular duct within the aberrant pancreatic tissue that may communicate with the main pancreatic duct [21].
Choledochocele
Choloedochoceles are rare abnormalities, possibly congenital, involving intramural ectasia of the terminal common bile duct within the ampulla of Vater. Despite its embryologic and functional distinctness [19, 22], it is included in the Todani classification (type III) of choledochal cysts.
Potential complications of choledochoceles include pancreatitis, biliary colic, and jaundice. Because the likelihood of associated malignancy is far lower for choledochoceles than for other choledochal cysts [23, 24], endoscopic therapy has gained favor over resection [25, 26].
Large choledochoceles may be detected on fluoroscopic upper GI series as an intraluminal filling defect within the descending duodenum. Although ultrasound is of low sensitivity for detecting choledochoceles [25], the visualization of a thick-walled periampullary cystic structure within the duodenal lumen may suggest the diagnosis, especially when calculi or sludge are detected within [27]. CT and MRI/MRCP may demonstrate a cystic structure involving the medial wall of the descending duodenum, with or without internal debris (Fig. 2).
Traditionally, choledochoceles can be either surgically excised or marsupialized into the duodenum. However, endoscopic papillotomy or fistulotomy with extension of incision over the cystic component has been described in the literature [28].
Malrotation
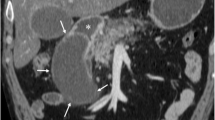
Malrotation refers to an abnormal position of the intestine within the peritoneal cavity due to faulty rotational development during the embryonic period. Under normal circumstances, the intestine undergoes a 270° rotation which results in a left-sided position of the duodenojejunal junction and produces a long mesenteric root for the small intestine. In malrotation, the bowel fails to complete its rotational sweep and, consequently, its mesenteric root is unusually short. Because the malrotated bowel is also inadequately fixated, these patients are predisposed to midgut volvulus [29], which usually occurs in infancy [29] and culminates in intestinal strangulation as it twists about its abbreviated mesenteric stalk.
Due to the life-threatening nature of midgut volvulus, any pediatric patients exhibiting the hallmark sign of bilious emesis should undergo emergent diagnostic workup. The fluoroscopic upper GI series is the mainstay of imaging in suspected malrotation [29, 30] (Fig. 3A). Visualization of the duodenojejunal junction to the left of the spine and above the level of the gastric pylorus virtually excludes a diagnosis of intestinal malrotation; in equivocal cases, identifying the cecum in the right lower quadrant may be reassuring [29].
28-year old man with malrotation. A Upper GI study showing right-sided jejunum (j), not crossing across the spine. B Axial contrast enhanced CT image through upper abdomen shows mid-line cecum (c), right-sided jejunum (j), and abnormal relationships between superior mesenteric vein (white arrow) artery (black arrow). C Malrotation is often an incidental finding in adults but could be associated with volvulus. Swirling mesentery and mesenteric vessels (whirl sign) associated with malrotation and proximal obstruction are a classical sign (different patients).
Intestinal malrotation is reliably demonstrated by MDCT as a failure of the duodenum to course between the aorta and the superior mesenteric artery (SMA). The normal relationship of the SMA and superior mesenteric vein may be reversed, so that the artery is located to the right of the vein (Fig. 3B) [1].
When present, midgut volvulus is classically depicted on upper GI fluoroscopy as a “corkscrew” appearance of the proximal small bowel about the SMA [29–31]. Although neither CT or US is the preferred modality for imaging suspected midgut volvulus, either study may demonstrate the condition as a clockwise wrapping of the mesenteric fat and superior mesenteric vein about the mesenteric artery (“swirling”) [29, 32] (Fig. 3C); under the appropriate clinical circumstances, such findings warrant an emergent fluoroscopic upper GI series and surgical consultation, if not already obtained.
Malrotation is associated with several other disorders of situs and/or GI development and is invariably present in the setting of omphalocele and gastroschisis [30]. Nonrotation, a subtype of malrotation that is less prone to torsion, appears as a predominantly right-sided position of the small bowel and predominantly left-sided position of the colon [30]. Because it is asymptomatic, it usually is an incidental finding in adults undergoing imaging for some other cause [33].
Duodenum inversum
Duodenum inversum is a rare developmental anomaly of retroperitoneal fixation, in which the third portion of the duodenum ascends to the right of the spine and crosses at or above the level of the duodenal bulb, rather than crossing leftward before ascending to the ligament of Treitz (Fig. 4). Although clinical symptoms may be absent or non-specific [34], instances of duodenal, biliary, and pancreatic obstruction have been reported [35, 36].
Mega-duodenum
The term “mega-duodenum” refers to a hereditary or acquired visceral myopathy that leads to dilatation and elongation of the duodenum, resulting in chronic intestinal pseudo-obstruction. The urinary tracts may also be involved, manifesting as recurrent infection [37].
Duodenal duplication cyst
Duplication cysts may occur anywhere within the alimentary tract; approximately 12% involve the gastroduodenal region, usually along the medial aspect of the descending or horizontal segment of the duodenum [1]. Although they are typically asymptomatic, symptoms related to obstruction or superinfection may be present [38].
The characteristic imaging appearance of a duodenal duplication cyst is that of a non-enhancing cystic mass that does not communicate with the duodenal lumen. Its internal contents measure fluid attenuation by CT and appear hyperintense on T2-weighted MR images (Fig. 5). When visualized sonographically, a duplication cyst manifests as a unilocular or multilocular anechoic or hypoechoic lesion with posterior acoustic enhancement [38].
Inflammatory conditions
Duodenal ulcer
Although duodenal ulcers are most commonly detected by upper endoscopy, they may be well demonstrated via fluoroscopic upper GI series. When the clinical presentation is non-specific, duodenal ulcers may be incidentally detected by cross-sectional imaging [1].
Peptic ulcer disease commonly involves the duodenal bulb; more distal involvement should raise concern for another underlying cause such as gastrinoma (as part of the Zollinger–Ellison syndrome) or Crohn’s Disease [39]. When visualized during barium fluoroscopy, duodenal ulcers appear as crater-like collections of barium that persist despite paddle compression and peristaltic motion. Mucosal folds should extend to the ulcer margin. Cross-sectional imaging may demonstrate exophytic wall thickening with associated edema (Fig. 6). The presence of ectopic gas, fluid, or contrast agent within the periduodenal fat or lesser sac is an ominous sign suggesting perforation (Fig. 7A, B). Frank pneumoperitoneum or remote fluid collections should raise concern for uncontained perforation.
53-year old man with perforated duodenal ulcer. A Axial contrast enhanced CT scan through the proximal duodenum shows an irregular air-fluid level (asterisk) adjacent to regions of ulcerated or inflamed duodenal wall (arrow head). Compare the bowel wall thickening and edema with normal duodenum (d). B Upper GI study performed with low-osmolar iodine contrast confirms contained perforation (asterisk) origination at the junction of 1st/2nd portion of duodenum (arrow).
Duodenal diverticulum
Both congenital (true) and acquired (pseudo-) diverticula may involve the duodenum, with the latter being more common. Acquired duodenal diverticula are usually formed by pulsion as the mucous and serous layers herniate through a focal mural defect along the pathway of penetrating blood vessels or ducts [40, 41]. In the duodenum, acquired diverticula most commonly involve the periampullary medial aspect of the descending segment, but may also involve the horizontal segment, ascending segment, or lateral descending wall [42].
Uncommon varieties of duodenal diverticula include acquired traction diverticula, which form in the setting of inflammatory fibrosis from peptic ulcer disease or cholecystitis [41], and intraluminal true diverticula, which are formed by membranous webs as an aberration of embryologic luminal recanalization [1]. Duodenal web refers to an intraluminal congenital membrane with a small aperture leading to complete or incomplete duodenal obstruction. They often occur in 2nd portion of duodenum, and identified as classical “windsock sign (due to gradual ballooning of the membranous web)” on upper GI studies. Of note, the characterization of the this entity as “true” diverticula has been contested [43].
Duodenal diverticulosis involves up to a quarter of the population and is more prevalent among older adults [40, 44]. Unlike diverticula occurring elsewhere in the bowel, duodenal diverticula rarely become inflamed, likely owing to their larger size and the regular flow of relatively sterile and liquid duodenal luminal contents [44, 45]. However, symptoms related to inflammation, compression of neighboring structures, or hemorrhage may be present in 5–10% of cases [40, 43] (Fig. 8A, B). Perforation of a duodenal diverticulum is a rare, but life-threatening condition that may be a consequence of diverticulitis, enterolithiasis, and iatrogenicor incidental trauma [40].
45-year old woman with duodenal diverticulum. A Coronal T2W MR image showing a heterogeneous out pouching or diverticulum (D) arising from duodenum (d). See the narrow neck (arrow) and dilated common bile duct (c). Coronal T2-W images better display the exact location of the neck and distance to the papillary region, and potential associated biliary dilation. B Axial T2W image demonstrates diverticulum (D) causing compression of lower common bile duct (black arrow head). The pancreatic duct is normal (white arrow).
Uncomplicated duodenal diverticula appear as saccular dilatations and are generally well detected by barium fluoroscopy and cross-sectional imaging. When filled with fluid, periampullary diverticula may mimic the appearance of a cystic pancreatic neoplasm, pancreatic pseudocyst or enteric duplication cyst; when filled with debris, they may be mistaken for tumor [45].
As with diverticulitis seen elsewhere in the bowel, the imaging hallmarks of duodenal diverticulitis include focal wall thickening with peridiverticular fat stranding and/or hemorrhage [44]. When inflammatory edema, phlegmon, or abscess involve the pancreaticoduodenal groove or lesser sac, it may be difficult to distinguish between periampullary diverticulitis or acute pancreatitis as the underlying cause. The presence of extra-luminal gas, ectopic contrast medium, phlegmon, or abscess in the vicinity of a duodenal diverticulum should raise concern for perforation; frank pneumoperitoneum is rare [45]. When diverticular perforation involves the duodenal bulb, peptic ulcer disease should be considered as a potential cause [46].
Duodenitis
Thickened duodenal wall with mucosal fold thickening is non-specific sign of duodenitis (Fig. 9A); the underlying etiology could be infection or inflammation.
Spectrum of duodenitis. A Non-specific duodenitis. Coronal contrast enhanced CT through duodenum shows non-specific mural thickening of duodenum (d) with mucosal enhancement (arrow). B Duodenitis secondary to groove pancreatitis. Coronal contrast enhanced CT through duodenum shows thickened edematous duodenal wall (arrows) with inflammation involving the pancraticoduodenal groove (asterisk). C Cryptosporidium duodenitis in a HIV patient. Upper GI study showing thickened mucosal folds involving the distal duodenum and proximal jejunum (arrows) giving rise to saw-toothed appearance. Note that mucosal thickening is irregular in appearance. D Crohn’s disease involving duodenum. Coronal contrast enhanced CT through duodenum shows diffuse mural thickening of 2nd and 3rd portion of duodenum (d) with short segment of narrowing in the distal 3rd portion (arrows).
The most common cause of duodenal inflammation is secondary involvement from pancreatitis. However, “Groove pancreatitis” is a distinctive form of the disease that involves the pancreaticoduodenal groove, which is a potential space between the pancreatic head, the duodenum, and the terminal common bile duct. The typical clinical scenario involves abdominal pain and nausea of variable acuity in a middle-aged male alcoholic. Uncommonly, the associated mucosal edema and/or hematoma can narrow the duodenal lumen, causing symptoms of gastric outlet obstruction [1]. Chronic inflammation of the distal common bile duct can result in weight loss and jaundice, potentially misleading the clinician to suspect underlying malignancy [47].
The hallmark of groove pancreatitis is inflammation in the pancreaticoduodenal groove, which can range from mild, hazy stranding to a frankly tumefactive infiltrate. In the “pure” form of the condition, the inflammation is confined to the groove and the pancreatic head is spared. In the “segmental” variant, the pancreatic head is inflamed and scar tissue forms in the groove [48]. The abnormal soft tissue often has a crescentic shape, which may be best demonstrated on coronal reformations [47, 49] (Fig. 9B). When viewed by MRI, this tissue will typically be mildly hypointense relative to the pancreas on T1-weighted sequences and, in its acute phase, will be T2-hyperintense. As fibrosis ensues, the soft tissue becomes increasingly hypointense on T2-weighted images and begins to exhibit delayed contrast enhancement [21, 47]. The adjacent duodenal wall is often thickened and tiny cysts can involve the duodenal wall (“cystic dystrophy”) or the groove itself; these cysts may be more conspicuous on MRI than on CT [21, 50]. Additionally, tapering stenosis of the pancreatic and/or common bile ducts may result in upstream distention.
The most common infectious organism to involve the duodenum is Helicobacter pylori. H. pylori has also been implicated as a causative organism in non-specific gastroduodenitis and peptic ulcer disease reported in uremic patients on long-term dialysis [51].Other potential causes for infectious duodenitis include cryptosporidium, which may be seen in immunocompromised states; Tropheryma whipplei, which can cause the multisystem syndrome known as Whipple disease; and Mycobacterium tuberculosis. Cryptosporidium duodenitis is exclusively seen in a HIV patient (Fig. 9C).
Crohn’s disease
Crohn’s disease is a relapsing and remitting enteropathy characterized by discontinuous segmental injury throughout the alimentary tract. The degree of damage ranges from mucosal edema and aphthous ulceration to transmural insult with fistulization and/or stricture. The terminal ileum is the most common site of involvement, followed by the ileocecal region, colon, and perianal region; duodenal involvement is comparatively rare, present in roughly 0.5–5% of cases [52, 53].
The diagnosis of Crohn’s disease is guided by clinical, laboratory, and histologic findings. However, diagnostic imaging is helpful to confirm the diagnosis and stage the severity of involvement. The barium small bowel follow-through has been a traditional means of assessing Crohn’s disease of the small bowel. However, it is limited by its operator dependence, the possibility of overlapping bowel loops, and its limited utility for evaluating extra-luminal disease [10]. For this reason, cross-sectional imaging has become a crucial component of evaluating Crohn’s disease.
Both CT and MR enterographic techniques are useful for evaluating Crohn’s disease, but MR offers a few distinct advantages. First, the excellent tissue contrast inherent to MRI improves the visualization of submucosal edema and fibrosis [10]. Moreover, TrueFISP dynamic cine sequences allow for evaluation of intestinal motility, which may be useful for distinguishing peristalsis from stricture [10]. On the other hand, CT does offer superior spatial resolution, rapidity, and availability when compared to MRI; evolving dose-reduction techniques and algorithms may mitigate concerns related to the harmful effects of exposure to ionizing radiation. In all, the selection of imaging modality should be guided by patient factors, clinical circumstances, and local resources.
Regardless of the modality used, the purpose of cross-sectional imaging in evaluating Crohn’s disease is to determine: the number, length, and location of intestinal lesions; to identify areas of stenosis and characterize them as being inflammatory or fibrotic in nature; to stage the severity of inflammatory lesions as either mild, moderate, or severe; and to identify mesenteric complications such as abscesses or fistula [10]. Maglinte et al. [54] have proposed an image-guided classification to guide the clinical management of Crohn’s disease, with cases sorted into the following categories: active inflammatory, perforating and fistulating, fibrostenotic, and reparative/regenerative.
The signs of active inflammatory Crohn’s disease include wall thickening greater than 3 mm, mucosal hyperenhancement, aphthous ulceration, mural laminar stratification, stricture, mesenteric fat stranding, and engorgement of the vasa recta that penetrate the intestinal wall perpendicularly (Fig. 9D). Submucosal edema appears as focal hyperintensity on T2-weighted images. Active intestinal inflammation in Crohn’s disease often shows restricted diffusion [15]. An aphthous ulcer appears as a hyperintense nidus surrounded by a halo or moderate signal intensity [5, 10].
The fistulizing–perforating subtype of Crohn’s disease is characterized by transmural ulceration with perforation; fistulization with adjacent bowel loops, neighboring organs, or the skin; and/or formation of an abscess. Fistulae may be appear as a discrete enhancing tract, however, these may be subtle or non-detectable on imaging; secondary signs may include ectopic gas or contrast agent, tethering of adjacent bowel loops, and mesenteric fat stranding [8]. Abscesses appear as extra-luminal fluid collections and often demonstrate restricted diffusion restriction and peripheral enhancement; gas may be present within.
In the chronic, fibrostenotic phase of Crohn’s disease, the development of intestinal strictures may lead to bowel obstruction. Intestinal strictures appear as a narrowed, aperistaltic segment of bowel with proximal distention. In contradistinction to the acute phase of the disease, mucosal edema is generally absent and the degree of mural enhancement is mild and heterogeneous [10]. Intramural deposition of fat is a non-specific finding that may be present in cases of chronic inflammation, but also may be seen in the setting of obesity, chronic corticosteroid use, and uncontrolled diabetes [8].
When Crohn’s disease is in its reparative or regenerative state, mucosal atrophy is often present and regenerative polyps may be seen. The aforementioned features of active inflammation are characteristically absent [10].
Duodenal neoplasms
Duodenal neoplasms are rare, but they may cause significant morbidity and mortality if undetected. Although many duodenal tumors will be detected by upper endoscopy, radiologic workup is important for characterizing tumors and staging the extent of disease.
Duodenal lipoma
Duodenal lipomas are rare, Slow-growing, benign mesenchymal tumors composed primarily of adipose tissue. They are usually asymptomatic and occur most commonly in older men. Epigastric pain and bleeding secondary to mucosal erosions is rare. Lipomas measuring 2 cm may cause obstruction. Their appearance on cross-sectional imaging is defined by their fatty composition, with corresponding hypoattenuation on CT and signal dropout on fat-suppressed MR sequences [1, 55] (Fig. 10). They are often incidentally found in any portion of the duodenum, but most common in 2nd portion.
Endoscopic ultrasound (EUS) may demonstrate a homogeneously hyperechoic submucosal mass [56].
However, they can present as either a submucosal or intraluminal mass
Duodenal polyps
Duodenal polyps are typically solitary lesions, except for in the setting of polyposis syndromes. In familial adenomatous polyposis, an autosomal dominant mutation in the APC gene results in the formation of hundreds of adenomas throughout the small and large intestine. Peutz–Jeghers syndrome is an autosomal dominant condition characterized by mucocutaneous hyperpigmentation and the proliferation of hamartomatous polyps throughout the GI tract. Most sporadic duodenal adenomas are flat or sessile and involve the descending duodenum [57].
Although they are rarely symptomatic, solitary adenomas are usually resected because the villous histologic subtype has malignant potential [55, 57] (Fig.11A, B).
40-year old man with villous adenoma. A Axial and coronal contrast enhanced CT scan through duodenum shows an soft tissue lesion projecting within the duodenal (2nd portion) lumen (small solid arrows) and unremarkable duodenal wall (large hallow arrow). B Endoscopy and biopsy confirmed the presence of a villous adenoma (asterisk).
Patients with familial adenomatous polyposis are at a particularly high risk for small bowel adenocarcinoma and typically undergo prophylactic colectomy with variable combinations of surveillance and surgery for more proximal disease [58]. The hamartomatous polyps seen in Peutz–Jeghers syndrome are often pedunculated and may be resected due to intussusception, obstruction, or bleeding; only anecdotal reports of malignant degeneration exist [59].
Gastrointestinal stromal tumor (GIST)
GISTs are uncommon tumors arising from the interstitial cells of Cajal, which are peristaltic pacemakers within the myenteric plexus. They are characterized by a mutation in the KIT gene (CD117) which leads to overexpression of a tyrosine kinase growth receptor.
Duodenal GISTs account for roughly 5% of cases; the stomach, jejunum, and ileum are more common sites of involvement [60]. Even when large, these tumors are often asymptomatic. However, patients may present with signs of obstruction or bleeding. Surgical resection is the treatment of choice, but medical therapy with tyrosine kinase inhibitors such as Imatinib (Gleevec) or Sinitinib malate (Sutent) is often employed, especially for patients with unresectable or metastatic disease [61]. The most common sites of metastasis are the liver and peritoneum, whereas lymphatic involvement is comparatively rare [38].
Imaging pattern of GIST is quite variable due to coexisting areas of hemorrhage, necrosis, or cyst formation. They may extend extra-luminally and exert considerable mass effect on the surrounding viscera (Figs. 12, 13A, B). Signal intensity on both T1 and T2w sequences is highly dependent on degree of hemorrhage and necrosis (Fig. 14A, B). GISTs often appear as a heterogeneously enhancing intramural mass on venous phase with variable necrosis [38] (Fig. 14C).
51-year old man with exophytic GIST. A Coronal BFFE/T2W image showing an exophytic mass lesion arising from the genu of duodenum (thick arrow). Note the lesion shows slightly higher intensity than muscles or duodenal wall on this sequence. The central necrosis appears hyperintense (thin arrow). B Fat-suppressed T2W axial image confirms the origin (thick arrow) from duodenum (d). C Coronal T1W post-contrast images show enhancing mass arising from the duodenum (thick arrow); the lesion is enhancing more than duodenal wall.
Carcinoids
Carcinoids consist of about 2–3% of all GI neuroendocrine neoplasms. These are often discovered incidentally or may produce symptoms from hormonal or peptide production. Most common carcinoids are G-cell tumors; 85% of which are associated with gastrin production (“Gastrinomas”) and less than 20% are somatostatin-producing (D-cell) tumors (“Somatostatinomas”).
The majority of gastrin-secreting neuroendocrine neoplasms arise within the “gastrinoma triangle,” which is bounded by the cystic duct confluence superiorly, the duodenal genu inferiorly and the pancreatic neck–body junction medially. Gastrinomas may occur as a component of multiple endocrine neoplasia-1 (MEN1) syndrome or may occur sporadically; in either case, they may or may not be associated with the Zollinger–Ellison syndrome, which is a gastrinoma-related, hypersecretory bleeding ulcer diathesis. Post bulbar ulcers are often associated with Zollinger–Ellison syndrome. When associated MEN-1, these are usually multiple, less than 5 mm and localized in proximal duodenum.
Somatostatinomas exclusively occur in and around the papilla of Vater and can be associated with neurofibromatosis type 1 (NF-1).
Preoperative imaging helps to ensure curative resection, while minimizing surgical morbidity. Side-viewing endoscopy, endoscopic ultrasound, and somatostatin-receptor scintigraphy have been successfully used to localize gastrinomas before surgery [62].
On imaging, 50% carcinoids manifest as polypoid masses and 40% as intramural masses. However, conventional ultrasound, CT, and MR techniques are limited in their ability to visualize duodenal neuroendocrine tumors, which are often much smaller than their pancreatic counterparts [62]. However, when present, these tumors tend to be arterially enhancing on CT and MR, and may be hyperintense on T2-weighted MR sequences [38] (Fig. 15A, B). Accurate timing in arterial phase is crucial for the detection of GI neuroendocrine tumors [63].
Duodenal and ampullary adenocarcinoma
Adenocarcinomas are the most common primary malignant neoplasms of the small intestine, with the majority involving either the periampullary duodenum or proximal jejunum. Patients usually present in the 5th through 7th decades of life [1, 38]. On cross-sectional imaging, duodenal adenocarcinoma may appear as a polypoid or fungating intraluminal mass, as eccentric wall thickening, or as an infiltrating annular stricture [55, 64] (Fig. 16A, B). Patients will present with nodal metastases in roughly half of all cases [38, 64].
60-year old man duodenal adenocarcinoma. A Coronal T2W showing short stenotic segment in 2nd portion of duodenum (asterisk) due to mural thickening (arrows). The stomach (S) slightly dilated and fluid filled. Duodenum (asterisk) and incidental simple cystic lesion is seen in the head of pancreas (c). B Axial T1W image showing diffuse isointense mural thickening (arrows) of duodenum. C, D Coronal and axial post-contrast T1W images showing diffuse enhancement of thickened wall (arrows). Incidentally, the right kidney is strophic and shows hydronephrosis (asterisk).
Peripapillary carcinomas arise within 2 cm of the major papilla in the duodenum and include four different origins: (a) the ampulla of Vater, (b) the intrapancreatic duct, (c) the head and uncinate process of the pancreas, and (d) the duodenal mucosa. Practically all “ampullary carcinomas” arise from the glandular epithelium of the ampulla of Vater and should be classified as a duodenal cancer because both share the same molecular development [64]. However, one distinguishing factor is that ampullary carcinoma almost invariably becomes symptomatic during relatively early stages of disease.
Ampullary carcinomas have a better prognosis than periampullary duodenal cancers or adenocarcinomas of the bile ducts or pancreas. Primarily, this difference is likely due to its tendency for intraluminal growth, which confers an earlier clinical presentation due to ductal obstruction. Consequently, ampullary cancers are more likely to be resectable and less likely to have nodal involvement at the time of presentation [65].
Duodenal adenocarcinoma frequently presents with luminal stenosis and/or common bile duct obstruction due to a polypoid intraluminal mass or an intramural thickening. However, because of their tendency to present earlier, ampullary carcinomas are often small at the time of diagnosis and, therefore, may be inconspicuous on endoscopy or conventional cross-sectional imaging [64]; the addition of diffusion-weighted sequences may raise the sensitivity of 3.0 Tesla MRI for detecting subtle ampullary tumors [66]. Ductal imaging (as with ERCP, MRCP, or antegrade catheter cholangiography) may demonstrate a polypoid or concentric filling defect at the level of the ampulla; variable upstream dilation of the common bile and/or pancreatic ducts is often present [4] (Fig. 17A, B). However, EUS is the mainstay of imaging in locally staging ampullary carcinoma [67]; either CT or MRI can be useful for detecting distant disease.
Lymphoma
The small intestine accounts for approximately one-third of all GI lymphomas [61]. Duodenal lymphoma may be an isolated phenomenon or a component of systemic disease. Cross-sectional imaging often shows segmental concentric wall thickening with mucosal effacement and/or nodularity [1, 55]. Infiltration and obliteration of the myenteric plexus may result in intestinal dilation [68]. Accompanying splenomegaly, when present, may assist the radiologist in distinguishing intestinal lymphoma from Crohn’s disease [5].
Duodenal metastasis
Secondary intestinal cancers may occur through direct local invasion or metastasis. Melanoma is the most common malignancy to metastasize to the small bowel; breast, lung, and renal cancers may also involve the intestine via hematogenous spread [61] (Fig. 18). The appearance of duodenal metastases on cross-sectional imaging is generally non-specific. Duodenal involvement by pancreatic, gastric, and colonic, and hepatic carcinomas often occurs via direct invasion [1] and may manifest as duodenal displacement, obstruction, or fistulization.
Vascular pathologies
SMA syndrome
SMA syndrome is a unique entity presenting with chronic epigastric pain, nausea, voluminous vomiting (bilious or partially digested food), and postprandial discomfort due to compression of horizontal portion of duodenum secondary to acute angulation of SMA. It is an uncommon diagnosis with an incidence of 0.1–0.3%.
A wide range of etiological factors have been linked to the syndrome, but the fundamental underlying cause is acute loss of retroperitoneal fatty tissue as a result of wide range of debilitating conditions leading to acute angulation of SMA. The causes may include rapid intentional or non-intentional weight loss, prolonged bed rest or illnesses, abdominal surgeries, or lordosis.
Diagnostic criteria on imaging may include acute angulation of SMA (aortomesenteric angle less than 22° and aortomesenteric distance less than 8–10 mm) with obstruction of 3rd part of duodenum [69, 70] (Fig. 19A, B). Sagittal reconstruction on CT or MR play vital role to diagnose this condition. Contrary to conventional thought, an upper GI study can miss the diagnosis, but can rule out mechanical obstruction [69].
24-year old woman with SMA syndrome. A Coronal T2W MR image through 3rd portion of duodenum shows dilated proximal duodenum (d) with abrupt narrowing of mid-3rd portion corresponding to the course of SMA (arrow). B Post Gd axial T1W MR image showing compression of horizontal duodenum (d) between SMA (arrow) and aorta.
Aortoenteric fistula (AEF)
AEF is a life-threatening condition and can be classified as primary or secondary. Primary fistulae between the native aorta and the adjacent bowel form spontaneously without previous history of aortic surgery or trauma, and often associated with a preexisting aortic aneurysm. Whereas, the secondary fistula usually forms as a complication of reconstructive aortic surgery with or without the placement of an aortic stent-graft.
Primary AEF is very rare (incidence 0.4–0.7%), and secondary AEF can occur in 0.3–1.6% cases [71].
The etiopathogenesis is complex and multifactorial; combination of chronic low-grade post-surgical infection of the aortic stent-graft and repetitive pressure (from aortic pulsations) leads to the formation of fistula between aorta and an overlying portion of intestine.
Majority (80%) of secondary aortoenteric fistulas involves the duodenum, mostly 3rd or 4th portions [72]. The clinical presentation of this entity is ambiguous, non-specific, and requires high suspicion for correct and timely diagnosis; common clinical presentations may include GI bleeding (80%), sepsis (44%), or abdominal pain (30%) [72].
On cross-sectional imaging, presence of perigraft soft tissue edema, fluid, and ectopic gas, loss of the normal fat plane between the aorta and the duodenum, and disruption of the aortic wall can be seen after 3–4 weeks of surgery [72] (Fig. 20).
64-year old man with aortoenteric fistula. Three month follow-up after open aorto-biiliac stent placement. Axial post-contrast CT through 3rd portion of duodenum shows the presence of air foci within the aortic lumen (arrow) concerning for aorto-duodenal fistulization. The horizontal portion of duodenum is decompressed (asterisk).
Duodenal trauma
The primarily retroperitoneal location of the duodenum affords it relative protection from injury due to low mechanism abdominal trauma. Accordingly, duodenal injuries are relatively rare, accounting for less than 2% of all abdominal injuries [73]. They usually do not occur in isolation and are often associated with pancreatic, hepatic, vascular, renal, and splenic injury. Penetrating trauma is the most common mechanism resulting in duodenal injury, followed by blunt abdominal trauma in the form of duodenal compression against the spinal column (such as by a seat belt, steering wheel, or a handlebar), or abrupt deceleration with resultant shearing injury at the junction of the retroperitoneal 3rd duodenal segment and the intraperitoneal 4th segment [74, 75]. Non-motor vehicle related trauma to the duodenum in children, particularly under the age of five, is concerning for non-accidental trauma due to the well-protected position of the duodenum and pancreas, and the associated large forces required to injure these structures [76].
The clinical presentation of duodenal injury is non-specific but typically consists of epigastric pain, back pain, and vomiting. Unfortunately, these symptoms may not be recognized as signs of an injured duodenum in the setting of multiple coexisting injuries. While early mortality in these patients can is typically be attributed to coexisting injuries and hemorrhage, duodenal injury may contribute to later morbidity and mortality due to duodenal fistula or leakage of enteric contents and enzymes into the retroperitoneum with associated inflammation, infection, and sepsis [77, 78]. Mortality from delayed diagnosis of duodenal injury beyond 24 h has been reported to increase from 11% to 40%, therefore, the emergency department physician and radiologist must remain vigilant, when evaluating a patient with significant abdominal trauma [75, 79].
Injury of the duodenum may be difficult for the radiologist to diagnose due to the frequently subtle nature of the imaging findings. Injury severity ranges from contusion or duodenal wall hematoma to full thickness laceration or disruption [73]. The latter conditions are considered indications for surgical exploration, and it is, therefore, critical to differentiate perforating from non-perforating injuries [80]. CT is the primary modality for evaluation abdominal trauma, including the duodenum; however, this type of injury can be very subtle.
Duodenal contusions and hematomas are a relatively common manifestation of duodenal injury in younger patients. Damage to the extensive submucosal and sub-serosal duodenal vascular plexuses can result in hemorrhage into the wall of the duodenum [38, 81]. This may manifest as focal bowel wall thickening, heterogeneous attenuation within the bowel wall, intramural gas, and/or periduodenal hemorrhage [73, 82] (Fig. 21A, B). Management of isolated duodenal hematomas is generally conservative; however, mass effect from the hematoma may result in progression to gastric outlet obstruction over 1–2 days post injury [81].
The overall sensitivity and specificity of MDCT for diagnosing duodenal injury is not well known; however, the presence of extra-luminal air and/or enteric contrast and focal wall discontinuity is considered the most specific signs of a transmural injury [73, 74, 83]. Non-specific findings of injury, both intramural and transmural, include fluid or hemorrhage in the retroperitoneum, stranding of peripancreatic or periduodenal fat, and significant pancreatic injury, such as pancreatic transection [1]. Fluoroscopic evaluation of the duodenum for suspected full thickness injury has been demonstrated to have poor sensitivity, and is, therefore, not likely to be a useful adjunct examination in the setting of an equivocal MDCT [84].
The American Association for the Surgery of Trauma (AAST) has developed an injury scoring scale for the duodenum; however, it is not an imaging-based system and is difficult to apply to CT findings. Primary consideration for grading a duodenal injury includes the number of segments involved, the percent of circumferential involvement, involvement of the ampulla or distal bile duct, and extent of devascularization [85].
Conclusion
The duodenal abnormalities are frequently missed or misdiagnosed on account of its retroperitoneal location and rarity of disorders that affect the duodenum. Cross-sectional imaging modalities in conjunction with endoscopy permit comprehensive evaluation of duodenal disorders that range from congenital abnormalities to traumatic, inflammatory, and neoplastic conditions.
References
Jayaraman MV, Mayo-Smith WW, Movson JS, Dupuy DE, Wallach MT (2001) CT of the duodenum: an overlooked segment gets its due. Radiographics: a review publication of the Radiological Society of North America, Inc., 21 Spec No: S147–S160
Cronin CG, Delappe E, Lohan DG, Roche C, Murphy JM (2010) Normal small bowel wall characteristics on MR enterography. Eur J Radiol 75(2):207–211. doi:10.1016/j.ejrad.2009.04.066
Avisse C, Flament JB, Delattre JF (2000) Ampulla of Vater. Anatomic, embryologic, and surgical aspects. Surg Clin North Am 80(1):201–212
Buck JL, Elsayed AM (1993) Ampullary tumors: radiologic–pathologic correlation. Radiographics 13(1):193–212
Masselli G, Gualdi G (2012) MR imaging of the small bowel. Radiology 264(2):333–348. doi:10.1148/radiol.12111658
Kuehle CA, Ajaj W, Ladd SC, et al. (2006) Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol 187(4):W375–W385. doi:10.2214/AJR.05.1079
Masselli G, Gualdi G (2013) CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging 38(2):249–259. doi:10.1007/s00261-012-9961-8
Raman SP, Horton KM, Fishman EK (2013) Computed tomography of Crohn’s disease: the role of three dimensional technique. World J Radiol 5(5):193–201. doi:10.4329/wjr.v5.i5.193
Cronin CG, Dowd G, Mhuircheartaigh JN, et al. (2009) Hypotonic MR duodenography with water ingestion alone: feasibility and technique. Eur Radiol 19(7):1731–1735. doi:10.1007/s00330-009-1346-1
Costa-Silva L, Brandao AC (2013) MR enterography for the assessment of small bowel diseases. Magn Reson Imaging Clin North Am 21(2):365–383. doi:10.1016/j.mric.2013.01.005
Zamboni GA, Raptopoulos V (2010) CT enterography. Gastrointest Endosc Clin North Am 20(2):347–366. doi:10.1016/j.giec.2010.02.017
Grand DJ, Mayo-Smith WW, Woodfield CA (2012) Practical body MRI: protocols, applications, and image interpretation. Cambridge: Cambridge Medicine, Cambridge University Press
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188(6):1622–1635. doi:10.2214/AJR.06.1403
Yacoub JH, Obara P, Oto A (2013) Evolving role of MRI in Crohn’s disease. J Magn Reson Imaging: JMRI 37(6):1277–1289. doi:10.1002/jmri.24081
Oto A, Kayhan A, Williams JT, et al. (2011) Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging: JMRI 33(3):615–624. doi:10.1002/jmri.22435
Zyromski NJ, Sandoval JA, Pitt HA, et al. (2008) Annular pancreas: dramatic differences between children and adults. J Am Coll Surg 206(5):1019–1025 (discussion 1025–1017). doi:10.1016/j.jamcollsurg.2007.12.009
Chen YC, Yeh CN, Tseng JH (2003) Symptomatic adult annular pancreas. J Clin Gastroenterol 36(5):446–450
Urayama S, Kozarek R, Ball T, et al. (1995) Presentation and treatment of annular pancreas in an adult population. Am J Gastroenterol 90(6):995–999
Mortele KJ, Rocha TC, Streeter JL, Taylor AJ (2006) Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics 26(3):715–731. doi:10.1148/rg.263055164
Sandrasegaran K, Patel A, Fogel EL, Zyromski NJ, Pitt HA (2009) Annular pancreas in adults. AJR Am J Roentgenol 193(2):455–460. doi:10.2214/AJR.08.1596
Shanbhogue AK, Fasih N, Surabhi VR, et al. (2009) A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics 29(4):1003–1026. doi:10.1148/rg.294085748
Ziegler KM, Zyromski NJ (2011) Choledochoceles: are they choledochal cysts? Adv Surg 45(1):211–224. doi:10.1016/j.yasu.2011.03.019
Horaguchi J, Fujita N, Kobayashi G, et al. (2005) Clinical study of choledochocele: is it a risk factor for biliary malignancies? J Gastroenterol 40(4):396–401. doi:10.1007/s00535-005-1554-7
Kim TH, Park JS, Lee SS, Lee SK, Kim M (2002) Carcinoma arising in choledochocele: is choledochocele innocent bystander or culprit? Endoscopy 34(8):675–676 (author reply 677). doi:10.1055/s-2002-33242
Masetti R, Antinori A, Coppola R, et al. (1996) Choledochocele: changing trends in diagnosis and management. Surg Today 26(4):281–285
Berger A, Douard R, Landi B, et al. (2007) Endoscopic management of a large choledochocele associated with choledocholithiasis. Gastroenterol Clin Biol 31(2):200–203
Cakmakci E, Ugurlar OY, Erturk SM, Ozel A, Basak M (2012) Sonographic diagnosis of choledochocele. J Clin Ultrasound: JCU 40(7):448–450. doi:10.1002/jcu.20877
Ghazi A, Slone E (1987) Endoscopic management of choledochocele. A case report and review of the English literature. Surg Endosc 1(3):151–154
Strouse PJ (2008) Malrotation. Semin Roentgenol 43(1):7–14. doi:10.1053/j.ro.2007.08.002
Applegate KE, Anderson JM, Klatte EC (2006) Intestinal malrotation in children: a problem-solving approach to the upper gastrointestinal series. Radiographics 26(5):1485–1500. doi:10.1148/rg.265055167
Berrocal T, Torres I, Gutierrez J, et al. (1999) Congenital anomalies of the upper gastrointestinal tract. Radiographics 19(4):855–872
Pracros JP, Sann L, Genin G, et al. (1992) Ultrasound diagnosis of midgut volvulus: the “whirlpool” sign. Pediatr Radiol 22(1):18–20
Gamblin TC, Stephens RE Jr, Johnson RK, Rothwell M (2003) Adult malrotation: a case report and review of the literature. Curr Surg 60(5):517–520. doi:10.1016/S0149-7944(03)00030-8
Kim ME, Fallon SC, Bisset GS, Mazziotti MV, Brandt ML (2013) Duodenum inversum: a report and review of the literature. J Pediatr Surg 48(1):e47–e49. doi:10.1016/j.jpedsurg.2012.10.066
Long FR, Mutabagani KH, Caniano DA, Dumont RC (1999) Duodenum inversum mimicking mesenteric artery syndrome. Pediatr Radiol 29(8):602–604
Rozek EC, Graney CM (1951) Duodenum inversum; a report of two cases. Radiology 57(1):66–69
Basilisco G (1997) Hereditary megaduodenum. Am J Gastroenterol 92(1):150–153
Hernandez-Jover D, Pernas JC, Gonzalez-Ceballos S, et al. (2011) Pancreatoduodenal junction: review of anatomy and pathologic conditions. J Gastrointest Surg 15(7):1269–1281. doi:10.1007/s11605-011-1443-8
Roy PK, Venzon DJ, Shojamanesh H, et al. (2000) Zollinger–Ellison syndrome Clinical presentation in 261 patients. Medicine 79(6):379–411
Thorson CM, Paz Ruiz PS, Roeder RA, Sleeman D, Casillas VJ (2012) The perforated duodenal diverticulum. Arch Surg 147(1):81–88. doi:10.1001/archsurg.2011.821
Yin WY, Chen HT, Huang SM, Lin HH, Chang TM (2001) Clinical analysis and literature review of massive duodenal diverticular bleeding. World J Surg 25(7):848–855
Lapin R, Kamath ML, Engler J, Friedman H (1974) Massive gastrointestinal hemorrhage from duodenal diverticula. Am J Gastroenterol 61(3):185–189
Bittle MM, Gunn ML, Gross JA, Rohrmann CA (2012) Imaging of duodenal diverticula and their complications. Curr Probl Diagn Radiol 41(1):20–29. doi:10.1067/j.cpradiol.2011.07.001
Pearl MS, Hill MC, Zeman RK (2006) CT findings in duodenal diverticulitis. AJR Am J Roentgenol 187(4):W392–W395. doi:10.2214/AJR.06.0215
Gore RM, Ghahremani GG, Kirsch MD, Nemcek AA Jr, Karoll MP (1991) Diverticulitis of the duodenum: clinical and radiological manifestations of seven cases. Am J Gastroenterol 86(8):981–985
Ames JT, Federle MP, Pealer KM (2009) Perforated duodenal diverticulum: clinical and imaging findings in eight patients. Abdom Imaging 34(2):135–139. doi:10.1007/s00261-008-9374-x
Raman SP, Salaria SN, Hruban RH, Fishman EK (2013) Groove pancreatitis: spectrum of imaging findings and radiology–pathology correlation. AJR Am J Roentgenol 201(1):W29–W39. doi:10.2214/AJR.12.9956
Stolte M, Weiss W, Volkholz H, Rosch W (1982) A special form of segmental pancreatitis: “groove pancreatitis”. Hepatogastroenterology 29(5):198–208
Kwak SW, Kim S, Lee JW, et al. (2009) Evaluation of unusual causes of pancreatitis: role of cross-sectional imaging. Eur J Radiol 71(2):296–312. doi:10.1016/j.ejrad.2008.04.006
Itoh S, Yamakawa K, Shimamoto K, Endo T, Ishigaki T (1994) CT findings in groove pancreatitis: correlation with histopathological findings. J Comput Assist Tomogr 18(6):911–915
Var C, Gultekin F, Candan F, et al. (1996) The effects of hemodialysis on duodenal and gastric mucosal changes in uremic patients. Clin Nephrol 45(5):310–314
Wagtmans MJ, van Hogezand RA, Griffioen G, Verspaget HW, Lamers CB (1997) Crohn’s disease of the upper gastrointestinal tract. Netherlands J Med 50(2):S2–S7
Reynolds HL Jr, Stellato TA (2001) Crohn’s disease of the foregut. Surg Clin North Am 81(1):117–135 (viii)
Maglinte DD, Gourtsoyiannis N, Rex D, Howard TJ, Kelvin FM (2003) Classification of small bowel Crohn’s subtypes based on multimodality imaging. Radiol Clin North Am 41(2):285–303
Cronin CG, Lohan DG, DeLappe E, Roche C, Murphy JM (2008) Duodenal abnormalities at MR small-bowel follow-through. AJR Am J Roentgenol 191(4):1082–1092. doi:10.2214/AJR.07.3756
Chen HT, Xu GQ, Wang LJ, Chen YP, Li YM (2011) Sonographic features of duodenal lipomas in eight clinicopathologically diagnosed patients. World J Gastroenterol: WJG 17(23):2855–2859. doi:10.3748/wjg.v17.i23.2855
Basford PJ, Bhandari P (2012) Endoscopic management of nonampullary duodenal polyps. Ther Adv Gastroenterol 5(2):127–138. doi:10.1177/1756283X11429590
Johnson MD, Mackey R, Brown N, et al. (2010) Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 14(2):229–235. doi:10.1007/s11605-009-1091-4
Beggs AD, Latchford AR, Vasen HF, et al. (2010) Peutz–Jeghers syndrome: a systematic review and recommendations for management. Gut 59(7):975–986. doi:10.1136/gut.2009.198499
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83
Paski SC, Semrad CE (2009) Small bowel tumors. Gastrointest Endosc Clin North Am 19(3):461–479. doi:10.1016/j.giec.2009.04.012
Khan RN, Bansal VK, Kumar S, et al. (2009) Duodenal gastrinoma: a diagnostic dilemma. Am J Surg 197(5):e48–e50. doi:10.1016/j.amjsurg.2008.06.042
Wang D, Zhang GB, Yan L, et al. (2012) CT and enhanced CT in diagnosis of gastrointestinal neuroendocrine carcinomas. Abdom Imaging 37(5):738–745. doi:10.1007/s00261-011-9836-4
Kim JH, Kim MJ, Chung JJ, et al. (2002) Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics 22(6):1335–1352
Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW (2008) Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg 207(2):210–218. doi:10.1016/j.jamcollsurg.2008.01.028
Jang KM, Kim SH, Lee SJ, et al. (2013) Added value of diffusion-weighted MR imaging in the diagnosis of ampullary carcinoma. Radiology 266(2):491–501. doi:10.1148/radiol.12121106
Zbar AP, Maor Y, Czerniak A (2012) Imaging tumours of the ampulla of Vater. Surg Oncol 21(4):293–298. doi:10.1016/j.suronc.2012.07.005
Montgomery M, Chew FS (1997) Primary lymphoma of the colon. AJR Am J Roentgenol 168(3):688. doi:10.2214/ajr.168.3.9057516
Konen E, Amitai M, Apter S, et al. (1998) CT angiography of superior mesenteric artery syndrome. AJR Am J Roentgenol 171(5):1279–1281. doi:10.2214/ajr.171.5.9798861
Agrawal GA, Johnson PT, Fishman EK (2007) Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol 41(1):62–65. doi:10.1097/MCG.0b013e31802dee64
Xiromeritis K, Dalainas I, Stamatakos M, Filis K (2011) Aortoenteric fistulae: present-day management. Int Surg 96(3):266–273
Vu QD, Menias CO, Bhalla S, et al. (2009) Aortoenteric fistulas: CT features and potential mimics. Radiographics 29(1):197–209. doi:10.1148/rg.291075185
Linsenmaier U, Wirth S, Reiser M, Korner M (2008) Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics 28(6):1591–1602. doi:10.1148/rg.286085524
Pandey S, Niranjan A, Mishra S, et al. (2011) Retrospective analysis of duodenal injuries: a comprehensive overview. Saudi J Gastroenterol 17(2):142–144. doi:10.4103/1319-3767.77247
Degiannis E, Boffard K (2000) Duodenal injuries. Br J Surg 87(11):1473–1479. doi:10.1046/j.1365-2168.2000.01594.x
Maguire SA, Upadhyaya M, Evans A, et al. (2013) A systematic review of abusive visceral injuries in childhood—their range and recognition. Child Abuse Negl 37(7):430–445. doi:10.1016/j.chiabu.2012.10.009
Blocksom JM, Tyburski JG, Sohn RL, et al. (2004) Prognostic determinants in duodenal injuries. Am Surg 70(3):248–255 (discussion 255)
LeBedis CA, Anderson SW, Soto JA (2012) CT imaging of blunt traumatic bowel and mesenteric injuries. Radiol Clin North Am 50(1):123–136. doi:10.1016/j.rcl.2011.08.003
Lucas CE, Ledgerwood AM (1975) Factors influencing outcome after blunt duodenal injury. J Trauma 15(10):839–846
Weigelt JA (1990) Duodenal injuries. Surg Clin North Am 70(3):529–539
Kurkchubasche AG, Fendya DG, Tracy TF Jr, Silen ML, Weber TR (1997) Blunt intestinal injury in children. Diagnostic and therapeutic considerations. Arch Surg 132(6):652–657 (discussion 657–658)
Brody JM, Leighton DB, Murphy BL, et al. (2000) CT of blunt trauma bowel and mesenteric injury: typical findings and pitfalls in diagnosis. Radiographics 20(6):1525–1536 (discussion 1536–1527)
Shilyansky J, Pearl RH, Kreller M, Sena LM, Babyn PS (1997) Diagnosis and management of duodenal injuries in children. J Pediatr Surg 32(6):880–886
Timaran CH, Daley BJ, Enderson BL (2001) Role of duodenography in the diagnosis of blunt duodenal injuries. J Trauma 51(4):648–651
Moore EE, Cogbill TH, Malangoni MA, et al. (1990) Organ injury scaling, II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma 30(11):1427–1429
Acknowledgments
The authors sincerely thank Charles A. Rohrmann, Jr, M.D. and Joel E. Lichtenstein, M.D., for their valuable contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNeeley, M.F., Lalwani, N., Dhakshina Moorthy, G. et al. Multimodality imaging of diseases of the duodenum. Abdom Imaging 39, 1330–1349 (2014). https://doi.org/10.1007/s00261-014-0157-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0157-2