Abstract
Anal carcinoma is an important but rare condition, managed in specialist centres. Both endoanal ultrasound and magnetic resonance imaging (MRI) can be used in the locoregional staging and follow-up of patients with anal cancer, and both may assist in treatment planning and prognosis. Recent guidelines published by the European Society for Medical Oncology have recommended MRI as the technique of choice for assessment of locoregional disease. This paper describes the techniques for both endoanal ultrasound and MRI, and compares the relative merits and disadvantages of each in the local assessment of anal carcinoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary carcinoma of the anal canal is rare, accounting for only 0.3% of all cancers, 3–3.5% of anorectal malignancies and 2% of all large bowel cancers [1]. It is consequently managed in specialist centres. Its incidence has been reported to be 1.5:100,000 per year, with 300 new cancers per year diagnosed in the UK and 5000 in the USA [2, 3]. Its incidence is believed to be rising, with twice as many women diagnosed with anal canal cancer, and with the median age at diagnosis of 60 years. Most anal cancers are linked to infection with human papilloma virus (HPV), particularly HPV 16, which is detected in approximately 70% of cases [4]. Anal cancer is also strongly associated with human immunodeficiency virus and immune suppression in transplant recipients. For primary tumours, prognosis largely depends on sex, grade, tumour size, depth of invasion and presence of nodal metastases [5, 6].
Until the mid 1980s, surgery was the treatment of choice. Today, the standard treatment for anal canal cancer is chemoradiotherapy, which offers superior local control and survival. In general, radiation fields should encompass the primary tumour and any sites of suspected nodal involvement on cross sectional imaging. Although not routine practice in the UK, suspected metastatic disease to the inguinal nodes in other centres (eg, in the United States) can be confirmed with the use of positron emission tomography (PET) or ultrasound guided biopsy. This then leads to booster radiation doses to the involved inguinal regions, Radiotherapy may lead to 5-year survival rates of up to 70%, but with the higher doses needed to achieve these survival rates there is the risk of radiation necrosis, stenosis and fistulae in 10–30% of patients [6–10]. Therefore radiotherapy at lower doses is usually combined with chemotherapy using 5-fluorouracil (5-FU) and mitomycin C or 5-FU and cisplatin. Surgery is reserved for tumours that fail to respond to chemoradiotherapy, large tumours causing proximal large bowel obstruction or small T1 tumours at the anal margin without sphincter involvement. Standard salvage therapy for patients with persistent or recurrent disease is a radical abdomino-perineal excision.
Accurate tumour staging in anal cancer is thus essential for prognostic information and for treatment planning. The most widely employed clinical staging system is the AJCC (American Joint Committee on Cancer)/UICC (International Union against Cancer) TNM classification (Table 1) which defines T-stage by maximum tumour diameter. This does not take into account sphincter muscle involvement which is an important prognostic factor [11]. In the last decade there has been increasing use of endoanal ultrasound (EAUS) and magnetic resonance imaging (MRI) for locoregional staging [12–15]. There appears to be only one paper to date [16] that directly compares EAUS (using 2-dimensional imaging) with MRI in the primary staging of anal carcinoma. Recently, the European Society for Medical Oncology guidelines have recommended both EAUS and MRI in the primary staging of anal carcinoma, although MRI is currently considered the technique of choice for assessing locoregional disease [17]. The guidelines also suggest that MRI can be used to complement clinical assessment in response evaluation [17].
This paper reviews the role of EAUS and MRI in the evaluation of anal carcinoma.
Anatomy of the anal canal
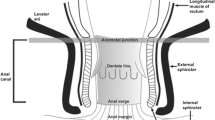
The anal canal is a 4-cm long structure extending from the anorectal junction above to the anal verge below. The dentate line lies approximately 2.5–3 cm from the anal verge and divides the anal canal into the upper canal lined by rectal glandular or transitional mucosa, and a lower part lined by non keratinising stratified squamous mucosa. The anal verge or anal margin is the mucocutaneous junction between the non hair-bearing squamous mucosa and the hair-bearing perianal skin. The anal canal is surrounded by 2 sphincters; the internal anal sphincter (IAS) consisting of smooth muscle which is a continuation of the circular muscle layer of the rectum, and the external anal sphincter (EAS) which is composed of striated muscle and is continuous with the puborectalis and levator ani muscles. Traditionally the EAS is believed to have 3 segments—deep, superficial and subcutaneous. The IAS extends from the anorectal junction to 1 cm below the dentate line. The EAS extends inferiorly approximately 1 cm beyond the IAS and is continuous posteriorly with the anococcygeal ligament. The intersphincteric space is a plane, composed of fat, lying between the IAS and the EAS.
Technique and imaging characteristics of the normal anal canal
Imaging on EAUS
At our centre, EAUS is performed using a variable frequency (6–16 MHz) rotating transducer (9 mm in diameter) that is encased in a sealed parallel walled, plastic cone, with 3D acquisition. The wide frequency range allows for high resolution and adequate depth penetration. The probe is introduced into the rectum with the anterior end uppermost or at the12 o’clock position and then slowly withdrawn down the anal canal until the hyperechoic puborectalis muscle is seen as a ‘U’ shaped structure around the posterior wall of the anal canal. The patient is examined either in the left lateral position or prone position. Real-time axial 3-dimensional images are acquired. Several layers are identified on EAUS (Fig. 1). The plastic cone is seen as an innermost well-defined hyperechoic ring. The subepithelium appears moderately hyperechoic with prominent vascular channels often demonstrated, representing haemorrhoids. This layer is surrounded by a wide hypoechoic band which represents the IAS which has an average thickness of 2 mm. The intersphincteric space appears hyperechoic. The final layer is the EAS, which has mixed echogenicity, similar to the subepithelium. The EAS is related anteriorly to the superficial transverse perineal muscle that is seen as a hypoechoic anterior region. EAUS has been shown to be comparable to MRI in the evaluation of anal canal anatomy [18].
Imaging on MRI
At our institution, magnetic resonance imaging is performed on a 1.5 Tesla magnet with the patient in the supine position. Either an endoanal or pelvic phased array coil can be used, but an endoanal coil suffers from a reduced field of view and is a relatively invasive technique, so the authors prefer to use a pelvic phased array coil. Our protocol for imaging anal carcinoma includes turbo spin echo T1- and T2-weighted sequences as shown in Table 2. The T2-weighted sequences provide high spatial and contrast resolution, giving detailed anatomical imaging and demonstrating the relationship of the tumour to the anal sphincter complex. We do not advocate the use of STIR sequences to increase tumour conspicuity (where anal carcinomas would appear of high signal intensity) because anatomical boundaries are more difficult to delineate compared to T2-weighted MRI. We also do not advocate the use of gadolinium contrast since this does not add to the information gained from the intrinsic soft tissue contrast of T2-weighted MRI.
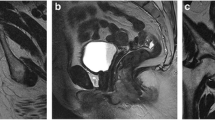
On T1-weighted sequences the anal sphincters are of low signal intensity, contrasting against the high signal intensity of the fat in the ischioanal fossa. On T2-weighted sequences (Fig. 2) the IAS is seen as a cylindrical structure of intermediate to high signal intensity surrounding the anal canal. The dentate line is not directly visualised on MRI. The surrounding longitudinal muscle and EAS are of lower signal intensity than the IAS, identical to skeletal muscle elsewhere (e.g. the gluteus maximus). The intervening intersphincteric plane is of high signal intensity on both T1- and T2-weighted sequences.
Normal anatomy of the anal canal on MRI using pelvic phased array coil: a) Coronal T2-weighted image and b) axial T2-weighted image through the pelvis demonstrating the normal signal characteristics of the IAS (white arrow) and EAS (black arrow); U: urethra; V: vagina. EAS is continuous with the puborectalis (PR) and levator ani (LA) muscles
Histopathological types and patterns of behaviour in anal carcinoma
Squamous cell carcinoma (or epidermoid cancer) represents about 80% of anal cancers. Eighty-five percent of tumours arise in the anal canal and 15% at the anal margin. Tumours arising below the dentate line are predominantly keratinising squamous cell neoplasms. Those arising at or just above the dentate line are non-keratinising and show mixed patterns of adenocarcinoma and squamous cell carcinoma, also defined as transitional, cloacogenic or basaloid carcinoma. Tumours of the anal canal are usually poorly differentiated, more common in women and have a worse prognosis. Tumours of the anal margin are usually well differentiated, more common in men and have a better prognosis
Anal cancer is an indolent disease. At the time of presentation, approximately 50% of patients have a superficial mass (T1/T2 disease) and approximately 25% have regional lymph node involvement. The pattern of lymph node spread reflects the site of origin of the tumour within the anal canal. Tumours above the dentate line spread to the peri-rectal, internal iliac and retroperitoneal nodes. Tumours below the dentate line spread to the inguinal nodes [19].
Primary staging of anal carcinoma
Role of EAUS
Endoanal ultrasound is a relatively cheap, safe, well-tolerated examination which is useful for local disease evaluation [20]. However the field of view is limited and distant mesorectal nodes, inguinal and iliac nodes cannot be assessed. It is also operator dependent and is unable to assess stenotic tumours.
Tumours usually appear as vascular hypoechoic central masses that may involve the sphincters and invade deeply (Fig. 3a). Involved perirectal lymph nodes (Fig. 3b) may be identified on EAUS as enlarged (≥1 cm), round hypoechoic structures. EAUS has been shown to be superior to MRI in the detection of small superficial tumours of the anal canal [16]. Staging systems on EAUS have been suggested [13, 21] which emphasise depth of invasion over size of tumour (Table 3), similar to the staging classification in rectal carcinoma. This staging system is not as widely used as the AJCC staging system.
Primary staging of anal carcinoma. 2-dimensional still image taken from a 3-dimensional EAUS data set: a) T4 anal tumour appears as a large hypoechoic mass (asterisks) between the 12 and 6 o’clock positions encroaching into both the IAS and EAS. The EAS is denoted on the non-involved side; b) enlarged mesorectal lymph nodes are seen (in a different patient) as round hypoechoic structures in the mesorectum (arrows); the primary tumour involves the lower rectum and extends into the mesorectum (arrowheads)
Role of MRI
Magnetic resonance imaging is useful for demonstrating local extent of pelvic disease because of its intrinsic soft tissue contrast and clear delineation of anatomical boundaries. However it is a more expensive and time-consuming examination than EAUS and may be contra-indicated in certain patients (e.g. in the presence of a pacemaker). Studies have shown good agreement in local tumour staging by MRI versus clinical digital examination [15].
Compared with the signal intensity of the gluteus muscles, anal carcinomas appear as intermediate signal intensity (SI) on T2-weighted MRI and isointense on T1-weighted MRI (Figs. 4, 5). Primary tumours tend to show infiltration of local structures with frequent involvement of the sphincter complex (external sphincter, levator ani, puborectalis) and the anterior urogenital triangle (urethra and vagina), possibly because of the thin anatomical separation between the anal canal, the urethra and the vagina. Tumour extension to adjacent structures is seen on T2-weighted MRI as ill-defined intermediate signal intensity infiltration or encasement of 1) lower signal intensity structures (such as the EAS, puborectalis muscle, levator ani muscle, superficial transverse perineal muscle or coccygeus muscle), 2) higher signal intensity structures (such as the ischiorectal fossa, perianal subcutaneous tissue, perianal skin or retropubic space) (Fig. 6), or 3) isointense structures (such as the vagina, prostate or urethra) (Fig. 7). Extension laterally into the ischiorectal fat is less common, suggesting that the levators act as a barrier to lateral tumour growth [15].
Primary staging of an anal carcinoma on MRI—T2 N0 M0: Coronal T2-weighted MRI demonstrating a 4-cm long intermediate signal intensity mass arising from the upper right anterolateral anal canal. T2-weighted MRI clearly delineates the tumour (white arrow) from the low signal intensity right EAS/levator plate (black arrows)
Primary staging of an anal carcinoma on MRI—T3 N0 M0: Axial T2-weighted MRI demonstrating a bulky intermediate signal intensity mass protruding from the anal margin to the perianal skin. Tumour conspicuity is enhanced by the intermediate signal intensity of the tumour (black arrow) against the high signal intensity of the ischiorectal fat (black asterisk)
Primary staging and restaging of anal carcinoma on MRI—T4 N0 M0: a) Axial T2-weighted MRI demonstrating a bulky intermediate signal intensity mass filling the anal canal and extending anteriorly to invade the posterior wall of the vagina with loss of the low signal intensity outline of the posterior vaginal wall (white arrow); b) axial T2-weighted image of the same patient 8 months following treatment. There has been a complete radiological response with restoration of the low signal intensity outline of the vagina wall (white arrow)
Nodal involvement is demonstrated on both T1-weighted and T2-weighted MRI with metastatic nodes demonstrating similar signal intensity to the primary tumour. Peri-rectal nodes with a maximum short-axis diameter of more than 5 mm and inguinal and pelvic side wall nodes with a maximum short-axis diameter of more than 10 mm are considered to be involved (Figs. 8, 9). This arbitrary size criterion may lead to both false-positives (because of reactive lymph nodes) and false-negatives (arising from microscopic nodal involvement). It has been shown in rectal cancer that size criteria alone has relatively poor predictive value for metastatic nodal involvement and that higher sensitivity (85%) and specificity (98%) can be obtained when using irregular border and mixed signal intensity as criteria for nodal disease [22].
Staging nodal disease on MRI—T2 N2 M0: Axial T1-weighted MRI of a patient with a T2 anal tumour (not shown), with a metastasis to a unilateral right internal iliac node (arrow) in keeping with N2 disease; b) axial T1-weighted MRI of the same patient following chemoradiation demonstrating complete resolution of the nodal metastasis (black arrow)
Evaluation of anal carcinoma following chemoradiation treatment
Role of EAUS
The value of EAUS in the follow-up of treated anal carcinoma is still controversial. Oedema and scar tissue may be difficult to distinguish from persistent or recurrent tumour on EAUS, although the former is more likely to have a mixed echogenic appearance (Fig. 10) compared with tumour, which is usually hypoechoic. It is suggested that 16–20 weeks after radiation treatment is sufficient time frame to allow for resolution of oedema and for accurate ultrasound imaging [13]. Serial follow-up examination with EAUS can monitor changes in the size of residual scar tissue and detect local recurrence [23]. One study suggests some superiority of EAUS over clinical examination in predicting local recurrence [12]. Colour Doppler may increase the specificity of EAUS, with vascularity more likely within tumour relapse rather than fibrosis [23].
Role of MRI
Following chemoradiation treatment there is usually a reduction in tumour size and signal intensity, with treated tumour appearing as homogeneously low signal intensity on T2-weighted MRI (Figs. 11, 12). Stabilisation of T2-weighted signal intensity and size of any residual abnormality more than one year after chemoradiation is associated with a good outcome [15]. Size involution is most evident at 6 months post-treatment compared with the immediate post-treatment stage where inflammation is superimposed on treated disease. Low signal intensity appearance of treated anal canal carcinoma on T2-weighted MRI is thought to represent fibrosis. Follow-up imaging is important for ensuring stability of appearances and detecting early recurrence. Re-staging of treated disease with MRI can lead to both over- and under-staging because of treatment-related tumour fibrosis and treatment-related reactive lymph nodes.
Restaging of the anal carcinoma following chemoradiation on MRI: Coronal T2-weighted pre-treatment MRI shows a 4-cm-long T2 N0 M0 tumour confined to the IAS (black arrows); b) coronal T2-weighted MRI 5 months post-chemoradiation treatment—the image shows a complete radiological response with no residual tumour
Restaging of an anal carcinoma following chemoradiation on MRI: a Axial T2-weighted pre-treatment MRI shows a bulky heterogeneous tumour involving the puborectalis muscle (black arrow); b axial T2-weighted MRI taken 4 months after chemoradiation treatment shows marked reduction in the size, extent and signal intensity of the tumour (short black arrow). The puborectalis muscle is now more distinct and also of low signal intensity (longer black arrow)
Persistent and locally recurrent tumours have a different pattern of disease with more aggressive and extensive involvement of the adjacent organs and the pelvic skeleton (Fig. 13) [14]. The pattern of lymph node spread also differs with locally recurrent disease, with the inguinal nodes less commonly involved, and the peri-rectal, presacral and internal iliac nodes more commonly involved than in the primary disease [14]. The MR signal characteristics of recurrent disease are similar to those of the primary disease [14].
Progression of disease following chemoradiation on MRI: a Axial T2-weighted MRI pre-treatment demonstrate a 4-cm-long anal canal tumour involving the left puborectalis (white arrow); b Axial T2-weighted MRI taken 7 months later post-chemoradiation shows resistance to treatment with disease progression—the mass has further extended through the left puborectalis muscle (white arrow). There is a new right inguinal nodal metastasis (black arrow)
Conclusion
Both EAUS and MRI play an established role in the locoregional staging of primary anal carcinoma. EAUS provides accurate staging of the primary tumour, allowing assessment of sphincter involvement which has both prognostic and therapeutic implications. MR imaging of primary anal carcinomas is an excellent, non-invasive, accurate means of assessing tumour extent, local tumour stage and nodal involvement, thus facilitating treatment planning. The use of imaging in patients following chemoradiotherapy is more debatable, with treatment-related changes causing difficulties in interpretation especially on EAUS. Preliminary studies suggest that MRI may be a more robust imaging technique, being able to accurately demonstrate response to chemoradiation treatment where a reduction in the size and signal intensity of primary tumour has been shown [14, 15]. MRI complements clinical assessment in response evaluation and serves as a useful baseline following treatment. Long-term follow-up and surveillance of patients is chiefly by clinical examination, although MRI or EAUS may assist in the early detection of disease relapse.
References
Deans GT, McAleer JJ, Spence RA (1994) Malignant anal tumours. Br J Surg 81:500–508
Ries LAG, Harkins D, Krapcho M et al (eds) (2005) SEER Cancer Statistics Review, 1975–2003. Available via National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2003/, Accessed 8 Aug 2010
Greenlee RT, Murray T, Bolden S et al (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33
Daling JR, Madeleine MM, Johnson LG et al (2004) Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 101:270–280
Goldman S, Auer G, Erhardt K, Seligson U (1987) Prognostic significance of clinical stage, histologic grade, and nuclear DNA content in squamous-cell carcinoma of the anus. Dis Colon Rectum 30:444–8
Klas J, Rothenberger D, Wong W, Madoff R (1999) Malignant tumours of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 85:1686–1693
Allal A, Kurtz JM, Pipard G, Marti MC, Miralbell R, Popowski Y et al (1993) Chemoradiotherapy versus radiotherapy alone for anal cancer: a retrospective comparison. Int J Radiat Oncol Biol Phys 27:59–66
Doggett SW, Green JP, Cantril ST (1988) Efficacy of radiation therapy alone for limited squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 15:1069–1072
Martenson JAJ, Gunderson LL (1993) External radiation therapy without chemotherapy in the management of anal cancer. Cancer 71:1736–1740
Allal AS, Mermillod B, Roth AD, Marti MC, Kurtz JM (1997) The impact of treatment factors on local control in T2–T3 anal carcinomas treated by radiotherapy with or without chemotherapy. Cancer 79:2329–2335
Boman BM, Moertell CG, O’Connell MJ et al (1984) Carcinoma of the anal canal: a clinical and pathological study of 188 cases. Cancer 54:114–125
Tarantino D, Bernstein MA (2002) Endoanal ultrasound in the staging and management of squamous cell carcinoma of the anal canal: potential implication of a new ultrasound staging system. Dis Colon Rectum 45:16–22
Giovannini M, Bardou VJ, Barclay R et al (2001) Anal carcinoma: prognostic value of endorectal ultrasound (ERUS) Results of a prospective multicentre study. Endoscopy 33:231–236
Roach SC, Hulse PA, Moulding FJ, Wilson R, Carrington BM (2005) Magnetic resonance imaging of anal cancer. Clin Radiol 60:1111–1119
Koh DM, Dzik-Jurasz A, O’Neill B, Tait D, Husband JE, Brown G (2008) Pelvic phased-array MR imaging of anal carcinoma before and after chemoradiation. Br J Radiol 81:91–98
Otto SD, Lee L, Buhr HJ, Frericks B, Hocht S, Kroesen AJ (2009) Staging anal cancer: prospective comparison of transanal endoscopic ultrasound and magnetic resonance imaging. J Gastrointest Surg 13:1292–1298
Glynne-Jones R, Northover J, Olivera J (2009) Anal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20:iv57–iv60
Williams AB, Bartram CI, Halligan S, Marshall MM, Nicholls RJ, Kmiot WA (2002) Endosonographic anatomy of the normal anal canal compared with endocoil magnetic resonance imaging. Dis Colon Rectum 45:176–183
Uronis HE, Bendell JC (2007) Anal cancer: an overview. Oncologist 12:524–534
Williams A, Bartram C, Halligan S (2000) Endoanal ultrasound (Normal and abnormal) Techniques in Gastrointestinal Endoscopy 2: 101–109
Magdeburg B, Fried M, Meyenberger C (1999) Endoscopic ultrasonography in the diagnosis, staging, and follow-up of anal carcinomas. Endoscopy 31:359–364
Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Gastrointestinal Imaging 227:371–377
Drudi FM, Giovagnorio F, Raffetto N, Ricci P, Cascone F, Santarelli M, Trippa F, Passariello R (2003) Transrectal ultrasound color Doppler in the evaluation of recurrence of anal canal cancer. Eur J Radiol 47:142–148
Acknowledgements
Work originated from Guys and St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, United Kingdom.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parikh, J., Shaw, A., Grant, L.A. et al. Anal carcinomas: the role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur Radiol 21, 776–785 (2011). https://doi.org/10.1007/s00330-010-1980-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1980-7