Abstract
To correlate posttreatment radiologic and pathologic findings in patients who underwent transarterial chemoembolization before transplantation or resection. Thirty-five patients with postchemoembolization follow-up imaging underwent liver transplantation/resection. Pre- and posttreatment contrast-enhanced magnetic resonance imaging were used to evaluate radiologic findings. Imaging characteristics using World Health Organization (WHO) and European Association for the Study of the Liver (EASL) criteria after treatment were evaluated. Treated lesions were examined by pathology (gold standard) for the assessment of necrosis. Radiologic findings on magnetic resonance imaging were correlated to pathologic findings to assess the predictability by imaging of actual necrosis. Kappa (κ) statistics were used to determine intermethod agreement between WHO and EASL criteria. Fourteen (40%) of 35 lesions had biopsy-proven hepatocellular carcinoma. Thirteen (37%) of 35 target lesions showed complete pathologic necrosis. Complete pathologic necrosis was seen in 35% of lesions with pretreatment size <3 cm. Complete pathologic necrosis was seen in 1 (100%) of 1, 6 (67%) of 9, 6 (33%) of 18, and 0 (0%) of 7 of the lesions that exhibited complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) by WHO criteria, respectively. Complete pathologic necrosis was seen in 9 (82%) of 11, 4 (36%) of 11, 0 (0%) of 8, and 0 (0%) of 5 of the lesions that showed CR, PR, SD, or PD by EASL criteria, respectively. EASL CR and WHO response were shown to have ≥85% specificity for predicting complete pathologic necrosis. The κ coefficient for agreement between WHO and EASL was 0.29. EASL and WHO criteria had minimal intermethod agreement. EASL CR and WHO response were able to predict pathologic necrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide incidence of hepatocellular carcinoma (HCC) is increasing [1]. Orthotopic liver transplantation (OLT) and resection remain the only available curative options for patients whose disease falls within the Milan criteria [2]. Unfortunately, the limited number of organs, advanced stage of disease, and medical comorbidities limit the role of curative options [3]. Locoregional therapies have been shown to bridge and downstage disease to permit OLT [3–5].
Diffusion-weighted magnetic resonance imaging (MRI) has been shown to detect histologic necrosis after chemoembolization [6, 7]. However, anatomic imaging guidelines are still considered the standard for response measurement after therapy for HCC [8]. The purpose of this study was to correlate patterns of imaging findings in HCC patients after chemoembolization with the level of tumor necrosis achieved. It has long been speculated that posttreatment decrease in size and amount of enhancing tissue on imaging represents tissue necrosis. There are few studies supporting this concept that use strict radiologic criteria for assessing the correlation of imaging response and explant pathology as the gold standard [9, 10]. World Health Organization (WHO) size criteria (which measure change in tumor size) and European Association of Study of the Liver (EASL) necrosis criteria (which measure change in amount of enhancing tissue only) were used to assess tumor necrosis by imaging [11, 12]. Pathologic assessment of the explanted tissue was used as the gold standard for assessing degree of necrosis. This is important information because transarterial chemoembolization is considered one of the standards of care for HCC.
Materials and Methods
This is a single-institution retrospective analysis of consecutive HCC patients who underwent chemoembolization as the sole bridging therapy and went on to receive OLT/resection. This study was performed following an institutional review board–approved protocol.
Pretreatment Evaluation
All patients underwent baseline assessment including history, laboratory tests (liver functions tests and analysis of alfa-fetoprotein levels), and contrast-enhanced MRI. Biopsy was performed to confirm HCC only if the lesion was >2 cm but did not meet the American Association for the Study of Liver Diseases and EASL criteria [11, 13, 14], and if the maximum dimension of the lesion was 1–2 cm [11]. The disease of all patients was classified according to Child-Pugh, United Network for Organ Sharing (UNOS), and Barcelona Clinic Liver Cancer (BCLC) classification systems [13, 15]. To minimize understaging bias, radiologists performing baseline assessments were blinded to whether patients had undergone OLT or resection [16]. All scans were evaluated by three expert radiologists (R.J.L., F.H.M., R.S.) who were well versed in the radiologic changes that follow locoregional therapies. Any ambiguity was clarified by consensus.
Patients with early/intermediate HCC (BCLC class A/B) were classified as BCLC class C (advanced) only if they exhibited cancer-related symptoms (e.g., pain). The presence of extrahepatic metastases is an absolute contraindication for OLT/resection [2]. For this analysis, we applied the imaging criterion of lymph node >2 cm in size as a sign of extrahepatic disease [17]. Index lesions were identified and measured; response assessment was based on the measurement of the index lesion only, which was defined as the largest measurable lesion that was targeted during the first treatment session.
Chemoembolization
The selection of chemoembolization was based on the consensus of a multidisciplinary team in conference. Patients underwent diagnostic angiography at treatment to determine vascular anatomy and variants, and to assess portal flow [18]. The preferential blood supply by the hepatic artery to HCC forms the basis of the safety and efficacy of transarterial therapies such as chemoembolization [19]. Thirty milligrams of doxorubicin, 30 mg of mitomycin, and 100 mg of cisplatin were mixed with 10 ml of lipiodol and selectively (distal to lobar artery) injected to minimize injury to normal parenchyma [20]. This was followed by the injection of bland embolic particles to increase dwell time of the chemotherapeutic agents [18].
Posttreatment Evaluation
Before the advent of magnetic resonance imaging, response assessment after chemoembolization by computed tomographic scan has been difficult because measuring necrosis may be obscured by the radiopaque lipiodol and because decrease in or lack of contrast enhancement is a direct result of occlusive macroembolization and not necessarily tumor death [21, 22]. This study used gadolinium-enhanced MRI to determine EASL response because the lipiodol has minimal effect on signal intensity [9, 23, 24]. Patients had MRI 1 month after treatment to assess response in the treated lesion, and subsequently every 3 months [11]. Postchemoembolization characteristics on T1-weighted postcontrast MRI demonstrated lack of enhancement due to necrosis [9, 25].
WHO/EASL responses were only categorized as such if maintained up to the last scan before explantation [16]. For the purposes of this study, response rates refer to combined rates of complete response (CR) and partial response (PR).
Tumor Size
Index lesions were classified into three groups on the basis of pretreatment imaging (1–2.9, 3–5, and >5 cm) [16]. This was done to assess imaging response/pathological analyses stratified by baseline tumor size.
WHO Size Criteria
Forner et al. concluded in a recent study that application of the unidimensional Response Evaluation Criteria in Solid Tumors criteria played a limited role in assessing treatment response [12]. Thus, response according to size was assessed by WHO criteria. These criteria measure changes in the sum of the cross-products of the lesion after treatment, irrespective of the amount of enhancing tissue. CR indicates 100% decrease in size (complete disappearance of all lesions); PR, ≥50% decrease the sum of the cross-products of all lesions; and progressive disease (PD), >25% increase in the sum of cross-products of all lesions from maximum response. All other findings were classified as stable disease (SD) [11, 16, 26].
EASL Necrosis Criteria
Assessing size without necrosis plays a limited role in determining response [11, 22]. Thus, the amount of enhancing tissue in the treated lesion was also studied [23]. EASL criteria are defined as follows: CR, absence of any enhancing tissue; PR, ≥50% decrease in enhancing tissue; and SD, <50% decrease in enhancing tissue [11, 12, 16]. PD according to EASL was defined as any increase in the amount of enhancing tissue that clinically would translate into additional therapy such as repeat chemoembolization. A conservative approach was used in reporting EASL CR; any enhancing tissue, no matter how minimal, in a lesion that seemed to be nearly completely necrotic was classified as PR. Given the difficulty in determining necrosis by EASL criteria in hypovascular tumors, they were classified as EASL SD unless they met the criteria for PD.
Pathologic Evaluation
The explant was evaluated by pathology for evidence of gross and histologic necrosis. One-centimeter sections of the treated lesion were taken following our institutional protocol of serial sectioning of the liver; routine hematoxylin and eosin staining was used to prepare the slides. This protocol has been used in previous studies [27]. The target lesion was examined for the presence of viable neoplastic tissue. Percentage of necrosis of the treated lesions was tabulated using the following classification as described by the pathologist: 100% necrosis, absence of any viable tissue; >50% necrosis, significant necrosis with presence of any viable tissue; and <50% necrosis, minimal necrosis [16]. A conservative approach was used in reporting pathologic necrosis; if any viable malignant cells were seen in the treated lesion, that tumor could not be classified as 100% (complete) pathologic necrosis.
Statistical Analyses
Fisher’s exact test was used to determine the statistical significance between the differences observed in the imaging and pathologic parameters. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated for the favorable radiologic changes after treatment, and the gold standard for calculation was taken as 100% pathologic necrosis [16]. Kappa (κ) statistics were used to determine the intermethod agreement between WHO size and EASL necrosis criteria [12].
Results
Patient Sample
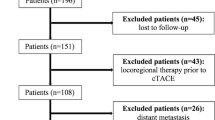
A total of 172 patients received chemoembolization for HCC over a 9-year period. A chart review was performed after obtaining institutional review board approval. This study was Health Insurance Portability and Accountability Act compliant. Thirty-five consecutive patients who were treated with chemoembolization only and had posttreatment follow-up imaging subsequently underwent OLT (n = 33) or resection (n = 2). This permitted pathologic analysis of the treated lesions and a retrospective correlation to the radiologic findings. Figure 1 presents a summary of the analysis.
The demographics of the patient population for this study are presented in Table 1. The median age was 55 (range, 48–79) years; most patients were men. The majority of disease was classified as Eastern Cooperative Oncology Group performance status 0 (63%); 48.5% were classified as Child-Pugh class A. Disease of 60% of patients was diagnosed by imaging. Forty percent had biopsy-proven HCC. One patient had lymphadenopathy at baseline. Disease of 51% was classified as BCLC class A; 40%, BCLC class B; 6%, BCLC class C; and 3%, BCLC class D. At the time of treatment, 3% of disease was UNOS stage T1; 51%, UNOS stage T2; 26%, UNOS stage T3; 17%, UNOS stage T4a; 3%, UNOS stage T4aN. One patient underwent transplantation despite the retrospective blinded assessment showing one lymph node >2 cm at baseline. After chemoembolization, there was no change in UNOS stage except for four of the nine T3 patients, whose disease was downstaged to T2. Thirty-three (94%) of 35 of the index lesions were hypervascular at baseline MRI.
Treatment and Follow-up
The median number of treatments delivered per patient was two (95% confidence interval [95% CI], 1.9–2.1). The median time between treatments was 1.9 months (95% CI, 1.4–2.8). The median number of treatments delivered to the target lesion was two (95% CI, 1–2). Thirteen (37%) of 35 patients received one treatment to the target lesion. Median time from time of first treatment to explantation was 6.6 months (95% CI, 3.9–9.4).
Tumor Response
The median time from the last cross-sectional imaging scan before explantation to the time of explantation was 1.5 months (95% CI, 0.9–1.9).
Radiologic Findings
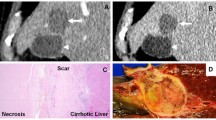
Table 2 summarizes the degree of posttreatment radiologic findings stratified by baseline tumor size. One lesion demonstrated WHO CR in our analysis. The complete disappearance of a lesion (CR by WHO guidelines) is extremely rare after therapy for HCC. The six lesions with maximum diameters of >5 cm had a mean diameter of 6.5 (range, 5.1–16.5) cm. Twenty-six (74%) of 35 lesions showed decrease in size after treatment. Ten (29%) of 35 lesions responded according to WHO criteria. For patients with >4 months’ imaging follow-up, the WHO response rate was 36%. EASL CR and PR were achieved in 11 (31%) and 11 (31%) of 35 lesions, respectively. Figure 2 shows a lesion demonstrating EASL CR and WHO PR after chemoembolization.
Pathologic Findings
Table 2 also presents the degree of pathologic necrosis in tumors stratified according to the pretreatment maximum dimension. Lesions with the maximum diameter between 1–2.9, 3–5, and >5 cm had 35, 42, and 33% complete pathologic necrosis, respectively. All lesions showed some degree of pathologic necrosis at explant. “Ghost cells” indicative of coagulative necrosis are seen in Fig. 3. Thirty-seven percent showed complete pathologic necrosis, 29% showed >50% necrosis, and 34% showed < 50% pathologic necrosis.
Effect of Time Between Treatment and Explant
Table 3 demonstrates the effect of the duration between chemoembolization and explantation of the liver on degree of pathologic necrosis in the treated lesion. Organs that were explanted at <3, 3–6, and >6 months’ postchemoembolization had complete pathologic necrosis in 1 (17%) of 6, 6 (55%) of 11, and 6 (33%) of 18 target lesions, respectively.
Effect of Time Between Treatment and Radiologic Evaluation
Table 3 also presents the evolution of radiologic characteristics with time after first treatment. Median time to WHO response was 9.6 months (95% CI, 7.3–12.5). Median time to EASL response was 3 months (95% CI, 1.5–9.1). Percentage of lesions showing response (EASL or WHO) was seen to increase with time.
Correlation of Radiologic and Pathologic Findings
Table 4 presents the degree of pathologic necrosis in lesions according to response observed by WHO and EASL criteria. By applying WHO criteria, CR, PR, SD, and PD were found to have complete pathologic necrosis in 100, 67, 33, and 0% of the lesions, respectively. By applying EASL necrosis criteria, CR, PR, SD, and PD had complete pathologic necrosis in 82, 36, 0, and 0% of the lesions, respectively.
The ability of the combined effect of WHO and EASL response to predict pathologic necrosis is shown in Table 4. For all 35 patients, lesions that demonstrated both EASL and WHO PR (n = 4) showed complete pathologic necrosis in 75% of the cases. Lesions that showed both WHO and EASL PD (n = 5) after chemoembolization showed < 50% necrosis in 100% of the cases.
Table 5 summarizes the sensitivity, specificity, PPV, and NPV of radiologic responses by EASL/WHO criteria as predictors of necrosis. EASL response had 100% NPV and sensitivity. EASL CR had 82% PPV and 91% specificity. WHO response showed 70% PPV and 86% specificity. The specificity and PPV increased upon application of stricter criteria, such as ≥60% decrease in size by WHO criteria. Lesions demonstrating both EASL and WHO response were studied. Specificity and PPV of the combination of WHO and EASL response was 91 and 78%, respectively.
The κ coefficient for the intermethod agreement between WHO size and EASL necrosis criteria was found to be 0.29 (95% CI, 0.07–0.51).
Discussion
The development of effective locoregional therapies for HCC necessitates the ability to accurately assess treatment response. There is inconsistency in the imaging characteristics seen after ablative and transarterial locoregional therapies. Thirty-five HCC patients treated with chemoembolization who underwent OLT/resection were studied for this analysis. This cohort provided the opportunity to analyze the treated lesion pathologically and assess therapeutic efficacy of chemoembolization. Explant pathology was correlated to radiologic findings after treatment to determine the ability to radiologically predict actual necrosis. This study shows that the application of strict radiologic response criteria (EASL and WHO) after chemoembolization have a reasonable ability to predict actual tumor necrosis. These data consolidate the belief that postchemoembolization changes in tumor size and amount of enhancing tissue correspond to actual necrosis. These data are of interest not only to the radiologist performing the interpretation, but also to the whole team involved in the management of HCC patients.
In our series, complete necrosis was seen in 37% of the treated lesions. This was similar to other reports, where 59% of the dominant lesions showed >90% necrosis after chemoembolization [3]. Veltri et al. showed >90% pathologic necrosis in 24% of the lesions after chemoembolization, 80% of the lesions after percutaneous ethanol injection, and in all lesions after chemoembolization and percutaneous ethanol injection [28]. The complete pathologic necrosis rate after radiofrequency ablation according to Lu et al. was 65.7% [29]. Our group recently published a complete pathologic necrosis rate of 61% after radioembolization [16].
The data presented here suggest that pretreatment tumor size does not permit us to predict the degree of pathologic necrosis after chemoembolization. This is contrary to current data presented on radiofrequency ablation and radioembolization, which suggest that smaller tumors show a higher rate of complete pathologic necrosis [16, 29]. The small number of lesions >5 cm in size limits our ability to assess radiologic and pathologic response criteria in this group.
Table 3 may suggest that pathologic necrosis seen after chemoembolization was not time dependent. Six lesions (33%) showed complete pathologic necrosis in the index lesion even when the liver was explanted >6 months after treatment. Five of these six lesions received multiple treatments to the target lesion. These data favor the application of scheduled treatments when using chemoembolization [30]. On the other hand, one (17%) of the six lesions explanted <3 months after treatment showed complete pathologic necrosis. This may indicate that chemoembolization-induced ischemia, which occurs acutely, may play an important role in inducing necrosis. This difference may also indicate a difference in biological behavior. Table 3 suggests that radiologic response (EASL and WHO) seen after chemoembolization evolves with time, but this may be the effect of multiple treatments.
The cohort in this study demonstrated a WHO response rate of 29% (Table 4). Seventy percent of the lesions showing WHO response showed complete pathologic necrosis. Lesions showing SD by WHO criteria showed complete pathologic necrosis in 33% of the lesions. WHO criteria remain important because WHO response may represent the ability of the surrounding parenchyma to regenerate normally. EASL criteria, which measure the amount of enhancing tissue only, should be concomitantly studied to improve the predictability of actual necrosis by radiologic evaluation. The cohort in this analysis had an EASL response rate of 62%. A total of 82% of the lesions showing EASL CR showed complete pathologic necrosis. This is in contrast to complete pathologic necrosis seen in all lesions demonstrating EASL CR after radioembolization. This may be due to vascular occlusion and subsequent decrease in contrast uptake by the tumor from arterial occlusion after chemoembolization. SD and PD by EASL criteria correlated with <50% pathologic necrosis in 75 and 100% of the lesions, respectively. The low intermethod agreement as represented by the low κ value of 0.29 is in keeping with the recently published data by Forner et al. [12]. The data presented may suggest guidelines combining EASL and WHO criteria.
EASL response resulted in 100% sensitivity, while the application of stricter criteria, such as EASL CR, only resulted in 90% specificity (Table 5). A similar trend was seen when stricter criteria, such as ≥60% decrease in cross-product, was applied. The combined effect of EASL and WHO response had higher PPV and specificity than response by either of these criteria applied alone, further supporting the notion of combined EASL and WHO criteria.
We present what is to our knowledge the first comprehensive analysis of radiologic–pathologic correlation after chemoembolization for HCC using EASL necrosis and WHO size criteria. There are limitations to this analysis. The analysis presented is relative to the first treatment. However, 37% of patients received only one treatment to the target lesion, and the radiologic characteristics of the target lesions were followed to transplant in all cases. The small sample size of patients with target lesions that received multiple treatments (n = 22) limited our ability to study the effect of multiple treatments on response. Explantation within a median time of 6.6 months after the first treatment restricted the ability of this analysis to study long-term response or time to progression before OLT. Because the time of OLT is unpredictable, not all lesions (i.e., in the case of multifocal disease) were treated before explantation; the radiologic–pathologic analysis is therefore based only on treated lesions.
Future studies will examine correlation of treatment response to risk of posttransplant HCC recurrence in larger cohorts. In order to eliminate the effect of untreated lesions, studies that focus on solitary hepatomas may be able to better correlate radiologic and pathologic findings after locoregional therapies, as well as long-term outcomes.
In conclusion, the data presented in this study support the notion that decreases in size and amount of enhancing tissue in the target lesion correspond to actual pathologic necrosis. EASL CR and WHO PR correlate well with pathologic necrosis. This study uses similar radiologic response criteria that were used by investigators for radioembolization and suggests that these criteria can reasonably predict actual tumor necrosis after transarterial locoregional therapies [16].
References
El-Serag HB (2002) Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 36(5 suppl 1):S74–S83
Mazzaferro V, Regalia E, Doci R et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699
Maddala YK, Stadheim L, Andrews JC et al (2004) Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl 10:449–455
Kulik LM, Atassi B, van Holsbeeck L et al (2006) Yttrium-90 microspheres (TheraSphere(R)) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 94:572–586
Lu DS, Yu NC, Raman SS et al (2005) Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 234:954–960
Geschwind JF, Artemov D, Abraham S et al (2000) Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol 11:1245–1255
Kamel IR, Bluemke DA, Ramsey D et al (2003) Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol 181:708–710
Llovet JM, Di Bisceglie AM, Bruix J et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711
Hunt SJ, Yu W, Weintraub J, Prince MR, Kothary N (2009) Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol 20:30–38
Waldmann TA, White JD, Carrasquillo JA et al (1995) Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood 86:4063–4075
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Forner A, Ayuso C, Varela M et al (2009) Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 115:616–623
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134:1752–1763
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Llovet JM, Fuster J, Bruix J (2004) The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 10(2 suppl 1):S115–S120
Riaz A, Kulik L, Lewandowski RJ et al (2009) Radiologic–pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 49:1185–1193
Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL (2000) Extrahepatic metastases of hepatocellular carcinoma. Radiology 216:698–703
Coldwell DM, Stokes KR, Yakes WF (1994) Embolotherapy: agents, clinical applications, and techniques. Radiographics 14:623–643
Nakashima T, Kojiro M (1986) Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis 6:259–266
Bruix J, Sala M, Llovet JM (2004) Chemoembolization for hepatocellular carcinoma. Gastroenterology 127(5 suppl 1):S179–S188
Salem R, Thurston KG (2006) Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol 17:1571–1593
Takayasu K, Arii S, Ikai I et al (2006) Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 131:461–469
Kamel IR, Bluemke DA, Eng J et al (2006) The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol 17:505–512
De Santis M, Alborino S, Tartoni PL, Torricelli P, Casolo A, Romagnoli R (1997) Effects of lipiodol retention on MRI signal intensity from hepatocellular carcinoma and surrounding liver treated by chemoembolization. Eur Radiol 7:10–16
Yoshioka H, Nakagawa K, Shindou H et al (1990) MR imaging of the liver before and after transcatheter hepatic chemo-embolization for hepatocellular carcinoma. Acta Radiol 31:63–67
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Marin HL, Furth EE, Olthoff K, Shaked A, Soulen MC (2009) Histopathologic outcome of neoadjuvant image-guided therapy of hepatocellular carcinoma. J Gastrointestin Liver Dis 18:169–176
Veltri A, Grosso M, Martina MC et al (1998) Effect of preoperative radiological treatment of hepatocellular carcinoma before liver transplantation: a retrospective study. Cardiovasc Intervent Radiol 21:393–398
Lu DS, Yu NC, Raman SS et al (2005) Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 41:1130–1137
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359(9319):1734–1739
Author information
Authors and Affiliations
Corresponding author
Additional information
Riad Salem and Reed Omary supported in part by NIH R01 CA126809.
Rights and permissions
About this article
Cite this article
Riaz, A., Lewandowski, R.J., Kulik, L. et al. Radiologic–Pathologic Correlation of Hepatocellular Carcinoma Treated with Chemoembolization. Cardiovasc Intervent Radiol 33, 1143–1152 (2010). https://doi.org/10.1007/s00270-009-9766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9766-5