Abstract
Magnetic resonance imaging (MRI) of the small bowel has become widely accepted at centers dedicated to the diagnosis and treatment of inflammatory bowel disease, due to the method’s diagnostic efficacy. MR enteroclysis is an imaging modality that combines the advantages of enteroclysis and multiplanar MR and allows the detection and the manifestations of small bowel diseases wherever they are located (intraluminal, intramural, or extramural). Magnetic resonance enteroclysis (MRE) is an emerging technique used for the detection and evaluation of small bowel neoplasms. This article illustrates the imaging appearances of small bowel tumors on MRI and the usefulness of MR enteroclysis in the diagnosis and categorization of these tumors, also discussing the role of MRE in comparison with other diagnostic modalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Small bowel tumors are quite uncommon, accounting for less than 5% of all gastrointestinal tract neoplasms, although the small intestine accounts for more than 90% of the mucosal surface area of the gastrointestinal tract [1, 2].
The clinical symptoms are non-specific and for this reason the detection of these tumors represents a challenge for both the physician and the radiologist. A mean delay of up to 3 years from first symptoms has been reported to diagnose benign tumors, and 18 months for malignant neoplasms [3, 4].
A method of small bowel examination with a high negative predictive value and high sensitivity is needed to evaluate for the small bowel tumors owing to the non-specificity of the presenting symptoms.
Magnetic resonance imaging (MRI) is having an increasing diagnostic impact on patients suffering from inflammatory bowel disease [5, 6]. In particular MR enteroclysis offers numerous advantages with its superb soft-tissue contrast resolution, multiplanar imaging capabilities, and the lack of associated ionizing radiation exposure that allow repeated data acquisition over time and thus functional evaluation of the small bowel mobility [7–10]. MRE overcomes the principal disadvantages of conventional enteroclysis, that are the limited indirect information on the state of the bowel wall and extramural extension of small bowel disease, and its effectiveness may be hindered owing to overlapping bowel loops [11, 12, 13].
The differential diagnosis for small bowel tumors is extensive; however, the various tumors have characteristic appearances on MR that can help in the diagnosis.
MR technique
Enteroclysis vs. enterography
Adequate distention of the bowel lumen is mandatory in MRI, as it facilitates demonstration of morphological changes and allows identification of subtle abnormalities. Collapsed bowel loops can hide lesions or mimic disease by suggesting pathologically thickened bowel wall in collapsed segments; moreover, collapsed normal bowel loops can exhibit enhancement after administration of intravenous contrast medium similar to the diseased segments [13].
Different methods have been used to achieve an adequate distention of the entire small bowel for MR imaging [7–16]. Most of the studies published to date on MR imaging of the small bowel have been performed with enteroclysis techniques. Although enteroclysis techniques provide better small bowel distention, there is a paucity of data available comparing the sensitivity of enteroclysis and enterography in various small bowel disorders.
Although some authors have reported that similar diagnostic results can also be obtained if patients simply drink oral contrast, rather than being intubated [17]; according to most of the studies [7, 8, 9–16], enteroclysis is the only method that provides adequate distention of the entire small bowel, and permits visualization of mucosal abnormalities and obtains functional information about the small bowel mobility.
Two major techniques are used to achieve bowel distention using MR: MR enteroclysis (MRE) with infusion of the contrast through a nasojejunal tube and MR enterography with oral contrast administration. MRE provides a superior small bowel distention and the optimal distention of small bowel loops is crucial to evaluate bowel wall pathologies correctly, because collapsed bowel loops can hide lesions or mimic disease by suggesting pathologically thickened bowel wall in collapsed segments, and the visualization of small polypoid masses that do not produce obstruction is difficult [18].
MR enteroclysis delineates superficial changes better than MR enterography in patients with Crohn’s disease, and this aspect has to influence the revealing and localizing of the disease in patients with only superficial manifestations [16]. Evaluation of superficial abnormalities is of particular importance in the depiction of small bowel neoplasm in an early stage.
MR enteroclysis with fluoroscopic sequences can help to determine the distensibility of narrowed areas, to improve the differentiation of contractions from strictures and differentiation between a fixed and an unfixed stenosis [10, 13].
Several authors performed MR enteroclysis as a first line imaging modality [7, 12, 13]. Other authors have chosen to perform enterography as the routine technique for both CT and MR imaging, reserving enteroclysis for selective indications such as low-grade small bowel obstruction when needed [11]. In the author’s opinion both approaches are valid, and the choice should be determined by the clinical setting, the patient population, the radiology practice, and the diagnostic algorithms in different hospitals.
Combing the functional and morphologic capabilities in evaluating intraluminal, mural, and extraparietal findings MR enteroclysis could be the one stop shop modality in the majority of the cases. For these reasons, we prefer to perform MR enteroclysis as the initial evaluation in patients with suspicion of a small bowel neoplastic vs. inflammatory diseases or with obscure gastrointestinal bleeding, whereas MR enterography approach is used for the follow-up of the patients with Crohn’s disease.
Pulse sequences
Several different pulse sequences are available for imaging the small bowel. The main diagnostic sequences can be divided into the T2-weighted sequences that consist of the single-shot HASTE Techniques (single-shot fast spin echo [SSFSE], HASTE, single-shot turbo spin echo) and the balanced gradient echo (Fast Imaging Employing Steady-State Acquisition, True Fast Imaging with Steady-state Precession [FISP], balanced fast field echo) sequences. Contrast enhanced T1-weighted gradient echo sequences with fat suppression also are routinely performed to look for areas of increased enhancement.
Combining T2-weighted half-Fourier rapid acquisition with relaxation enhancement (RARE) or half-Fourier acquisition single-shot turbo spin echo (HASTE) and T1-weighted gadolinium-enhanced spoiled gradient echo (SGE) sequences, it is possible to assess small bowel diseases, because these sequences complement each other for the evaluation of location, extent, and severity of the small bowel diseases.
The lack of magnetic susceptibility artefacts and lack of artefacts from bowel peristalsis theoretically makes the HASTE sequence ideal for imaging bowel. A limitation of HASTE is its sensitivity to intraluminal flow voids, while another disadvantage is that no information on mesenteries can be obtained due to K-space filtering effects [10].
Another sequence promoted for the evaluation of small bowel diseases is the true fast imaging with steady-state precession (true-FISP) sequence, which is the proprietary name of a completely refocused steady-state gradient echo sequence (also called balanced fast-field echo and FIESTA by other vendors) [13]. The true-FISP sequence is particularly good for obtaining information about the mural and extraintestinal complications; the mural ulcers and mesenteries are very well visualized and lymph nodes are very conspicuous with this technique [5, 19]. The black boundary artefact encountered with the true-FISP sequence at fat–water interfaces may hamper the perception of subtle thickening of the bowel wall.
While steady-state sequence with fat saturation has an advantage over the same sequence without fat saturation, because of the elimination of black boundary artefacts. The detection of subtle bowel wall thickness is therefore improved [5].
Malignant peritoneal tissue enhances moderately to substantially on interstitial phase gadolinium enhanced images and appears as nodular or irregular thickened peritoneal or serosal diseases.
Gadolinium-enhanced fat suppressed imaging has been shown to be more sensitive than CT imaging in detecting small tumor nodules [19].
MR enteroclysis appearance of the small bowel tumors
Benign tumors
Benign neoplasms account for approximately 1%–2% of all GI tract tumors; leiomyoma, adenoma, and lipoma constitute the most common primary benign small intestinal tumors [20, 21].
At MR, adenomas appear as a well-defined soft-tissue mass showing homogeneous moderate enhancement on arterial and venous phases, with clear fat planes around the tumor. MR fluoroscopy sequence shows an intraluminal filling with no evidence of prestenotic dilatation.
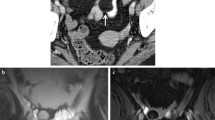
Leiomyoma may be located submucosal, intramural, or subserosal and appear as a sharply defined lesion showing uniform enhancement greater than that of adjacent bowel on post gadolinium images [22]. At MR fluoroscopy the leiomyoma appears as a smooth, round, (or semilunar) mural defect that is demarcated by sharp angles to the intestinal wall (Fig. 1). The typical findings and the absence of mesenteric changes and metastases helps in the diagnosis and mostly rules out malignant differential diagnosis.
Ileal leiomyoma in 37-year-old patient with unexplained gastrointestinal bleeding. Coronal FIESTA sequence (A) shows a well-defined soft-tissue intraluminal lesion (arrow); note that the high signal intensity of intraluminal PEG solution within the terminal ileum clearly delineates the lesion, which appears moderately low in signal intensity. MR fluoroscopic sequence (B) shows reduction of the caliber of terminal ileum without prestenotic dilatation.
Lipomas that arise in the submucosal, are high in signal on T1-weighted images and have signal intensity comparable to intraabdominal fat on T2-weighted images. On T1- and T2-weighted fat-suppressed images these lesions show a loss of signal intensity [23].
Intestinal hemangiomas are usually submucosal tumors, and they are sessile or pedunculated.
Diffuse angiomatosis of the ileum appears as multiple nodules that show low-signal intensity on T1-weighted images and marked hypersignal on T2-weigthed images; central nodular enhancement is seen within the tumor in the arterial phase with centrifugal enhancement on delayed phase (Fig. 2).
Diffuse angiomatosis of the ileum in a 22-year-old patient with recurrent abdominal pain. Axial T2-Haste-Fat Sat (A) shows multiple nodules in the small bowel with marked hypersignal on T2-weigthed images; central nodular enhancement within the tumor is seen in the arterial phase (B) with centrifugal enhancement enhancement on delayed phase (C).
Hemangioma shows relatively low-signal on T1-weighted images and rather higher signal intensity on T2-weighted images with central nodular enhancement into the mass [24].
Polyps can be seen as several soft-tissue masses spread throughout the entire small bowel in patients with polyposis syndromes.
Malignant tumors
Up to 70% of all symptomatic small bowel tumors are found to be malignant [25].
On MR adenocarcinoma may have a variable appearance. It typically appears as a focal mass with intra and extraluminal growth or with circumferential constricting lesions, which narrow the bowel lumen. It presents heterogeneous signal on T2-weighted images and heterogeneous moderate enhancement on gadolinium images [22]. MR fluoroscopy sequence shows the luminal high grade irregular stenosis with a fixed, unchanging appearance during the infusion of the intraluminal contrast (Fig. 3).
Primary adenocarcinoma in a 71-year-old patient with low-grade small bowel obstruction. MR fluoroscopic sequence (A) shows an irregular annular stenosis of the lumen (arrow) with prestenotic dilatation, suggesting the mucosal origin of the neoplasm. Coronal FIESTA (B) sequences show a focal wall thickening (arrows) involving a short segment of ileal small bowel which causes luminal narrowing. Coronal T1-GRE sequence (C) shows a heterogeneous enhancement of the bowel wall with evidence of extraluminal extension.
Carcinoid tumors cause focal, asymmetric bowel wall thickening, and usually manifest as nodular wall thickening or a smooth submucosal mass [25]. On unenhanced sequences, these lesions are isointense to muscle on T1-weighted images and iso or mildly hyperintense to muscle on T2-weighted images. The primary lesions show contrast enhancement. Mesenteric masses range between 2 and 4 cm and are typically isointense to muscle on T1- and T2-weighted images (Fig. 4).
Ileal carcinoid tumor in a 38-year-old patient with unexplained gastrointestinal bleeding. Coronal FIESTA (A) and axial FIESTA with fat saturation (B) shows a lobulated mesenteric mass (arrow) with desmoplastic reaction. Coronal (C) and axial (D) contrast T1-GRE show a marked enhancement of the mass.
As previous reports on ileal carcinoid described the mesenteric mass with radiating strands of tissue; this constellation of imaging findings has been described as not uncommon for these tumors [25].
The characteristic desmoplastic changes in the mesentery and retroperitoneum that occur in response to the secretion of serotonin and tryptophan are low-signal on both T1- and T2-weighted images and show negligible enhancement after contrast [22, 25]. Carcinoid neoplasms rarely cause annular narrowing, but kinking of the bowel wall with narrowing of the lumen.
Gastrointestinal lymphomas comprise 1%–2% of all gastrointestinal malignancies [26, 27] and can assume different gross appearances: (1) diffusely infiltrating lesions that often produce full-thickness mural thickening with effacement of overlying mucosal folds (Fig. 5); (2) polypoid lesions that protrude into the lumen; (3) large, exophytic, and fungating masses that are prone to ulceration and fistula formation (Fig. 6).
Jejunal lymphoma in a 24-year-old patient with celiac disease and chronic abdominal pain. Coronal Haste sequence (A) shows a long segment of jejunum (arrow) with abnormal thickening, smooth margins, and luminal narrowing with loss of normal mucosal folds. Contrast Axial T1-GRE sequences (B–C) show mild wall thickening (arrows) with mesenteric nodes encircling the vessels.
Jejunal lymphoma in a 68-year-old patient with acute abdominal pain. Coronal Haste A sequence shows an irregular mass (arrow) that envelops multiple small bowel segments. Coronal contrast T1-LAVA B sequence shows minimal enhancement of the mass (long arrow) infiltrating the small bowel loop. Note large mesenteric lymph nodes (small arrows in A and B).
The diverse appearances of the small intestine lymphoma on MR studies reflect the gross morphology of the disease. In the setting of diffuse infiltrating lesions, the bowel walls appear dilatated, possibly because of the interference with the normal innervations and regulation of smooth muscle bowel wall contraction. The presence of bowel wall mass and dilatation without proximal bowel obstruction is suggestive of lymphoma. The presence of diffuse splenomegaly and mesenteric and retroperitoneal lymphadenopathy support the diagnosis.
Smooth mural contour, diffuse segmental bowel loop aneurysmal dilatation and absence of a distinct mesenteric or antimesenteric distribution are highly suggestive of the presence of lymphoma in celiac disease patients. An association has been observed between certain mural characteristics and the presence of celiac disease, most notably that of a smooth marginal component (Fig. 5) [26].
On MR, GIST typically appears as an exophytic, sometimes bulky mass, with moderate heterogeneous contrast enhancement, and tends to show central necrosis [22]. GIST can extend to a size of several centimeters, displacing bowel loops. Unlike adenocarcinoma and lymphoma, lymphatic spread does not usually occur in patients with GIST. Mesenteric masses are usually smooth surfaced and do not show spiculation or in drawing of the mesentery. The predominant MR features of GIST are a heterogeneously enhancing exophytic mass, with regions of necrosis.
Metastases account for approximately 50% of all small bowel neoplasms [25]. Indeed in a patient with a known neoplasm, a small bowel neoplasm is most likely a metastasis. Metastases develop through four major pathways: direct extension, intraperitoneal seeding, lymphatic, and haematogenous spreads [22, 28]. Metastatic lesions often lodge on the antimesenteric border of the small bowel [22]. On gadolinium-enhanced fat-suppressed SGE images, hypervascular metastases are moderately high in signal intensity in contrast to the low-signal intensity of intraabdominal fat [28] (Fig. 7). Gadolinium-enhanced fat suppressed imaging has been shown to be more sensitive than CT imaging in detecting small neoplastic tumor nodules [19].
Ovarian metastasis in a 64-year-old patient with unexplained gastrointestinal bleeding. Axial CT A shows a mass (arrow) at the level of medial duodenal wall that looks like a cystic lesion. Axial B Haste sequence shows a rounded lesion with high signal (arrow). Axial GRE sequence c demonstrates enhancement of the lesion (arrow), indicative of the solid nature.
MR enteroclysis and other imaging modality in the assessment of small bowel neoplasms
Radiologists assume a major role in the detection of small bowel tumors [29].
Endoscopy provides the advantage of obtaining biopsies, but only the terminal ileum, the duodenum, and the proximal ileum can be sufficiently explored in routine procedures. Ileoscopy is further compromised by the occasional inability to reach the cecum or to intubate the ileum during colonoscopy. Enteroscopy plays a significant role in the diagnosis of small bowel tumors, but may cover only a limited length of the small intestine and is not commonly available. The push enteroscopy and the double Balloon Enteroscopy are a newly developed endoscopic method allowing full-length exploration of the small bowel [30]. It is, however, invasive, time-consuming, and requires conscious sedation; for these reasons strict selection of patients is required [30].
Wireless capsule endoscopy (WCE) is probably the best method for visualizing mucosal abnormalities [31]. However, it is not very accurate in the estimation of location and size of the intraluminal abnormalities and it is contraindicated in patients suspected with bowel stricture, history of prior small bowel surgery, swallowing disorders, motility disorders, and intestinal obstruction [31, 32]. In patients with small bowel neoplasms capsule retention at the site of the lesion has been described in 10%–25% [33]. Capsule endoscopy can be complementary after a negative MRE in symptomatic patients visualizing tumors at early stages having only mucosal abnormalities.
Another limitation of all endoscopic methods is the inability to visualize submucosal or extramural neoplastic manifestations [31, 33].
For many years, “conventional” double contrast enteroclysis has been suggested as the technique of choice for the evaluation of the small intestine [34]. Adequate distention of the small bowel allows imaging of mucosal abnormalities and provides functional information by defining free peristaltic contraction or fixation of the small bowel loops.
The principal disadvantage of conventional enteroclysis is the limited information about the state of the bowel wall and extramural extension of tumor disease [34].
MDCT enteroclysis shows a good accuracy in the evaluation of small bowel neoplasms [35, 36].
MR enteroclysis was more sensitive in detecting lesions of the small bowel than CT enteroclysis in patients with Crohn’s disease [37] and for these reasons MR seems superior in the detection of segments with only superficial abnormalities. Moreover because of ionizing radiation exposure at CT, imaging can be obtained at only a few points in time, precluding repeated temporal imaging and hence assessment of small-bowel peristaltic activity. For these reasons an intermittent spasm or peristalsis contraction during the examination can also be misdiagnosed as a small bowel neoplasm.
In the author’s opinion MR enteroclysis could be superior in comparison of MDCT for the better soft-tissue contrast, that may be important for detecting subtle areas of pathology, and for the tissue characterization.
MR fluoroscopy sequences provide useful information in determining the distensibility of narrowed areas and improve differentiation of contractions from strictures, the evaluation of the prestenotic dilatation, and small bowel mobility, and in the visualization of findings similar to that obtained with barium studies useful in the differentiation between mucosal, submucosal, and extramural origin.
In conclusion MR enteroclysis with its anatomic, vascular, and functional informations and its ability to assess the intraluminal, mural, and extraluminal neoplastic manifestations could be the optimal imaging procedure for depiction and evaluation of the small bowel tumors.
For these reasons MRE is an accurate method that allows the visualization of small bowel diseases in the majority of the cases and in the author’s opinion should be the preferred diagnostic method in patients with suspected small bowel tumors.
References
Martin RG (1986) Malignant tumors of the small intestine. Surg Clin North Am 66:779–785
North JH, Pack MS (2000) Malignant tumors of the small intestine: a review of 144 cases. Am Surg 66:46–51
Gupta S, Gupta S (1982) Primary tumors of the small bowel: a clinico-pathological study of 58 cases. J Surg Oncol 20:161–167
Gill SS, Heuman DM, Mihas AA (2001) Small intestinal neoplasms. J Clin Gastroenterol 33:267–282
Gore R, Masselli G, Caroline D (2008) Crohn’s disease of the small bowel. In: Gore R, Levine M (eds) Textbook of gastrointestinal radiology. 3rd edn. Saunders Elsevier, Philadelphia, PA, pp 781–806
Furukawa A, Saotome T, Yamasaki M et al (2004) Cross-sectional imaging in Crohn’s disease. Radiographics 24:689–702
Umschaden HW, Szolar D, Gasser J, et al (2000) Small bowel disease: comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology 215:717–725
Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, et al. (2006) Imaging of small intestinal Crohn’s disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol 16(9):1915–1925
Prassopoulos P, Papanikolau N, Grammatikakis J, et al. (2001) MR enteroclysis imaging of Crohn disease. Radiographics 21:161–172
Masselli G, Vecchioli A, Gualdi GF (2006) Crohn disease of the small bowel: MR enteroclysis versus conventional enteroclysis. Abdom Imaging 31:400–409
Fidler J (2007) MR imaging of the small bowel. Radiol Clin N Am 45(2):317–331
Masselli G, Brizi GM, Parrella A, et al. (2004) Crohn disease: magnetic resonance enteroclysis. Abdom Imaging 29(3):326–334
Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Prassopoulos P (2002) MR enteroclysis: technical considerations, clinical applications. Eur Radiol 12(11):2651–2658
Laghi A, Carbone I, Catalano C, et al. (2001) Polyethylenglycol solution as an oral contrast agent for MR imaging of the small bowel. AJR 177:1333–1334
Maccioni F, Bruni A, Viscido, et al. (2006) MR imaging in patients with Crohn disease: value of T2- vs. T1-weighted gadolinium enhanced MR sequences with use of an oral supermagnetic contrast agent. Radiology 238(2):517–530
Masselli G, Casciani E, Polettini E, Gualdi G (2008) Comparison of MR-Enteroclysis with MR-Enterography and Conventional enteroclysis in patients with Crohn’s disease. Eur Radiol 18(3):438–447
Negaard A, Paulsen V, Sandvik L, et al. (2007) A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol 17(9):2294–301
Maglinte DD (2006) Invited commentary. Radiographics 26:657–662
Low RN, Barone RM, Lacey C, et al. (1997) Peritoneal tumor: MR imaging with diluite oral barium and intravenous gadolinium-containing contrast agents compared with unhanced MR imaging and CT. Radiology 204:513–520
Gore RM, Mehta UK, Berlin JW, Rao V, Newmark GM (2006) Diagnosis and staging of small bowel tumours. Cancer Imaging 6:209–212
Sailer J, Zacherl J, Schima W (2007) MDCT of small bowel tumours. Cancer Imaging 7:224–233
Semelka RC, John G, Kalekis N, Burderry DA, Ascher SM (1996) Small bowel neoplastic disease:demonstration by MRI. JMRI 6: 855–860
Kim KW, Ha HK (2003) MRI for small bowel diseases. SEM Ultrasound CT MR 24:387–402
Kazama T, Kurihara Y, Tani I, et al. (2000) MR appearance of the small bowel hemangioma J CAT 24(4): 655–656
Maglinte DD, Lappas JC, Sandrasegaran (2008) Malignat Tumors of the small bowel. In: Gore R, Levine M (eds) Textbook of gastrointestinal radiology. 3rd edn. Saunders Elsevier, Philadelphia, PA, pp 853–869
Lohan DG, Alhajeri AN, Cronin CG, Roche CJ, Murphy JM (2008) MR enterography of small bowel lymphoma: potential for suggestion of histologic subtype and the presence of underlying celiac disease. AJR 190:287–293
Chou CK, Chen LT, Sheu RS et al (1994) MRI manifestations of gastrointestinal lymphoma. Abdom Imaging 19:495–500
Masselli G, Brizi MG, Restaino G, Vecchioli A (2004) MR enteroclysis in solitary ileal metastasis from renal cell carcinoma. AJR 182:828–829
Maglinte DD (2005) Capsule imaging and the role of radiology in the investigation of diseases of the small bowel. Radiology 236:763–767
Cazzato IA, Cammarota G, Nista EC, et al. (2007) Diagnostic and therapeutic impact of double-balloon enteroscopy (DBE) in a series of 100 patients with suspected small bowel diseases. Dig Liver Dis 39:483–487
Fork FT, Aabakken L (2007) Capsule enteroscopy and radiology of the small intestine. Eur Radiology 17:3103–3111
Hara AK, Leighton JA, Sharma VK, Heigh RI, Fleischer DE (2006) Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiology 238(1):128–134
Pennazio M, Rondonotti E, De Franchis R (2008) Capsule endoscopy in neoplastic diseases. World J Gastroenterol 14(34):5245–5253
Nolan DJ, Traill ZC (1997) The current role of barium examination of the small intestine. Clin Radiol 52:809–820
Pilleul F, Penigaud M, Milot L, et al. (2006) Possible small-bowel neoplasm: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology 241:796–801
Boudiaf M, Jaff A, Soyer P, et al. (2004) Small bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology 233:338–344
Albert JG, Martiny F, Krummenerl A et al (2005) Diagnosis of small bowel Crohn’s disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut 54(12):1721–1727
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masselli, G., Gualdi, G. Evaluation of small bowel tumors: MR enteroclysis. Abdom Imaging 35, 23–30 (2010). https://doi.org/10.1007/s00261-008-9490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-008-9490-7