Abstract
Although the small intestine accounts for over 90% of the surface area of the alimentary tract, tumors of the small intestine represent less than 5% of all gastrointestinal tract neoplasms. Common small bowel tumors typically are well evaluated with cross-sectional imaging modalities such as CT and MR, but accurate identification and differentiation can be challenging. Differentiating normal bowel from abnormal tumor depends on imaging modality and the particular technique. While endoscopic evaluation is typically more sensitive for the detection of intraluminal tumors that can be reached, CT and MR, as well as select nuclear medicine studies, remain superior for evaluating extraluminal neoplasms. Understanding the imaging characteristics of typical benign and malignant small bowel tumors is critical, because of overlapping features and associated secondary complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small bowel neoplasms are uncommon, representing less than 5% of all tumors of the gastrointestinal tract [1,2,3]. The frequency of small bowel tumors diminishes more distally in the alimentary tract: duodenal, jejunal, then ileal [1]. However, certain tumors have a predilection for particular portions of the small bowel, such as duodenal adenocarcinoma or ileal neuroendocrine tumor. Presentation of small bowel tumors can be incidental in asymptomatic patients or related to vague symptoms of abdominal pain, weight loss, or GI bleeding. Complications such as intussusception, obstruction, or perforation can occur with either benign or malignant neoplasms; however, they are more common with the latter.
Benign lesions offer imaging clues to their nonaggressive nature. Typical findings suggestive of benignity are rounded, well-circumscribed lesions with smooth margins and homogeneous appearance on all imaging modalities [4]. Ulceration and bleeding are not uncommon features, however, and can confound the diagnosis. Malignant neoplasms of the small bowel most often demonstrate heterogeneous enhancement or signal intensity, with irregular margins and invasion of surrounding structures/tissues [5]. More obvious signals of malignancy include regional metastatic adenopathy and distant metastases.
Imaging of the small bowel is hampered by innate challenges, such as peristalsis and the natural mobility of abdominal structures. Traditional barium small bowel follow-through exams have not been shown to reliably detect small bower tumors [6]. Enteroscopy/enteroclysis, in contrast, has demonstrated an excellent ability to detect small bowel tumors, but it is more challenging for the radiologist to perform and the patient to endure [6]. Routine and capsule endoscopy are superior to cross-sectional imaging for detection of small intraluminal lesions [6, 7]. However, mural/extraluminal and larger luminal small bowel tumors can be identified and classified confidently with computed tomography (CT) or magnetic resonance (MR) imaging. Cross-sectional imaging provides the added benefit of completely evaluating the mural and extramural extent of the disease process [8]. The imaging goal is two-fold: (1) to differentiate bowel lumen from non-lumen, and (2) provide maximum contrast between normal bowel tissue and the abnormal bowel mass [9].
In this pictorial essay, we review the imaging features of typical small bowel neoplasms, summarized in Table 1. Small bowel tumors discussed in this review include the benign—lipoma, polyp, leiomyoma, and gastrointestinal stromal tumor (GIST)—as well as malignant—malignant GIST, lymphoma, neuroendocrine tumor, metastatic disease, and primary small bowel adenocarcinoma.
Imaging technique
Its near-universal availability and its speed make CT the most frequent primary imaging option. CT is less susceptible to motion artifact, while still providing a high degree of spatial resolution, and CT can evaluate the entirety of the abdominopelvic structures in a single exam. Limitations include exposure to ionizing radiation (of particular concern in younger patients), and the necessity of intravenous iodinated contrast administration, with its potential risks of adverse reactions and potential renal function compromise.
At our institution, an enterography protocol is the preferred CT approach for suspected small bowel tumors [10]. Patients must abstain from food and drink for at least 4–6 h prior to the study. Adequate distention of the small bowel lumen is key to accurate interpretation; hence, oral contrast is essential. While traditional, “positive” oral contrast agents, such as diluted iohexol (GE Healthcare, Chicago, IL), can be effective in the evaluation of small bowel tumors, “negative” oral contrast agents such as Breeza (Beekely Medical, Bristol, CT) or Volumen (Bracco Diagnostics, Monroe Township, NJ) are preferred [11]. Negative oral contrast agents generally allow superior assessment of mucosal enhancement, mural thickness, and the mesenteric vasculature [12]. Typically, 1.5–2 L of the agent are ingested. A helically acquired scan is performed from the lung bases to the femoral heads, following the administration of intravenous (IV) contrast. An arterial and/or venous phase of IV contrast can be performed with multiplanar reformatted images also obtained.
MR enterography is a viable alternative in the cross-sectional evaluation of small bowel neoplasms. As with CT enterography, patients should abstain from all food and drink for 4–6 h prior to the exam, and 1–1.5 L of Breeza or Volumen are also consumed prior to the exam to ensure adequate bowel distention. At our institution, 0.5–1.0 mL IV glucagon is also administered to decrease bowel peristalsis, additionally minimizing motion artifact. Centered on the bowel, a multiplanar, multisequence acquisition is obtained pre and post administration of an intravenous gadolinium-based contrast agent.
Images are acquired at multiple points in time, mitigating the effect of motion or bowel contraction in obscuring small bowel pathology, and thereby increasing the sensitivity for detecting small bowel tumors with MR. Some tumors demonstrate inherently different signal characteristics from adjacent normal small bowel, which can also increase tumor conspicuity. Lack of ionizing radiation is a key differentiator from CT and an important consideration for all age groups. Lengthy exam duration, variable availability, and a relative higher exam cost are notable downsides. Also, with an enterography technique, the abdominopelvic organs may not be as fully characterized compared to other dedicated MR exams [13].
Benign tumors
Benign small bowel tumors account for approximately 2% of all gastrointestinal tract neoplasms [9]. The most common benign small bowel tumors discussed in this review include lipoma, leiomyoma, polyp, and GIST. Clinical presentation is variable with up to 50% of patients asymptomatic.
Lipoma
Lipomas make up 2–3% of benign tumors of the GI tract [14]. Approximately 20–25% of lipomas involving the gastrointestinal tract are located within the small bowel, second only to the colon, although they can arise anywhere along the GI tract [15]. These tumors composed of adipose tissue are frequently identified in isolation as sessile submucosal lesions, though they can be pedunculated [16]. Well-encapsulated, homogeneous lesions of fat attenuation (− 60 to − 120 HU) on CT and uniform fat signal intensity on all MR sequences with absence of IV contrast enhancement are typical characteristics (Fig. 1).
Axial contrast-enhanced CT image (a) demonstrates a homogeneous, fat-density mass in the wall and lumen of the ileum (arrow), consistent with a lipoma. Coronal T2 weighted MR image (b) and steady-state free precession MR image with fat saturation (c) of the same patient illustrates how the lipoma follows the signal of fat
Lipomas carry no risk of malignant degeneration. Although rare, lipomas can present as a lead point for development of intussusception, and they are the most common benign tumor to do so [17]. Bowel obstructions, volvulus, or gastrointestinal bleeding are exceedingly uncommon associations; however, they most frequently occur in lesions larger than 2 cm [14]. Endoscopic or surgical resection is warranted in those tumors leading to such complications. In the absence of symptoms, treatment or routine surveillance of small bowel lipomas is not indicated [16].
Polyp
Polyps account for up to 20% of benign small bowel neoplasms, with their overall prevalence related to the presence of any underlying syndrome or inflammatory bowel disease [9, 16]. Pathologic subtypes include hamartomatous, hyperplastic, adenomatous, and inflammatory [18]. Polyps can be sporadic or multiple, but in affiliated genetic syndromes such as familial adenomatous polyposis (FAP), Gardner, Turcot, Lynch, and Peutz-Jegher, they are often quite numerous. Of particular concern is the adenomatous subtype, because of its association with polyposis syndromes and risk of malignant transformation [19]. Polyps generally are small (less than 2 cm), homogenous enhancing masses that protrude into the bowel lumen (Fig. 2) [4]. Given that polyps are frequently multiple, the presence of numerous, otherwise indeterminate, small bowel masses makes polyps a likely diagnosis.
Coronal contrast-enhanced CT image (a) in a patient with familial adenomatous polyposis demonstrates numerous soft tissue density masses scattered throughout the duodenum and small bowel (arrows), consistent with adenomatous polyps. Coronal T2 weighted MR image (b) of the same patient shows that the polyps are iso- to hypointense to the bowel wall
Though most often asymptomatic, polyps may grow large enough to lead to obstruction or intussusception. The risk of malignant transformation in small bowel adenomas rises when they exceed 1 cm in size [16]. Detection of malignant degeneration within a polyp is difficult, though a combination of size > 2 cm plus extraserosal extension are suggestive.
Asymptomatic polyps not affiliated with a polyposis syndrome and less than 2 cm in size are generally not treated unless able to be resected endoscopically. Larger polyps lending a greater malignant potential, or those causing obstructive symptoms, necessitate surgical removal [20]. In regards to polyposis syndromes, management recommendations vary by guidelines, though attempt to remove all polyps greater than 1 cm is the current consensus [21].
Leiomyoma
While accounting for 20–30% of benign tumors in the gastrointestinal tract [22], small bowel leiomyomas are rare mesenchymal neoplasms. The tumor has a predilection for the esophagus, though in the small bowel, they are more frequently in the jejunum rather than the ileum [23]. Leiomyomas typically appear as well-circumscribed, homogeneous, enhancing soft tissue masses [24]. Calcification (Fig. 3) and ulceration are more often seen with larger tumors [9].
Unfortunately, it is virtually impossible to differentiate a leiomyoma from GIST based on cross-sectional imaging alone, especially if necrosis and ulceration are present. The distinction between these two entities is important as GISTs have a greater malignant potential. Because of their propensity to ulcerate and bleed, similar to GIST, small bowel leiomyomas can give rise to pain, hemorrhage, or anemia [25]. Size greater than 6 cm and the presence of irregular margins, with or without surrounding lymphadenopathy, raises suspicion for malignancy—either a malignant GIST or, less frequently, leiomyosarcoma [26]. Treatment consists of surgical resection for lesions with suspicious features and larger lesions leading to recurrent bleeding or bowel obstruction [20].
Benign/malignant tumors
GIST
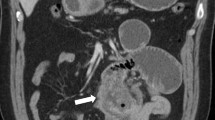
GIST is the most common mesenchymal neoplasm of the gastrointestinal tract, including both benign and malignant varieties, and they are most commonly seen in patients over 40 years of age [27]. They occur throughout the small bowel, classically as a well-marginated mass of variable size. Though they begin as mural masses, they can protrude into the lumen (Fig. 4) or grow exophytically. When presenting as an extraserosal mass, identifying the origin can be challenging. Tumors can be very large and markedly exophytic, with a heterogeneous appearance on CT and MR because of hemorrhage and necrosis. Calcification sometimes occurs, similar to leiomyoma (Fig. 5) [28]. These tumors are often hypervascular following IV contrast administration and can be hypermetabolic on PET imaging [29].
Axial (a) and coronal (b) contrast-enhanced CT images show a well-marginated mass (thin arrow) with a punctate calcification (arrowhead) and prominent arterial supply (thick arrow), consistent with GIST. This was subsequently resected. However, axial T2 weighted, fat saturated MR image (c) and axial T1 weighted post-contrast MR image (d) from a later study in the same patient demonstrate a new T2 intense, enhancing mass in the left abdomen (thin arrows), consistent with malignant GIST recurrence, confirmed by a subsequent surgical resection
CT imaging features can be unreliable in differentiating benign from malignant GISTs. Tumor size is particularly telling, with diameter > 10 cm a strong indicator of malignancy [30]. While even larger tumors tend to displace adjacent structures rather than invade [31], unequivocal local invasion is a specific indicator of malignancy. Malignant GISTs can present with metastases to the liver, omentum, and peritoneum [32]. Lymphatic spread and retraction of the mesentery are unusual features compared to other malignancies affecting the small bowel [4]. Recurrent disease following surgical resection is not uncommon in the setting of malignant GIST (Fig. 5c, d). All GISTs are now viewed as potentially malignant; therefore, if possible, surgical resection is warranted for all lesions regardless of size [33]. The use of adjuvant and neo-adjuvant therapy, particularly utilizing molecular-targeted therapy with Imatinib, may also play a role and depends upon the extent of disease [34].
Malignant tumors
The majority of symptomatic tumors, as high as 70%, are found to be malignant [3]. Often the disease process is not amenable to surgical intervention at the time of diagnosis, lending a poor prognosis. Hereditary conditions, inflammatory bowel disease, and conditions leading to immunosuppression are identifiable risk factors. Malignant tumors discussed in this review include neuroendocrine tumors, metastatic disease, adenocarcinoma, and lymphoma.
Location, depth of penetration, presence of nodal or systemic metastases, and histological tumor grade, if available, are a few of the considerations in the multifactorial approach to management of malignant small bowel tumors. Complete surgical resection is the aim for small bowel carcinoma, neuroendocrine tumors, and lower stage lymphoma, sometimes requiring more aggressive resections or multistage procedures depending on the extent of local or metastatic involvement [35]. In most cases, chemotherapy and/or radiation are often reserved for more advanced stage III or IV disease. Controversy surrounds the role of surgery even in early localized stages of lymphoma, with the need for surgical intervention diminishing as oncological treatment options have become more sophisticated. Accordingly, advanced stages of lymphoma are not considered surgical candidates unless for palliation purposes [35].
Neuroendocrine tumor
Well-differentiated neuroendocrine tumors (carcinoid tumors) originate from enterochromaffin cells within the gastro-entero-pancreatic and bronchopulmonary systems [36]. Gastrointestinal neuroendocrine tumors are rare in the general population, representing approximately 0.5% of all human cancers [37]. Reported as the second most common malignancy of the small bowel, neuroendocrine tumors account for approximately 20–25% of malignant neoplasms [38]. Approximately 30% of gastrointestinal tract neuroendocrine tumors arise from the small bowel with an affinity for the ileum [39]. A solitary enhancing mass within the small bowel wall/mucosa is the typical CT description (Fig. 6), though detection can be challenging. All too often, the initial scan depicts a spiculated mesenteric metastasis and avidly enhancing liver metastases, frequently without identification of the primary intraluminal small bowel tumor (Fig. 7) [40]. However, the primary tumor can be more conspicuous on MR compared to CT because of the former’s greater soft tissue contrast resolution [41].
Coronal contrast-enhanced CT image (a) demonstrates an enhancing mass in the jejunem (thin arrow) with an adjacent spiculated mesenteric metastasis (thick arrow), consistent with metastatic neuroendocrine tumor. This image is displayed with a narrow window specifically to increase the conspicuity of the small bowel primary. Also noted is a liver metastasis (asterisk) in the left hepatic lobe. Coronal T1 weighted post-contrast MR image (b) illustrates these findings as well. Compared to both, however, the primary bowel tumor is most conspicuous on the accompanying coronal T2 weighted MR image (c)
Mesenteric spread, either by direct extension or through the local lymphatics, occurs in approximately 40%–80% of gastrointestinal neuroendocrine tumors [42]. Mesenteric metastases calcify in approximately 70% of cases [43]. Additional characteristic features include a desmoplastic reaction surrounding the mesenteric lesions, with traction and tethering of adjacent small bowel, and possible encasement of mesenteric vasculature [44]. Though these features are suggestive of neuroendocrine lesions, similar findings can also be found with treated lymphoma or retractile mesenteritis [45].
Somatostatin-analog nuclear medicine imaging exams are useful in both diagnosing and staging disease. While traditional exams, such as 111In-pentetreotide imaging (Octreoscan), were long considered the standard for nuclear medicine imaging of neuroendocrine tumors (Fig. 8), newer analog agents such as 18F-FDOPA and 68Ga-DOTATATE are assuming a primary role for tumor detection and response to treatment (Fig. 9) [46, 47].
Whole body 24 h planar image (a) from an 111In-pentetreotide scan (Octreoscan) demonstrates a small focus of abnormal scintigraphic activity in the midline of the abdomen (arrowheads), consistent with a neuroendocrine tumor. Coronal T1 weighted post-contrast MR image (b) demonstrates a horseshoe-shaped mass in the ileum (arrow), corresponding with the finding seen on the Octreoscan. Whole body 48 h planar image from the same Octreoscan (c) demonstrates repositioning of the abnormal focus of scintigraphic activity to the right lower quadrant. Oblique coronal contrast-enhanced CT image (d) shows the same mass as in panel (b) has relocated to the right abdomen. Endoscopic image from the same patient (e) shows the mass. Upon surgery, a single small bowel neuroendocrine tumor was identified and resected
Axial fused image from 68Ga-DOTATATE PET-CT (a) shows a focus of increased radiotracer accumulation in the distal jejunum (thin arrow), corresponding with the primary tumor in this patient with known metastatic neuroendocrine tumor. Diffuse nonspecific lower-level radiotracer accumulation is also present in the more proximal jejunum (thick arrows) and liver (L). Corresponding axial T1 weighted post-contrast MR image (b) demonstrates only subtle enhancement and loss of normal mucosal folds in this segment of small bowel (arrow)
Metastatic disease
Metastatic spread to the small intestine is unusual, but tumors that most commonly metastasize to the small bowel include melanoma, lung cancer, breast cancer, and Kaposi sarcoma [20]. Metastatic lesions may be solitary or multiple, frequently with an intramural location (Figs. 10, 11, 12, 13, 14). These tumors have a variety of appearances, even mimicking benign lesions when discrete, smoothly marginated, or with homogenous enhancement. Large size, central ulceration and/or cavitation, invasion of adjacent structures, and intraperitoneal spread are features which raise concern for malignancy [48]. In the setting of a known primary malignancy, particularly one with a propensity to metastasize to small bowel, metastatic disease should always be considered when a solid small bowel mass is identified. Management of small bowel metastases varies on a host of factors, with surveillance imaging and treatment tailored to the aggressivity of the primary malignancy.
Axial contrast-enhanced CT image (a) demonstrates an irregular soft tissue density mass in the jejunum (thin arrows) in this patient with known melanoma. Corresponding fused axial 18F-FDG PET-CT image demonstrates marked hypermetabolic activity of this mass. Fused axial 18F-FDG PET-CT image in the pelvis (c) depicts a second hypermetabolic mass (thin arrow) that the corresponding axial diagnostic CT image (d) shows is causing an intussusception (thick arrows). Both masses were surgically resected and confirmed as metastases
Axial T1 weighted post-contrast MR image in this patient with primary peritoneal mesothelioma demonstrates numerous serosal and mucosal metastases to the small bowel (thin arrows), as well several nodular tumor deposits outside the bowel (thick arrows), with the bulk of tumor in the left abdomen (asterisk)
Small bowel carcinoma
Primary small bowel carcinoma is rare, comprising less than 2% of all GI tumors, yet it is the most common primary small bowel malignancy [49]. Greater than 50% of tumors are located in the duodenum, followed by the jejunum, then the ileum [50]. Those arising from the jejunum are most often within 30 cm of the ligament of Trietz [51]. Adenocarcinoma has been linked to both Crohn’s and Celiac diseases, most commonly seen in the ileum and jejunum, respectively. Up to 75% of small bowel adenocarcinomas occur in these settings [52, 53].
Typical imaging characteristics include asymmetric, nodular wall thickening with heterogeneous, moderate contrast enhancement [54]. Tumors also appear as an infiltrative or annular (“apple-core”) lesion, with circumferential luminal narrowing or irregularity, and sometimes ulceration (Fig. 15) [51]. Less often, small bowel carcinoma can appear as a small, polypoid sessile tumor with plaque-like growth. Intussusception is a common presentation, and larger small bowel carcinomas can lead to bowel obstruction. Periampullary tumors in the duodenum can obstruct the common bile duct (Fig. 16). Additional common findings include vascular invasion, locally enlarged mesenteric lymph nodes, and metastases, most frequently to the liver or peritoneum [1].
Oblique axial (a) and sagittal (b) contrast-enhanced CT images show focal circumferential irregular bowel wall thickening with mild luminal narrowing in the jejunum (arrows) with associated mesenteric adenopathy (asterisk). Endoscopic image from the same patient (c) demonstrates an irregular ulcerating small bowel mass, confirmed as adenocarcinoma on biopsy
Lymphoma
Lymphoma accounts for 15–20% of all malignant small bowel tumors, with more than 60% arising from the ileum [55,56,57]. Risk factors consist of immune suppression, such as in transplant recipients, celiac disease, and AIDS. Similar to renal lymphoma, involvement of the small bowel typically does not cause obstruction. Imaging features are varied, with the most frequent infiltrative form producing circumferential wall thickening, fold effacement, and irregular (“aneurysmal”) dilation of the bowel lumen (Fig. 17) [58]. The polypoid variant may present with a single or multiple mucosal or submucosal masses that vary in size and number [50]. The rarest variant, multiple lymphomatous polyposis, with numerous polypoid masses, can be mistaken for adenomatous or hamartomatous polyposis, in which histologic diagnosis is necessary [59].
Central ulceration has been described as a “target” lesion. The mesenteric form of small bowel lymphoma may demonstrate multiple small bowel masses, which displace or compress bowel loops or adjacent vessels. Coexisting lymphadenopathy encasing the mesenteric vasculature produces the classic “sandwich” sign [60]. The clinical presentation of small bowel lymphoma can be variable or asymptomatic. When present, however, lymphoma may cause secondary findings of obstruction, intussusception, bleeding, ischemia, or perforation [61].
While CT is commonly the diagnostic imaging modality of choice for small bowel lymphoma, MR diffusion weighted imaging sequences and/or 18F-FDG PET-CT certainly can aid in identifying less conspicuous lesions (Fig. 18). The clinical history is important, because overlapping imaging features can also be seen in post-transplant lymphoproliferative disorder (PTLD) following solid organ or stem cell transplant (Fig. 19). 18F-FDG PET-CT is generally the preferred imaging modality for lymphoma and PTLD, with greater sensitivity and specificity for tumor detection [62]. Distinction between primary adenocarcinoma and lymphoma on imaging can be challenging. Lymphoma can be suggested in the presence of significant wall thickening (> 2 cm), multiple lymphomatous nodules, and coexistent lymphadenopathy [60].
Axial contrast-enhanced CT image (a) shows a short segment of mildly dilated jejunum with thickened folds (arrows). Subsequent axial T2 weighted MR image (b) confirms the finding (arrows). However, the abnormal segment of bowel is strikingly conspicuous on the axial high b-value diffusion weighted MR image (c). Corresponding axial fused 18F-FDG PET-CT image (d) also shows hypermetabolic activity in this bowel segment, confirmed as lymphoma on endoscopic biopsy
Axial non-contrast CT image with narrow window in this patient post renal transplant demonstrates abnormal asymmetric soft tissue thickening along a segment of jejunum (arrows). This patient also had retroperitoneal adenopathy (not shown), which was biopsied, revealing lymphoma consistent with post-transplant lymphoproliferative disorder
Conclusion
Common small bowel neoplasms may be well evaluated with CT, MR, and select nuclear medicine techniques. Enterography protocols remain the best imaging technique for both CT and MR, with MR the more sensitive test for detecting small bowel tumors [63, 64]. Scintigraphy and PET are useful adjuncts to cross-sectional imaging, particularly for neuroendocrine tumors and other malignancies. While no imaging exam yet is as sensitive as endoscopy for detecting small intraluminal lesions, the utility of cross-sectional studies for the evaluation of extraluminal disease is clear.
References
Buckley JA, Fishman EK (1998) CT evaluation of small bowel neoplasms: Spectrum of disease. Radiographics 18(2):379-392. https://doi.org/10.1148/radiographics.18.2.9536485
Martin RG (1986) Malignant tumors of the small intestine. Surg Clin North Am 66(4), 779-785 https://doi.org/10.1016/s0039-6109(16)43988-5
North JH, Pack MS (2000) Malignant tumors of the small intestine: a review of 144 cases. Am Surg, 66(1):46–51.
Masselli G, Colaiacomo MC, Marcelli G, et al. (2012) MRI of the small-bowel: How to differentiate primary neoplasms and mimickers. Brit J Radiol 85(1014):824–837. https://doi.org/10.1259/bjr/14517468
Rummeny EJ, Reimer P, Heindel W. (2009) MR imaging of the body. Thieme Medical Pub. ISBN:3131358416.
Hara AK, Leighton JA, Sharma VK, et al. (2005) Imaging of small bowel disease: Comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics 25:697-718.
Howe JR, Karnell LH, Menck HR, et al. (1999) Adenocarcinoma of the small bowel. Cancer 86(12):2693-2706.
Masselli G, Casciani E, Polettini E, et al. (2013) Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging 13:92–99.
Sailer J. (2007) MDCT of small bowel tumours. Cancer Imaging 7(1):224-233. https://doi.org/10.1102/1470-7330.2007.0032
Sokhandon F, Al-katib S, Bahoura L, et al. (2017) Multidetector CT enterography of focal small bowel lesions: A radiological-pathological correlation. Abdom Radiol 42:1319-1341.
Paulsen SR, Huprich JE, Fletcher JG, et al. (2006) CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics 26:641–657.
Gauci J, Sammut L, Sciberras M, et al. (2018) Small bowel imaging in Crohn’s disease patients. Ann Gastroenterol 31(4):395–405. http://doi.org/10.20524/aog.2018.0268
Mollard BJ, Smith EA, Dillman JR. (2015) Pediatric MR enterography: Technique and approach to interpretation—how we do it. Radiology 274(1):29-43. https://doi.org/10.1148/radiol.141224
Spada C., Alfieri S, Familiari P, et al. (2013) Giant lipoma as an unusual cause of obscure gastrointestinal bleeding. Video Journal and Encyclopedia of GI Endoscopy 1(1):233-234.
Taylor AJ, Stewart ET, Dodds WJ. (1990) Gastrointestinal lipomas: A radiologic and pathologic review. Am J Roentgenol 155(6):1205-1210. https://doi.org/10.2214/ajr.155.6.2122666
de Latour RA, Kilaru SM, Gross SA. (2017) Management of small bowel polyps: A literature review. Best Pract Res Clin Gastroenterol 31(4):401-408. https://doi.org/10.1016/j.bpg.2017.06.003
Chiang J, Lin Y. (2008) Tumor spectrum of adult intussusception. J Surg Oncol 98(6):444-447. https://doi.org/10.1002/jso.21117
Colucci PM, Yale SH, Rall CJ. (2003) Colorectal polyps. Clin Med Res 1(3), 261–262.
Wood LD, Salaria SN, Cruise MW, et al. (2014) Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol 38(3):389-393.
Gill SS, Heuman DM, Mihas AA. (2001) Small intestinal neoplasms. J Clin Gastroenterol 33(4):267-82.
Syngal S, Brand RE, Church JM, et al. (2015) ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110(2):223-262. https://doi.org/10.1038/ajg.2014.435
Cengiz H, Yildiz S, Kaya C et al. (2014) A diagnostic dilemma of acute abdomen in pregnancy: Leiomyoma of the small intestine. J Turk Ger Gynecol Assoc 15(1):60-62. https://doi.org/10.5152/jtgga.2013.38233
Gourtsoyiannis N, Bays D, Malamas M, et al. (1992) Radiological appearances of small intestinal leiomyomas. Clin Radiol 45(2):94-103. https://doi.org/10.1016/s0009-9260(05)80063-7
Ramai D, Tan QT, Nigar S, et al. (2018) Ulcerated gastric leiomyoma causing massive upper gastrointestinal bleeding: A case report. Mol Clin Oncol 8(5):671–674. https://doi.org/10.3892/mco.2018.1597
Xynopoulos D, Mihas AA, Paraskevas E, et al. (2002) Small bowel tumors. Ann Gastroenterol 15(1):18-35.
Schindler R, Blomquist OA, et al. (1946) Leiomyosarcoma of the stomach; its roentgenologic and gastroscopic diagnosis and its possible relationship to pernicious anemia. Surg Gynecol Obstet 82:239–252.
Rabin I, Chikman B, Lavy R, et al. (2009) Gastrointestinal stromal tumors: A 19 year experience. Isr Med Assoc J 11(2):98-102.
Izawa N. (2012) Gastrointestinal stromal tumor presenting with prominent calcification. World J Gastroenterol 18(39):5645. https://doi.org/10.3748/wjg.v18.i39.5645
Kim S, Lee S. (2018) Performance of F-18 FDG PET/CT for predicting malignant potential of gastrointestinal stromal tumors: A systematic review and meta-analysis. J Gastroenterol Hepatol 33(3):576-582. https://doi.org/10.1111/jgh.14015
Levy AD, Remotti, HE, Thompson WM, et al. (2003) Gastrointestinal stromal tumors: Radiologic features with pathologic correlation. Radiographics 23(2):283-304. https://doi.org/10.1148/rg.232025146
Suster S. (1996) Gastrointestinal stromal tumors. Semin Diagn Pathol 13(4):297-313.
Crosby JA, Catton CN, Davis A, et al. (2001) Malignant gastrointestinal stromal tumors of the small intestine: A review of 50 cases from a prospective database. Ann Surg Oncol 8(1):50-59. https://doi.org/10.1007/s10434-001-0050-4
Fletcher CD, Berman JJ, Corless C, et al. (2002). Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 33(5):459-465. https://doi.org/10.1053/hupa.2002.123545
Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. (2005) Ann Oncol 16(6):993-993. https://doi.org/10.1093/annonc/mdi250
Talamonti MS, Goetz LH, Rao S, et al. (2002) Primary Cancers of the Small Bowel. Arch Surg 137(5):564-570. https://doi.org/10.1001/archsurg.137.5.564
Pinchot SN, Holen K, Sippel RS, et al. (2008) Carcinoid Tumors. Oncologist 13(12):1255-1269. https://doi.org/10.1634/theoncologist.2008-0207
Mantzoros I, Savvala NA, Ioannidis O, et al. (2017) Midgut neuroendocrine tumor presenting with acute intestinal ischemia. World J Gastroenterol 23(45):8090-8096. https://doi.org/10.3748/wjg.v23.i45.8090
Anzidei M, Napoli A, Zini C, et al. (2011) Malignant tumours of the small intestine: A review of histopathology, multidetector CT and MRI aspects. Brit J Radiol 84(1004):677-690. https://doi.org/10.1259/bjr/20673379
Boudreaux JP, Klimstra DS, Hassan MM, et al. (2010) The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors. Pancreas 39(6):753-766. https://doi.org/10.1097/mpa.0b013e3181ebb2a5
Sheth S, Horton KM, Garland MR, et al. (2003) Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics 23(2):457-473. https://doi.org/10.1148/rg.232025081
Masselli G, Gualdi G. (2008) Evaluation of small bowel tumors: MR enteroclysis. Abdom Imaging 35(1):23-30. https://doi.org/10.1007/s00261-008-9490-7
Burke AP, Thomas RM, Elsayed AM, et al. (1997) Carcinoids of the jejunum and ileum. Cancer 79(6):1086-1093. https://doi.org/10.1002/(sici)1097-0142(19970315)79:63.0.co;2-e
Pantongrag-Brown L, Buetow PC, Carr NJ, et al. (1995) Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation. Am J Roentgenol 164(2):387-391. https://doi.org/10.2214/ajr.164.2.7839976
Horton KM, Kamel I, Hofmann L, et al. (2004) Carcinoid tumors of the small bowel: A multitechnique imaging approach. Am J Roentgenol 182(3):559-567. https://doi.org/10.2214/ajr.182.3.1820559
Seigel RS, Kuhns LR, Borlaza GS, et al. (1980) Computed tomography and angiography in ileal carcinoid tumor and retractile mesenteritis. Radiology 134(2):437-440. https://doi.org/10.1148/radiology.134.2.7352226
Addeo P, Bachellier P, Goichot B, et al. (2018) Preoperative imaging with 18F-FDOPA PET/CT for small bowel neuroendocrine tumors. J Gastrointest Surg. https://doi.org/10.1007/s11605-018-3729-6
Bodei L, Ambrosini V, Herrmann K, et al. (2017) Current concepts in 68Ga-DOTATATE imaging of neuroendocrine neoplasms: Interpretation, biodistribution, dosimetry, and molecular strategies. J Nucl Med 58(11):1718-1726. https://doi.org/10.2967/jnumed.116.186361
O’Riordan BG, Vilor M, Herrera L. (1996) Small bowel tumors: An overview. Dig Dis 14(4):245-257. https://doi.org/10.1159/000171556
Maglinte DD, Lappas JC, Sandrasegaran K. Malignant tumors of the small-bowel. Gore R, Levine M, editors., Textbook of gastrointestinal radiology. 3rd edn Philadelphia, PA: Saunders Elsevier; 2008. pp. 853–69.
McLaughlin PD, Maher MM. (2013) Primary malignant diseases of the small intestine. Am J Roentgenol 201(1):W9-14. https://doi.org/10.2214/ajr.12.8492
Gore RM, Mehta UK, Berlin JW, et al. (2006) Diagnosis and staging of small bowel tumours. Cancer imaging 6(1):209-12. https://doi.org/10.1102/1470-7330.2006.0031
Dossett LA, White LM, Welch DC, et al. (2007) Small bowel adenocarcinoma complicating Crohn’s disease: case series and review of the literature. Am Surg 73:1181–1187.
Cahill C, Gordon PH, Petrucci A, et al. (2014) Small bowel adenocarcinoma and Crohn’s disease: Any further ahead than 50 years ago? World J Gastroenterol 20(33):11486-11495. https://doi.org/10.3748/wjg.v20.i33.11486
Romano S, Lutio ED, Rollandi GA, et al. (2005) Multidetector computed tomography enteroclysis (MDCT-E) with neutral enteral and IV contrast enhancement in tumor detection. Eur Radiol 15(6):1178-1183. https://doi.org/10.1007/s00330-005-2673-5
Cheung DY, Choi M. (2011) Current advance in small bowel tumors. Clin Endosc 44(1):13-21. https://doi.org/10.5946/ce.2011.44.1.13
Bäck H, Gustavsson B, Ridell B, et al. (1986) Primary gastrointestinal lymphoma incidence, clinical presentation, and surgical approach. J Surg Oncol 33(4):234-238. https://doi.org/10.1002/jso.2930330406
Ghimire P, Wu GY, Zhu L (2011) Primary gastrointestinal lymphoma. World J Gastroenterol 17(6):697-707. https://doi.org/10.3748/wjg.v17.i6.697
Horton KM, Fishman EK. (2004) Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms. J Comput Assist Tomogr 28(1):106-116. https://doi.org/10.1097/00004728-200401000-00019
Xie C, Xu X, Wei S. (2018) Multiple lymphomatous polyposis of the intestine with ileocecal intussusception due to mantle cell lymphoma: A case report of a 34-year-old man. Am J Case Rep 19:262–266. http://doi.org/10.12659/AJCR.907804
Lo Re G, Federica V, Midiri F, et al. (2016) Radiological features of gastrointestinal lymphoma. Gastroenterol Res Pract. 2016:2498143. https://doi.org/10.1155/2016/2498143
Smith C, Kubicka RA, Thomas CR. (1992) Non-Hodgkin lymphoma of the gastrointestinal tract. Radiographics 12(5):887-899. https://doi.org/10.1148/radiographics.12.5.1529131
Borhani AA, Hosseinzadeh K, Almusa O, et al. (2009) Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics 29(4):981-1000. https://doi.org/10.1148/rg.294095020
Masselli G, Polettini E, Casciani E, et al. (2009) Small-bowel neoplasms: Prospective evaluation of MR enteroclysis. Radiology 251(3):743-750. https://doi.org/10.1148/radiol.2513081819
Van Weyenberg SJ, Meijerink MR, Jacobs MA, et al. (2010) MR enteroclysis in the diagnosis of small-bowel neoplasms. Radiology 254(3):765-773. https://doi.org/10.1148/radiol.09090828
Acknowledgements
The authors would like to acknowledge Dr. David DiSantis for assistance in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Williams, E.A., Bowman, A.W. Multimodality imaging of small bowel neoplasms. Abdom Radiol 44, 2089–2103 (2019). https://doi.org/10.1007/s00261-019-01955-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-01955-y