Abstract
With the introduction of multidetector row computed tomography (MDCT), CT is being considered a potential diagnostic method for patients with acute gastrointestinal (GI) bleeding. On arterial phase MDCT images, active GI bleeding is typically identified as a focal area of high attenuation within the bowel lumen, which represents a collection of contrast material that has been extravasated in association with arterial bleeding. Additional CT findings suggestive of acute GI bleeding are focal dilatation of fluid-filled bowel segment noted on contrast-enhanced CT and acute hematoma on unenhanced CT. In addition to detection of active bleeding, an advantage of contrast-enhanced MDCT is the ability to demonstrate morphologic changes in the GI tract, which could suggest specific conditions that cause acute GI bleeding such as intestinal tumors. Arterial phase contrast-enhanced MDCT is rapid, noninvasive, and accurate in detecting and localizing sites of bleeding in patients with acute GI bleeding. Contrast-enhanced MDCT may be a promising diagnostic option in patients with acute GI bleeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acute gastrointestinal (GI) bleeding remains a common medical emergency. It is associated with substantial patient morbidity and mortality despite advances in diagnosis and therapy. Acute GI bleeding is divided into upper and lower intestinal bleeding by an anatomic landmark, the ligament of Treitz. Mortality rates have been reported to vary from 8% to 14% for patients with acute upper GI bleeding [1, 2] and from 3.6% to 18% for those with acute lower GI bleeding [3, 4]. Mortality rate increases to 21% to 40% in cases of massive bleeding, defined as hemodynamic instability or required transfusion of more than 4 U of packed red blood cells per 24 h [5, 6]. Patients with acute GI bleeding should undergo resuscitation, including stabilization of blood pressure and restoration of intravascular volume, before the initiation of definite diagnostic and therapeutic measures [7]. After initial resuscitation and patient assessment, further localization of the source of bleeding should be determined as soon as possible. Diagnostic modalities used for the detection and localization of acute GI bleeding include upper GI endoscopy, colonoscopy, enteroscopy, capsule endoscopy, radionuclide imaging, and angiography. At this time, computed tomography (CT) is not commonly performed for diagnosis of acute GI bleeding. The ability of contrast-enhanced CT to detect acute GI bleeding has been infrequently documented in case reports and a few retrospective series [8–12]. Recently, with the introduction of multidetector row CT (MDCT), CT is being investigated as a potential diagnostic method for patients with acute GI bleeding. MDCT markedly decreases scan time, enables acquisition of accurate arterial phase images, and thus allows identification of extravasation of contrast material into the intestinal lumen, a finding indicative of acute GI bleeding, before it is diluted with intestinal fluid. This article reviews the current status of diagnostic options for acute GI bleeding and discusses CT features, and the technique, advantage, and limitations of contrast-enhanced MDCT for the diagnosis of acute GI bleeding.
Endoscopy, radionuclide imaging, and angiography
The current main diagnostic modality in acute upper GI bleeding is endoscopy. Although endoscopy is effective in diagnosing and treating most causes of upper GI bleeding [13], emergent endoscopy often fails to locate the exact focus of bleeding when massive bleeding (>1 mL/min) occurs. Vreeburg et al. [14] reported that no diagnosis could be made at first endoscopy in 24% of patients with acute upper GI bleeding. In their study, excessive blood or clots in the gastroduodenal tract impaired endoscopic viewing in 15% of patients.
In contrast to upper GI bleeding, the diagnostic approach to acute lower GI bleeding remains controversial because of a lack of prospective controlled data. Currently, the diagnostic options used for acute lower GI bleeding include colonoscopy, enteroscopy, wireless capsule endoscopy, tagged red blood cell (RBC) scintigraphy, and visceral angiography.
It is generally believed that colonoscopy is the procedure of choice for the initial evaluation of acute lower GI bleeding [15]. It has been reported that urgent colonoscopy for acute lower GI bleeding has a diagnostic yield ranging from 48% to 90% [16]. The exception to the lower GI bleeding indication for urgent colonoscopy is the patient with massive bleeding because the detection rate of lesions is low when colonoscopy is performed in the setting of incomplete bowel preparation or continued massive bleeding [17].
Technetium 99m (99mTc) RBC scanning is another method used in the investigation of acute lower GI bleeding. Bleeding rates as low as 0.1 to 0.5 mL/min can be detected by 99mTc RBC scanning [15]. A review of published studies regarding the accuracy of RBC scan has shown that the scan was accurate in 78% of instances and inaccurate in 22% [16]. This high false localization rate would limit the use of a RBC scan for the purpose of localization of the bleeding site.
It is generally accepted that angiography is reserved for the patient who has massive lower GI bleeding that precludes colonoscopy, persistent or recurrent bleeding, or a colonoscopy that has failed to reveal the bleeding source [18, 19]. Pooled data from 14 studies involving 675 patients who presented with massive lower GI bleeding and underwent angiography indicated a mean positivity rate of 47% (range 27% to 77%) [16].
In cases of acute lower GI bleeding where no colonic source is identified, evaluation of the small bowel may be necessary. Endoscopic methods for evaluation of the small bowel include push enteroscopy and wireless capsule endoscopy. Push enteroscopy has a limited role in the evaluation of acute small bowel bleeding because it allows examination of the proximal jejunum only approximately 40 to 60 cm beyond the ligament of Treitz. Recently, the advent of wireless capsule endoscopy has allowed painless imaging of entire segments of the small bowel. Mylonaki et al. [20] reported that wireless capsule endoscopy was superior to push enteroscopy in the identification of bleeding abnormalities in the small bowel and was well tolerated by patients. However, at present, capsule endoscopy has a number of limitations including many technical problems, long examination time, low picture quality, and high cost. Wireless capsule endoscopy is currently an immature technology that needs to be improved in the future [20].
MDCT for acute gastrointestinal bleeding
CT technique
To prevent contrast-induced nephrotoxicity, patients are adequately hydrated with an intravenous infusion of 500 to 1000 mL of saline, which is commenced 1 h before CT study and continued for 12 h after CT study. When MDCT is performed to localize the acute GI bleeding, contrast material or water should not be given orally because these agents hamper accurate diagnosis: orally administered contrast material obscures the presence of extravasation of contrast material that is given intravenously and excessive water may dilute extravasated contrast material in the intestinal lumen. Before arterial phase scan, preliminary unenhanced CT scan should be obtained to detect preexisting hyperattenuating material in the bowel lumen such as metallic clips used for endoscopic hemostasis, suture materials, foreign bodies, or contrast material in intestinal lumen. At our institution, these unenhanced scans are obtained with a section thickness of 10 mm and a scan range from the hepatic dome to the inferior pubic ramus.
MDCT scan should be obtained during the arterial phase to identify active extravasation of contrast material within the bowel lumen, a finding diagnostic of active GI bleeding. We routinely administer 140 mL of contrast agent with 350 mg of iodine per milliliter intravenously with an automated injector at an injection rate of 3.5 to 4 mL/s. At our institution, imaging parameters for arterial phase MDCT scans using a four-detector row CT scanner are as follows: 2.5-mm nominal section thickness, beam pitch of 1.5, table speed of 15 mm/rotation, 2-mm reconstruction interval, 120 kV, and 200 mA. Scan delay is determined by using an automatic bolus triggering software program with a circular region of interest positioned at the level of the abdominal aorta and a predefined 100-HU enhancement threshold for triggering data acquisition. The duration of data acquisition ranges from 20 to 25 s. The scan range for the arterial phase scan is identical to that for unenhanced CT. In our experience, three-dimensional reconstruction of datasets is not necessary for the diagnosis of acute GI bleeding because it offers little additional information in most cases and, hence, is only a time-consuming process. At our institution, portal phase delayed scans are not routinely performed. In our experience, active extravasation of contrast material that is clearly seen on arterial phase images may or may not be detected on portal phase MDCT scan. Additional portal phase scans may be useful in determining the cause of acute GI bleeding, especially in cases of intestinal tumor.
CT findings
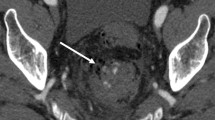
In arterial phase MDCT, the following two features are considered diagnostic of acute GI bleeding: (a) the presence of extravasation of contrast material in the bowel lumen and (b) extravasated contrast material greater than 90 HU. On arterial phase MDCT images, active GI bleeding is typically identified as a focal area of high attenuation within the bowel lumen, which represents a collection of contrast material that has been extravasated in association with arterial bleeding (Fig. 1). Active arterial extravasation can be differentiated from clotted blood by measuring CT attenuation. Willmann et al. [21] reported that the attenuation of active arterial extravasation on MDCT examination ranged from 91 to 274 HU (mean 155 HU), whereas that of clotted blood ranged from 28 to 82 HU (mean 54 HU).
A 65-year-old woman who presented with hematochezia of unknown cause. A Transverse, nonenhanced CT image shows no preexisting high-attenuated lesion in the bowel lumen at the level of the midpelvis. B At the same level, transverse, arterial phase MDCT image depicts extravasation of contrast material (arrows) in the ileum. The maximum measurement was 180 HU. C Superior mesenteric arteriogram reveals active bleeding (arrow) from the distal branch of the ileal artery. D Superior mesenteric arteriogram obtained after embolization with microcoils (arrows) shows no further bleeding.
In our experience, with the use of these diagnostic criteria, arterial phase MDCT scan is highly accurate in detection and localization of acute GI bleeding. When compared with angiography as the reference standard, MDCT had a sensitivity of 90.9% and a specificity of 99% in the detection of acute GI bleeding in 26 consecutive patients [22]. In addition, MDCT had an accuracy rate of 100% in localizing acute GI bleeding. The site of contrast extravasation on MDCT images corresponded exactly to the angiographically detected site of bleeding in all patients with a focus of bleeding on MDCT and angiography [22].
Two minor but useful CT findings suggestive of acute massive GI bleeding are focal dilatation (luminal distention) of fluid-filled bowel segment noted on contrast-enhanced CT scan (Fig. 2) and acute hematoma on unenhanced CT scan (Fig. 3). These CT findings are commonly seen in patients with massive bleeding. In addition to detection of active bleeding, an advantage of contrast-enhanced MDCT is the ability to demonstrate morphologic changes in the GI tract, which could suggest specific conditions causing acute GI bleeding such as intestinal tumors (Figs. 4, 5).
A 75-year-old man who presented with 5-day history of hematochezia due to postoperative stress ulceration. A Transverse, nonenhanced CT image shows focal distention of a fluid-filled small bowel loop in the left abdomen. B At the same level, transverse, arterial phase MDCT image demonstrates extravasated contrast material (arrows) in the dependent portion of the distended small bowel segment (arrowheads). C Corresponding superior mesenteric arteriogram reveals active bleeding (arrows) from the jejunal artery.
A 38-year-old man who presented with melena due to acute duodenal ulceration. A Transverse, nonenhanced CT image shows an acute hematoma (arrowheads) in the second portion of the duodenum. The maximum measurement was 52 HU. B Transverse, arterial phase MDCT image obtained superior to that obtained in A demonstrates active extravasation of contrast material (arrows) in the lumen of the duodenum.
A 53-year-old man with a 3-day history of melena. A Transverse, arterial phase MDCT image shows a well-defined mass (arrows) with active bleeding (arrowheads) in the second portion of the duodenum. B Transverse, portal phase MDCT scan shows a heterogeneously enhancing mass (arrows) with central low attenuation indicating necrosis. C Selective gastroduodenal arteriogram shows a hypervascular mass (arrows) with active bleeding (arrowheads). Pathologic examination of biopsy specimen obtained by surgery confirmed a gastrointestinal stromal tumor in the duodenum.
A 68-year-old man with massive hematochezia. A Transverse, nonenhanced CT image shows a distended sigmoid colonic loop with slightly high-attenuated luminal contents. B At the same level, transverse, arterial phase MDCT image demonstrates extravasated contrast material (arrows) in the dependent portion of the sigmoid colon and eccentric thickening of the colonic wall (arrowheads). Pathologic examination of biopsy specimen obtained by subsequent colonoscopy confirmed an adenocarcinoma in the sigmoid colon.
Published experience
In 2003, Kuhle and Sheiman [23] reported that single-detector helical CT angiography can depict active colonic hemorrhage extravasating at a rate of 0.3 mL/min in an animal model of colonic hemorrhage. They suggested that the ability of helical CT to depict acute lower GI bleeding may exceed the lower limit of 0.5 mL/min cited for mesenteric angiography and may approach the 0.2-mL/min limit cited for 99mTc RBC scanning.
The clinical use of helical CT angiography in the diagnosis of lower GI bleeding has infrequently been reported in the literature. In 1997, Ettorre et al. [8] published a series of 18 consecutive patients with acute GI bleeding who underwent catheterization of the abdominal aorta followed by single-detector helical CT angiography after intra-arterial injections of a contrast medium through an angiographic catheter positioned near the origin of the celiac trunk. In their series, helical CT angiography revealed the site of contrast extravasation in 72% (13 of 18) of patients. The site of bleeding was revealed by angiography in two of five patients with negative results on helical CT angiography. One limitation of their study was the complexity and invasiveness of the methods used for diagnosis, which included arterial catheterization. Recently, Ernst et al. [10] reported that single-detector helical CT examination after intravenous injection of contrast material located sites of bleeding in 79% (15 of 19) of patients with acute lower GI bleeding. In their study, minor or inconclusive CT findings, such as high-attenuated peri-bowel fat, intestinal wall thickening, polyp, tumor and vascular dilatation, and contrast extravasation, were also used as diagnostic criteria for acute GI bleeding. The fact that extravasation of contrast medium was found in only three of 15 patients suggests that single-detector helical CT examination has only a limited role in clinical diagnosis of active GI bleeding. More recently, Tew et al. [12] performed a retrospective review of 13 patients with acute lower GI bleeding who underwent MDCT angiography using a four-channel MDCT scanner before angiography. They reported that MDCT angiography depicted a site of bleeding in 54% (seven of 13) of patients, with all such sites confirmed on angiography. In their study, the six patients whose results of MDCT examination were negative exhibited resolution of bleeding without further intervention. They thus obtained no false-positive or false-negative findings with MDCT.
Advantages and limitations of MDCT
At present, it is reasonable to perform angiography first in cases of acute massive GI bleeding because there is a high probability of detecting a focus of bleeding in such cases and it can offer transcatheter therapy. However, we believe that there are several advantages of performing arterial phase MDCT scan before angiography. In our experience, arterial phase MDCT is highly accurate in localization of acute GI bleeding. The site of contrast material extravasation on MDCT scans corresponded to the site of bleeding identified on angiography in all patients with acute GI bleeding. Thus, if MDCT scan is performed before angiography and embolization procedures, interventional radiologists can use the location of contrast material extravasation on MDCT scans to direct the performance of more selective and superselective investigations of arteries that are most likely to be bleeding, which may increase the rate of angiographic detection of acute GI bleeding (Fig. 6).
A 58-year-old man with acute leukemia who presented with massive hematochezia. A Transverse, arterial phase MDCT image demonstrates a jet of extravasated contrast material (arrows) in the cecum. B Initial superior mesenteric arteriogram shows no definite focus of active bleeding. C Selective right colic and D superselective arteriograms with a microcatheter (arrowheads) clearly depict a focus of active bleeding (arrow) from a branch of the right colic artery.
In addition, interventional radiologists can confidently perform delayed follow-up examinations of arteries that supply specific areas when the first angiographic examination fails to demonstrate a site of bleeding despite the presence of contrast extravasation on MDCT scans (Fig. 7). This delayed follow-up examination might reveal a focus of bleeding not demonstrated at the first examination and thus increase the rate of angiographic detection of acute GI bleeding. Other clear advantages of MDCT are its noninvasiveness and rapidity compared with other diagnostic modalities such as endoscopy, scintigraphy, or angiography.
A 72-year-old man who presented with massive hematochezia due to acute stress ulceration during hospitalization for a fracture of the left femur. A Transverse, arterial phase MDCT image demonstrates active extravasation of contrast material (arrows) in the rectal lumen. B Initial inferior mesenteric arteriogram shows no definite bleeding focus. C A second inferior mesenteric arteriogram obtained 5 min later reveals a site of active bleeding (arrow) from a branch of the superior rectal artery.
There are several limitations for MDCT regarding MDCT diagnosis of acute GI bleeding. First, MDCT examination may not be feasible to perform in patients who have decreased renal function [24]. It is well known that preexisting impairment of renal function appears to be the most important risk factor of contrast-induced nephropathy. A second limitation is that CT artifact can obscure contrast extravasation in the bowel lumen. Metallic artifact from hemoclips used for endoscopic hemostasis may obscure contrast extravasation, which may result in false-negative MDCT diagnosis. The lack of therapeutic capability of MDCT is another limitation that would require further hemostatic procedures, such as transcatheter embolization, endoscopic hemostasis, or surgery.
Conclusions
With the introduction of MDCT, another step forward has been made in the diagnosis of acute GI bleeding. Arterial phase contrast-enhanced MDCT is rapid, noninvasive, and accurate in diagnosing and localizing sites of bleeding in patients with acute massive GI bleeding. We believe that contrast-enhanced MDCT may be a promising diagnostic option in patients with acute GI bleeding.
References
Sanders DS, Perry MJ, Jones SGW, et al. (2004) Effectiveness of an upper-gastrointestinal haemorrhage unit: a prospective analysis of 900 consecutive cases using the Rockall score as a method of risk standardization. Eur J Gastroenterol Hepatol 16:487–494
Van Leerdam ME, Vreeburg EM, Rauws EAJ, et al. (2003) Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 98:1494–1499
Longstreth GF (1997) Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 92:419–424
Anthony T, Penta P, Todd RD, et al. (2004) Rebleeding and survival after acute lower gastrointestinal bleeding. Am J Surg 188:485–490
Leitman IM, Paull DE, Shires GT (1989) Evaluation and management of massive lower gastrointestinal bleeding. Ann Surg 209:175–180
Walsh RM, Anain P, Geisinger M, et al. (1999) Role of angiography and embolization for massive gastroduodenal bleeding. J Gastrointest Surg 3:61–66
Barkun A, Bardou M, Marshall JK (2003) Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 139:843–857
Ettorre GC, Francioso G, Garribba AP, et al. (1997) Helical CT angiography in gastrointestinal bleeding of obscure origin. AJR 168:727–730
Krestan CR, Pokieser P, Wenzl E, Leitha T (2000) Localization of gastrointestinal bleeding with contrast-enhanced helical CT. AJR 174:265–266
Ernst O, Bulois P, Saint-Drenant S, et al. (2003) Helical CT in acute lower gastrointestinal bleeding. Eur Radiol 13:114–117
Yamaguchi T, Yoshikawa K (2003) Enhanced CT for initial localization of active lower gastrointestinal bleeding. Abdom Imaging 28:634–636
Tew K, Davies RP, Jadun CK, Kew J (2004) MDCT of acute lower gastrointestinal bleeding. AJR 182:427–430
American Society for Gastrointestinal Endoscopy (2004) ASGE guideline: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc 60:497–504
Vreeburg EM, Snel P, de Bruijne JW, et al. (1997) Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol 92:236–243
Bounds BC, Friedman LS (2003) Lower gastrointestinal bleeding. Gastroenterol Clin North Am 32:1107–1125
Zuckerman GR, Prakash C (1998) Acute lower intestinal bleeding. Part 1: clinical presentation and diagnosis. Gastrointest Endosc 48:606–616
Elta GH (2004) Urgent colonoscopy for acute lower GI bleeding. Gastrointest Endosc 59:402–408
Zuccaro G (1998) Management of the adult patient with acute lower gastrointestinal bleeding. Am J Gastroenterol 93:1202–1208
American Society for Gastrointestinal Endoscopy (2001) An annotated algorithmic approach to acute lower gastrointestinal bleeding. Gastrointest Endosc 53:859–863
Mylonaki M, Fritscher-Ravens A, Swain P (2003) Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut 52:1122–1126
Willmann JK, Roos JE, Platz A, et al. (2002) Multidetector CT: detection of active hemorrhage in patients with blunt abdominal trauma. AJR 179:437–444
Yoon W, Jeong YY, Shin SS, et al. (2005) Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology in press
Kuhle WG, Sheiman RG (2003) Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology 228:743–752
Gleeson TG, Bulugahapitiya S (2004) Contrast-induced nephropathy. AJR 183:1673–1689
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, W., Jeong, Y.Y. & Kim, J.K. Acute gastrointestinal bleeding: contrast-enhanced MDCT. Abdom Imaging 31, 1–8 (2006). https://doi.org/10.1007/s00261-005-0367-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-005-0367-8