Abstract
Purpose
The recent introduction of integrated PET-MRI systems into practice seems promising in oncologic imaging, and efforts are made to specify their added values. The current study evaluates the added values of PET-MRI over PET-CT in detecting active malignant hepatic lesions.
Methods
As part of an ongoing prospective study in our institution that assesses the added values of PET-MRI, subjects undergo PET-CT and subsequent PET-MRI after single radiotracer injection. The current study included 97 pairs of whole-body PET-CT and liver PET-MRI scans, of 61 patients (19/61 had ≥ 2 paired scans), all performed with [18F]FDG and interpreted as showing active malignant hepatic involvement. Primary malignancies were of colorectal/biliary/pancreatic/breast/other origins in 19/9/9/7/17 patients. Monitoring response to therapy was the indication in 86/97 cases. When PET-MRI detected additional malignant lesions over PET-CT, lesions size, their characteristics on PET-MRI, and the influence on the final report were recorded.
Results
In 37/97 (38.1%) cases, a total of 78 malignant lesions were identified on PET-MRI but not on PET-CT: 19 lesions (11 cases) were identified on PET of PET-MRI but not on PET of PET-CT; 37 lesions (14 cases) were small (≤ 0.8 cm) and identified on MRI only; 22 lesions (12 cases) were > 0.8 cm, had low/no [18F]FDG uptake, but were categorized as viable based on MRI. These 78 lesions caused major effect on final reports in 11/97 (11.3%) cases, changing reported response assessment category (10/86 cases) or defining malignant hepatic disease on staging/restaging scans (1/11 cases).
Conclusion
PET-MRI offers several advantages over PET-CT in assessing the extent and response to therapy of malignant hepatic involvement. Additional malignant lesions detected on PET-MRI are attributed to superior PET performance (compared with PET of PET-CT), greater spatial resolution provided by MRI, and improved multi-parametric viability assessment. In around one-tenth of cases, findings identified on PET-MRI but not on PET-CT significantly change the final report’s conclusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessing the extent of malignant hepatic involvement in oncologic patients, for either staging, restaging, or monitoring response to therapy, depends on the applied imaging modality. Not only potentially altering lesion detection rate, the selected imaging modality may affect the interpreting physician’s degree of confidence, and hence ease or complicate clinical workup [1,2,3,4,5,6,7,8,9,10,11,12,13].
Although CT remains the first-line imaging modality for assessing malignant hepatic involvement in most clinical scenarios [14, 15], the additional values offered by [18F]FDG PET-CT and by MRI were studied extensively during the past decades [1,2,3,4,5,6,7]. Retrospective fusion of [18F]FDG PET and MRI was also reported to improve detection rate and diagnostic confidence in small cohorts of patients with malignant hepatic lesions [8, 9].
The novel integrated hybrid PET-MRI technology has recently become available, and data on potential applications are being accumulated, particularly in the field of oncologic imaging [16, 17]. Conventional MRI sequences offer excellent soft tissue contrast, which serve as anatomical references for PET data. In addition, functional MRI techniques offer complementary parameters to the molecular-metabolic data obtained by PET. Altogether, the integrated PET-MRI system has the potential to provide multi-parametric assessment of tumors and their viability, enabling anatomic, functional, and metabolic characterization on one-stop. Despite these promising potential benefits, the design of PET-MRI systems that fully integrate both of the stand-alone modalities has been complex and faced physical and methodological barriers [18, 19]. In “real-life,” the additional value of the available fully integrated hybrid PET-MRI systems has yet to gain robust evidences.
In the context of assessing malignant hepatic involvement, only few previous studies focused on the role of integrated PET-MRI systems, reporting superior performance of PET-MRI over PET-CT [10,11,12,13]. Brendle et al. [10] evaluated combinations of anatomical and functional imaging techniques in PET-MRI and PET-CT for assessing metastatic lesions in 15 colorectal cancer patients that underwent [18F]FDG PET-CT and subsequent PET-MRI. They retrospectively compared the accuracy of different imaging combinations in detecting malignant lesions, including PET plus CT, PET plus morphologic MRI alone, and PET plus MRI with diffusion-weighted imaging (DWI). In a liver lesions subanalysis, the combination of PET plus MRI with DWI showed higher accuracy than PET plus CT in detecting 22 hepatic metastases among 37 hepatic lesions. In a study by Zhou et al. [11], that included 56 colorectal cancer patients, the clinical value of performing [18F]FDG PET-CT and subsequent liver PET-MRI was assessed. For liver lesion detection, PET-MRI showed higher detection ability than PET-CT, and the authors also found that adding [18F]FDG PET-CT and subsequent PET-MRI changed therapeutic strategies determined based on conventional imaging in one-fourth of cases. In another study that included 56 oncologic patients [12], compared with [18F]FDG PET-CT, a subsequent liver PET-MRI scan had higher sensitivity and specificity, and resulted in detecting additional liver metastases that were not detected on PET-CT, particularly small ones. The higher accuracy provided by [18F]FDG PET-MRI performed immediately after PET-CT was similarly reported in a study by Beiderwellen et al. [13], which included 26 patients with hepatic lesions, and highlighted the conspicuity provided by PET-MRI.
In late 2019, a SIGNA™ PET-MRI system [20] capable of simultaneous acquisition of both time-of-flight (TOF) PET and high resolution 3.0 T MR data was introduced in our facility at the nuclear medicine department of Tel Aviv Sourasky Medical Center. Since then, the PET-MRI system is used for research purposes, mainly in oncologic patients. After recruiting hundreds of patients, all of whom performed PET-CT and subsequent dedicated PET-MRI, we had the impression that PET-MRI has particular advantages over PET-CT in the context of imaging malignant hepatic lesions. In the current study, we aimed to summarize our experience with PET-MRI for assessing malignant hepatic involvement by detailing the major advantages provided by PET-MRI over PET-CT alone and assessing their influence on the final imaging report conclusion.
Methods
Between December 2019 and April 2022, a total of 768 paired PET-CT and subsequent PET-MRI scans were performed in our institution as part of an ongoing prospective study to evaluate the added value of PET-MRI over PET-CT in the imaging of various malignancies. The study was approved by the institutional review board, and written informed consents were obtained from included subjects. We reviewed the reports of all 768 above-mentioned paired scans and included in the current study all cases that met the following three inclusion criteria: (1) imaging was performed after injection of [18F]FDG, (2) the PET-MRI scan was directed to the liver, and (3) the scans were reported as having evidence of at least one active liver malignant lesion (primary or secondary). Among all 768 cases, 147 studies were performed with a radiotracer different than [18F]FDG (most commonly with a radiolabeled somatostatin-analog). In additional 485 cases, the PET-MRI scan was directed to locations different than the liver (most commonly to the pelvis). In other 39 cases, whole-body PET-CT and liver PET-MRI scans were performed with [18F]FDG, but no liver malignant lesions were identified. Accordingly, 97 paired scans, of 61 patients, were included in the study. Among the 61 patients, 42, 10, 3, 4, and 2 patients had 1, 2, 3, 4, and 5 paired scans, respectively.
Patients’ characteristics
The total 61 studied patients had a median age of 66.4 years. The primary tumor was of liver origin in 10 patients (16.4%), 9 of them had intrahepatic cholangiocarcinoma (IHC) and 1 had hepatocellular carcinoma (HCC). The most common primary malignancies among the other 51 patients were colorectal (n = 19), pancreatic (n = 9), and breast (n = 7) cancers. Among the 97 paired scans, 86 (88.7%) were performed for monitoring response to therapy (during the 3 months period prior to imaging, therapeutic regimen contained chemotherapy in 63 cases and biologic therapy in 37 cases), and in 56 (57.7%) cases, at least 4 (and up to numerous) malignant lesions were identified and reported. Table 1 summarizes the characteristics of the patients and paired scans included in the current study.
Image acquisition
The injected activity of [18F]FDG was 3.7 MBq/kg. Patients were instructed to fast for at least 4 h and to avoid physical exercise for 24 h prior to [18F]FDG administration. Before imaging, glucose levels were tested to ensure blood level < 180 mg/dl. Proper hydration was ensured as well. The whole-body PET-CT scan was acquired 1 h after intravenous injection of the radiotracer, and the liver PET-MRI scan was acquired immediately afterwards. Patients were instructed to void immediately prior to imaging acquisitions.
PET-CT
All whole-body PET-CT scans were performed with a 4-ring Discovery MI PET-CT system (GE Healthcare) with a PET axial field-of-view of 20 cm. The CT scans were performed with automatic mA-modulation and 120 kV, and reconstructed to a slice thickness of 2.5 mm. Contrast material was administered orally and intravenously, unless contraindicated. PET acquisition was performed with an acquisition time of 2.5–3 min per bed position. The scans were reconstructed in a matrix size of 256 × 256 with a pixel size of 2.7 mm and a slice thickness of 2.8 mm. The reconstruction method used TOF information and included normalization and image corrections for attenuation, scatter, randoms, and dead time. The data were reconstructed by means of the Bayesian penalized likelihood reconstruction algorithm (Q.clear; GE Healthcare), with a penalization factor (β) of 500.
PET-MRI
All liver PET-MRI scans were performed with a simultaneous TOF SIGNA™ PET-MRI system (GE Healthcare) scanner, having five rings of 28 detector blocks each, a PET axial field-of-view of 25 cm, and a TOF timing resolution of 400 ps. After performing a partial body MR localizer scan, PET acquisition of the liver area (one bed station, 20 min per bed position) was started. During PET scanning, a default 3D dual-echo, spoiled gradient recalled echo MR sequence was performed for PET attenuation correction (MRAC). Fat and water images were automatically reconstructed from the obtained in-phase and out-of-phase images. The PET attenuation correction algorithm uses a continuous fat–water-based attenuation correction method. Besides the MRAC sequence, the MRI protocol included axial and coronal single shot FSE T2-weighted images, axial fast recovery FSE T2-weighted images with fat suppression, DWI with apparent diffusion coefficient (ADC) map, and axial Lava MPH T-1 fat suppression images. Dynamic contrast enhanced (DCE) MRI that included arterial, portal, and delayed phase imaging was performed after the injection of gadolinium-based contrast agent as the last part of the PET-MRI scan, and was available for interpretation in 77/97 of the studied cases (in the other cases, contrast agent injection was either contraindicated or not performed due to patient request or lack of patient cooperation). The total imaging time for a full liver PET-MRI scan was around 40 min.
Image analysis and data categorization
For each of the paired scans, the net acquired data were interpreted and reported jointly in consensus by a nuclear medicine physician (EES) and a body radiologist (CL), each with decades of experience in reading oncologic imaging. In all of the included paired scans, the interpreting physicians reported the existence of at least one malignant liver lesion. Primary tumor type, the patient’s clinical history and course of the disease, the presence and location of other abnormal imaging findings, findings on previous imaging studies, and any available laboratory and pathological data were all used to assist in categorizing the nature of liver lesions. All cases were reviewed, and in cases when PET-MRI detected additional malignant lesions that were not identified on PET-CT, the number of these lesions in each case, the size of each lesion, as well as their imaging characteristics on the different components of the PET-MRI scan ([18F]FDG-avidity on the PET part of the PET-MRI, restrictive water diffusivity on DWI/ADC data, and contrast enhancement on DCE sequences when available) were recorded. The main advantage PET-MRI offered in each case, and the influence on the final report were recorded as follows.
PET-MRI advantages
were categorized as Advantage 1, when the additional lesions were identified as malignant mainly based on [18F]FDG-avidity that they demonstrated on the PET of the PET-MRI scan but not on the PET of the PET-CT scan; Advantage 2, when lesions ≤ 0.8 cm in size were identified as malignant based on MRI data only; or Advantage 3, when lesions larger than 0.8 cm in size were categorized as viable malignant lesions based on MRI data, despite showing low/no [18F]FDG uptake on both the PET of the PET-CT scan and the PET of the PET-MRI scan.
The influence on the final report
was considered Major, if the additional data on PET-MRI caused a significant change in the final report’s conclusion, namely, on staging/restaging cases, the PET-MRI data defined the existence of malignant hepatic disease that would have otherwise been missed on PET-CT, and when monitoring response to therapy, the PET-MRI data caused a change in the reported response assessment category (e.g., stable or progressive disease was reported, instead of remission that would have been reported based on PET-CT alone); Minor, if the final conclusion would have been similar on PET-CT alone, but the additional data identified on PET-MRI increased the level of confidence and caused the final conclusion to be more definitive; or Negligible, if the additional evident data on PET-MRI changed neither the final conclusion nor the degree of confidence in the final conclusion.
Statistical analysis
Categorical data were described with contingency tables that included frequency and percent. Continuous variables were evaluated for normal distribution and reported as median and interquartile range (IQR). Wilcoxon signed-rank test was used to compare medians of continuous variables between two paired groups. A two-sided P-value of < 0.05 was considered statistically significant. SPSS software (IBM SPSS Statistics for Windows, version 27, IBM Corp., Armonk, NY, USA, 2017) was used for statistical analysis.
Results
Malignant hepatic lesions detected on PET-MRI but not on PET-CT: rates and characteristics
In 37/97 (38.1%) cases, of 25/61 (41.0%) patients, PET-MRI proved advantageous over PET-CT in detecting malignant hepatic lesions. In 16, 9, and 12 of these cases, the number of additional lesions identified on PET-MRI but not on PET-CT was 1, 2, and ≥ 3 lesions per case, respectively, summing up to a total of 78 malignant lesions. Of these 78 lesions, 53 (67.9%) lesions were ≤ 0.8 cm and 25 (32.1%) were larger than 0.8 cm.
In terms of imaging characteristics, these 78 lesions demonstrated the following features on the different components of PET-MRI scans: on the PET part of the PET-MRI, 19/78 (24.4%) lesions showed [18F]FDG-avidity. On DWI/ADC data, 73/78 (93.6%) lesions demonstrated restrictive water diffusivity. As for DCE MRI, such data were not available for interpretation in 7/37 of the cases, which included 19/78 lesions. In the other 30/37 cases, which included 59/78 lesions reported as malignant based on PET-MRI only, 52/59 (88.1%) lesions demonstrated contrast enhancement on DCE sequences. Table 2 further details the imaging characteristics of the lesions identified on PET-MRI but not on PET-CT.
In 11 (11.3%), 9 (9.3%), and 17 (17.5%) of the total 97 studied cases, identifying additional malignant lesions on PET-MRI caused major, minor, and negligible effect on the final report, respectively.
The added values of PET-MRI over PET-CT in detecting malignant hepatic lesions
We hereby expand on three main advantages we identified as strengths provided by the integrated PET-MRI system over the PET-CT system (see illustration in Fig. 1), as well as on the influence these advantages had on final report conclusions.
Illustration of the study design and main results. Among the 97 pairs of whole-body [18F]FDG PET-CT and subsequent liver PET-MRI, all interpreted jointly and reported as having at least one active malignant hepatic lesion, a total of 78 malignant lesions were identified on PET-MRI but not on PET-CT. Focusing on these lesions, three main advantages provided by integrated PET-MRI over PET-CT are discussed in the current study and illustrated in the lower row of the figure. On the left, Advantage 1 is illustrated: [18F]FDG-avid lesions were identified as malignant based on the PET of the PET-MRI but not on the PET of the PET-CT. On the middle, Advantage 2 is illustrated: small lesions, below PET resolution, were categorized as malignant based on MRI data. On the right, Advantage 3 is illustrated: larger lesions, with no or only faint [18F]FDG uptake, were categorized as viable based on MRI data
Advantage 1: [18F]FDG-avid lesions were identified as malignant based on the PET of the PET-MRI but not on the PET of the PET-CT
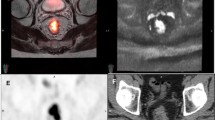
In 11 (11.3%) of the 97 included paired scans, a total of 19 malignant hepatic lesions were reported based on a clear focal “hot” spot that was evident on the PET of the PET-MRI scan only (Fig. 2). On a retrospective evaluation of these lesions on the PET part of the PET-CT scan, these lesions were very faint (n = 7) or not evident at all (n = 12), with significantly lower median target-to-background uptake ratio measured on the PET of the PET-CT scan compared with the PET of the PET-MRI (1 versus 1.61, Pv < 0.01). These lesions were either small (≤ 0.8 cm, n = 16) or larger with necrotic center and circumferential [18F]FDG uptake (n = 3).
Representative examples of [18F]FDG-avid lesions detected on the PET of the PET-MRI only. While clear focal “hot” spots were evident on the PET of the PET-MRI scan (red arrows, two left columns), matched locations in the PET of the PET-CT scan showed no focal [18F]FDG uptake (two right columns). Patients with gastric (A), pancreatic (B), and rectal (C) cancers underwent imaging for assessing response to systemic therapies, and these lesions that were evident on PET-MRI defined progressive (A, B) or stable (C) metabolic hepatic disease instead of a complete metabolic remission that would have been reported had PET-CT been performed alone. PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
Although categorized as malignant primarily based on their clear-cut [18F]FDG-avidity demonstrated on the PET part of the PET-MRI, 16/19 lesions had supporting MRI features (Table 2) on DWI/ADC data (16 lesions) and on DCE MRI sequences (14 lesions).
As for the influence on the final reports among these 11 cases: in 5 cases, all performed for monitoring response to therapy, the additional lesions on PET-MRI had major influence on the final report, by defining progressive (n = 3) or stable (n = 2) metabolic hepatic disease instead of complete metabolic remission that would have been reported had PET-CT been performed alone. In 4 cases, the additional lesions had minor influence on the final report, only increasing reading confidence. In the remaining 2 cases, the additional lesions had negligible influence on the final report.
Advantage 2: small lesions, below PET resolution, were categorized as malignant based on MRI data
In 14 (14.4%) of the 97 included paired scans, a total of 37 malignant hepatic lesions were reported based on MRI data only, and were [18F]FDG- “negative” on the PET data of both the PET-CT and the PET-MRI scans, due to their small size, all ≤ 0.8 cm (Fig. 3).
Representative examples of small lesions that were detected as malignant based on MRI data (A–C). While restricted water diffusivity was evident in these lesions (red arrows on high b-value DWI-MRI data), these lesions were too small for assessment on the PET of the PET-CT and the PET-MRI scans. Patients with gastric (A), gastroesophageal junction (B), and colorectal (C) cancers underwent imaging for assessing response to systemic therapies. Although these additional small lesions were identified on PET-MRI, progressive disease was already clearly evident based on the PET-CT data of these patients (for example, pre- and post-therapy fusion PET-CT of patient A are presented on D, with new lesions that defined progression marked with arrowheads). PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography; DWI, diffusion-weighted imaging
In 4/14 of these paired scans, DCE data were not available, and 14 lesions were hence categorized as malignant based on their restricted water diffusivity demonstrated on DWI/ADC data (Table 2). In the other 10/14 cases, DCE data were available, and the lesions demonstrated restricted diffusivity on DWI/ADC data (22/23 lesions) and/or enhancement on DCE sequences (19/23 lesions).
These 37 additional lesions, that were only evident due to the addition of the PET-MRI scan, had negligible influence on the final report in 11/14 cases, in 8 of which a progressive disease was already clearly evident on PET-CT. In two cases, the influence was minor, and in only one case, the effect on the final report was major.
Advantage 3: larger lesions, with no or only faint [18F]FDG uptake, were categorized as viable based on MRI data
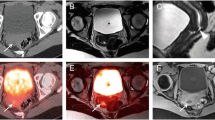
In 12 (12.4%) of the 97 included paired scans, a total of 22 malignant hepatic lesions, all larger than 0.8 cm (median size 1.6 cm, IQR 1.1–2.3 cm), were reported based on MRI data only, even though they demonstrated low [18F]FDG uptake (not higher than that observed in the near liver) on the PET data of both the PET-CT and the PET-MRI scans.
In 2/12 of these cases, of patients with malignancies with low [18F]FDG-avidity (one with HCC and the other with RCC), a total of 3 lesions were identified as viable based on MRI data: 2/3 and 3/3 lesions demonstrated restrictive diffusivity and contrast enhancement, respectively.
In the other 10/12 cases, all performed for monitoring response to therapy, a total of 19 lesions demonstrated restrictive diffusivity on DWI/ADC data (19/19 lesions) and contrast enhancement on DCE sequences (16/19 lesions), and were hence reported as viable lesions despite the low [18F]FDG uptake they demonstrated on PET (Fig. 4). In these cases, lesion characteristics were attributed to therapy-induced metabolic shutdown, and these viable hepatic lesions would have been missed had PET-CT been applied alone.
Representative examples of lesions with no or faint [18F]FDG uptake that were identified as viable malignant lesions based on MRI data. These lesions were not small, and despite the low [18F]FDG uptake they demonstrated on PET, they were interpreted as viable based on DWI and/or DCE MRI data (red arrows). Patients with GIST (A), colorectal (B), and hepatocellular (C) carcinomas underwent imaging for assessing response to systemic therapies (A, B) or for staging (C). Despite the PET-negativity of the marked lesions, caused by either therapy-induced metabolic shutdown (A, B) or by inherent low [18F]FDG-avidity (C), the multi-parametric viability assessment offered by PET-MRI enabled the identification of these viable lesions. PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography; DWI, diffusion-weighted imaging; DCE, dynamic contrast enhanced
Among the 12 cases described in this section, major, minor, and negligible effects on the final reports were recorded in 5, 3, and 4 cases, respectively.
Discussion
Given the potential advantages the integrated PET-MRI system offers in oncologic imaging, expanding its clinical application awaits gaining real-life robust evidences of the added values it offers in predefined clinical settings. In the context of assessing malignant hepatic involvement, previous data support the benefit provided by [18F]FDG PET-CT, MRI, and retrospective fusion of [18F]FDG-PET and MRI [1,2,3,4,5,6,7,8,9]. However, data on the use of integrated PET-MRI systems are still very limited [10,11,12,13].
As part of an ongoing mission to refine our community’s ability to correctly detect and assess malignant lesions, the current study was designed to trace and specify the main advantages integrated PET-MRI systems offer to interpreting physicians, who routinely read [18F]FDG PET-CT scans. We intentionally aimed to focus on the findings that we might be “missing” on our routine clinical work, but would potentially be locating with the aid of integrated PET-MRI. Beyond the somewhat expected improved detection rate of malignant hepatic lesions, stemming from both excellent PET and MRI performance and covered in depth in our data, we shed a light on the multi-parametric complementary data obtained with PET-MRI for viability assessment, one of the fundamental concepts in oncologic imaging. To frame our data in clinical setting, we also evaluated the possible effect that the integrated PET-MRI system has on interpreting physicians in terms of altering their final report conclusion and their degree of confidence.
One major result presented in this paper is the added value of the integrated PET-MRI system in detecting “hot” [18F]FDG-positive hepatic lesions that were not evident on the PET part of the paired PET-CT scan. Given the physiologic [18F]FDG activity in the liver, the quality of PET data is extremely crucial for identification of “hot” pathologic spots. The better PET performance of the PET-MRI system (compared with the PET-CT system) that is demonstrated in our data, which proved advantageous in around one-tenth of the current study’s cases, was previously described only by Zhou et al. [12]. Although the data obtained in the current study cannot fully provide definitive explanations to this intriguing yet important clinical observation, after extensive review of literature, we hereby suggest four possible factors that may be related to the different PET performance documented in our data in two TOF PET systems. First, Zhou et al. [12] attributed this phenomenon to later image acquisition (time from radiotracer injection), raising the possibility that [18F]FDG continues to “seep into” malignant lesions during the time interval between the two paired scans. Although some studies support that tumor concentration of [18F]FDG does not reach a plateau within the first 90 min post-injection [21], later studies concluded that optimizing the post-injection imaging time in oncologic PET is complex [22] and current well-verified guidelines recommend standardizing PET imaging to around 60 min post-injection [23], making this explanation alone unsatisfactory. Second, longer PET acquisition time per bed position (2.5–3 min in the PET-CT scan vs 20 min in the PET-MRI scan) can theoretically improve lesion detectability [24]. Here again, this explanation is not likely to solely explain the observed phenomenon, as both acquisition time lengths are well-above “acceptable” acquisition lengths [24, 25]. Third, differences in detector configuration between PET systems (in the current study, 4-ring 20 cm axial field-of-view PET-CT system vs 5-ring 25 cm axial field-of-view PET-MRI system) have been shown to affect PET performance in phantom-based studies, particularly photon sensitivity and background variability [26, 27]. Fourth, the conceptually different attenuation-correction techniques applied in the two PET systems (CT-based vs MRAC sequences-based) should be noted as another possible cause to incongruent PET findings [28]. This “real-life” phenomenon is probably the result of some combination of the possible contributing factors mentioned above. Either way, this result should raise our community’s awareness to the fact that in non-negligible proportion of cases, PET-CT might be underestimating the real [18F]FDG-positive extent of hepatic malignant involvement.
In terms of detection rate, our finding that lesion detection of small lesions was improved by MRI was relatively straightforward, given the intrinsic higher spatial resolution offered by conventional and advanced MRI techniques. Still, it seems that in our cohort, these small lesions that were evident on MRI and not on PET-CT had relatively limited effect on final reports, since in most of such cases (more than three-quarters), PET-CT alone could have identified the existence of hepatic malignant lesions or their response to therapy, with the additional lesions detected on MRI not changing the final conclusion nor the interpretation confidence. Previous cohorts, however, that did focus on the better conspicuity offered by the MRI part of the PET-MRI scan, described that PET-MRI did aid in clinical decision-making [12] and in increasing interpretation confidence [13].
Although the current study does not aim to evaluate the role of MRI in assessing hepatic malignant lesions, nor to make claims about optimizing MRI protocols, a worth-mentioning observation that arises from our data is that among the total studied cases, only 2 malignant hepatic lesions were categorized as such based on DCE imaging alone (without demonstrating accompanying [18F]FDG-avidity, nor restricted water diffusivity). Given that contrast agent injection is contraindicated in some patients and requires patient cooperation, it seems possible that one-stop liver PET-MRI scan that contains DWI/ADC can be considered sufficient for assessing malignant hepatic disease in frail oncologic patients. This possible approach should be further studied.
Assessing tumor viability is one of the cornerstones of oncologic imaging. The possibility of false-negativity on [18F]FDG PET, mainly in the context of therapy-induced metabolic shutdown of viable lesions [29,30,31], highlights the important complementary role that MRI can play in tumor viability assessment when PET-MRI is applied. With therapies potentially affecting GLUT expression and thus altering [18F]FDG uptake [31], the assessment of lesion viability based on water diffusivity through DWI-MRI, irrespectively of glucose transport and metabolism, ended up in the current cohort at identifying around one-quarter of the total number of additional lesions. When this advantage was evident on PET-MRI, major influence on the final conclusion was indeed common.
The current study summarizes our initial experience with the integrated PET-MRI system in the context of liver malignancies. Still, given the study design of sequential application of liver PET-MRI after whole-body PET-CT, the results cannot be directly translated into real-life clinical practice, particularly not to application and interpretation of liver PET-MRI without paired PET-CT, or to image acquisition of liver PET-MRI starting 1-h post tracer injection. Even though this study includes a large number of cases compared with previous studies aimed to assess the role of PET-MRI in liver malignancies, the relatively small cohort, the heterogeneity in tumor origins, as well as in indications for imaging and recent therapies given to included patients are all limiting the generalizability of the results.
Conclusion
Assessing hepatic involvement in malignancy with integrated PET-MRI system has several added values over PET-CT-based assessment. Not only aiding in lesion detection due to excellent PET performance and MRI spatial resolution, the complementary data obtained simultaneously by PET-MRI have added value in assessing lesion viability. While these advantages of PET-MRI over PET-CT are met commonly when both modalities are applied sequentially, significant changes in the final report’s conclusion provided by interpreting physicians seem less common. We believe that the presented data, together with previous and future relevant data, will pave the way to defining liver oncologic assessment as a potential clinical indication for performing PET-MRI.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- [18F]FDG:
-
18F-fluorodeoxyglucose
- PET-MRI:
-
Positron emission tomography-magnetic resonance imaging
- PET-CT:
-
Positron emission tomography-computed tomography
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- DCE:
-
Dynamic contrast enhanced
- TOF:
-
Time of flight
- Pv:
-
P-Value
- IQR:
-
Interquartile range
References
Hectors SJ, Wagner M, Besa C, Huang W, Taouli B. Multiparametric FDG-PET/MRI of hepatocellular carcinoma: initial experience. Contrast Media Mol Imaging. 2018;3:2018.
Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis. Meta-analysis. In Database of abstracts of reviews of effects (DARE): quality-assessed reviews [Internet] 2005. Centre for Reviews and Dissemination (UK).
Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. In Database of abstracts of reviews of effects (DARE): quality-assessed reviews [Internet] 2010. Centre for Reviews and Dissemination (UK).
Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31(1):19–31.
Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26(12):4595–615.
Renzulli M, Clemente A, Ierardi AM, Pettinari I, Tovoli F, Brocchi S, Peta G, Cappabianca S, Carrafiello G, Golfieri R. Imaging of colorectal liver metastases: new developments and pending issues. Cancers. 2020;12(1):151.
Tahtabasi M, Erturk SM, Basak M. Comparison of MRI and 18F-FDG PET/CT in the liver metastases of gastrointestinal and pancreaticobiliary tumors. Med Bull Sisli Etfal Hosp. 2021;55(1):12.
Yong TW, Yuan ZZ, Jun Z, Lin Z, He WZ, Juanqi Z. Sensitivity of PET/MR images in liver metastases from colorectal carcinoma. Hell J Nucl Med. 2011;14(3):264–8.
Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B, Weishaupt D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA–enhanced MRI. J Nucl Med. 2010;51(5):692–9.
Brendle C, Schwenzer NF, Rempp H, Schmidt H, Pfannenberg C, La Fougère C, Nikolaou K, Schraml C. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43(1):123–32.
Zhou N, Guo X, Sun H, Yu B, Zhu H, Li N, Yang Z. The value of 18F-FDG PET/CT and abdominal PET/MRI as a one-stop protocol in patients with potentially resectable colorectal liver metastases. Front Oncol. 2021;11:714948. https://doi.org/10.3389/fonc.2021.714948.
Zhou N, Meng X, Zhang Y, Yu B, Yuan J, Yu J, Zhu H, Yang Z. Diagnostic value of delayed PET/MR in liver metastasis in comparison with PET/CT. Front Oncol. 2021;11:717687. https://doi.org/10.3389/fonc.2021.717687
Beiderwellen K, Geraldo L, Ruhlmann V, Heusch P, Gomez B, Nensa F, Umutlu L, Lauenstein TC. Accuracy of [18F] FDG PET/MRI for the detection of liver metastases. PLoS One. 2015;10(9): e0137285.
Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS SSO and TOS. Annals Oncol. 2018;29(1):44–70.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology colon cancer version 1. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 13 Nov 2022.
Martin O, Schaarschmidt BM, Kirchner J, Suntharalingam S, Grueneisen J, Demircioglu A, et al. PET/MRI versus PET/CT in whole-body staging: results from a unicenter observational study in 1003 subsequent examinations. J Nucl Med. 2020;61(8):1131–6.
Mayerhoefer ME, Prosch H, Beer L, Tamandl D, Beyer T, Hoeller C, Berzaczy D, Raderer M, Preusser M, Hochmair M, Kiesewetter B. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging. 2020;47(1):51–60.
Musafargani S, Ghosh KK, Mishra S, Mahalakshmi P, Padmanabhan P, Gulyás B. PET/MRI: a frontier in era of complementary hybrid imaging. Eur J Hybrid Imaging. 2018;2(1):1–28.
Torrado-Carvajal A, Catana C. PET/MRI: technical and methodological aspects. Clinical PET/MRI. 2023;1:1–33.
GE Healthcare. SIGNA™ PET/MR technical data. http://promed-sa.com/wp-content/uploads/2020/08/PET-MR_Datasheet_DOC1545629.pdf. Accessed 13 Nov 2022.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35(8):1308–12.
Thie JA, Hubner KF, Smith GT. Optimizing imaging time for improved performance in oncology PET studies. Mol Imag Biol. 2002;4(3):238–44.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Lasnon C, Coudrais N, Houdu B, Nganoa C, Salomon T, Enilorac B, Aide N. How fast can we scan patients with modern (digital) PET/CT systems? Eur J Radiol. 2020;1(129): 109144.
Fragoso Costa P, Jentzen W, Brahmer A, Mavroeidi IA, Zarrad F, Umutlu L, Fendler WP, Rischpler C, Herrmann K, Conti M, Seifert R. Phantom-based acquisition time and image reconstruction parameter optimisation for oncologic FDG PET/CT examinations using a digital system. BMC Cancer. 2022;22(1):1–8.
Pan T, Einstein SA, Kappadath SC, Grogg KS, Lois Gomez C, Alessio AM, Hunter WC, El Fakhri G, Kinahan PE, Mawlawi OR. Performance evaluation of the 5-ring GE discovery MI PET/CT system using the national electrical manufacturers association NU 2–2012 standard. Med Phys. 2019;46(7):3025–33.
Levin C, Peterson W, Ross S, Stearns C, Uribe J. PET performance as a function of axial field of view for a new silicon photomultiplier-based whole body TOF PET/CT system. J Nucl Med. 2016;57(suppl 2):200.
Wagenknecht G, Kaiser HJ, Mottaghy FM, Herzog H. MRI for attenuation correction in PET: methods and challenges. Magn Reson Mater Phys, Biol Med. 2013;26(1):99–113.
Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med. 2003;44(2):224–39.
Glazer ES, Beaty K, Abdalla EK, Vauthey JN, Curley SA. Effectiveness of positron emission tomography for predicting chemotherapy response in colorectal cancer liver metastases. Arch Surg. 2010;145(4):340–5.
Akhurst T, Kates TJ, Mazumdar M, Yeung H, Riedel ER, Burt BM, Blumgart L, Jarnagin W, Larson SM, Fong Y. Recent chemotherapy reduces the sensitivity of [18F] fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol. 2005;23(34):8713–6.
Acknowledgements
We thank our department’s research assistants Irina Dana and Liron Maor for their assistance in conducting this study, and the anonymous reviewers of the European Journal of Nuclear Medicine and Molecular Imaging for their insightful feedbacks.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients were enrolled as part of an ongoing prospective study to evaluate the added values of PET-MRI over PET-CT in the imaging of various malignancies (ClinicalTrials.gov identifier: NCT04158414, registered November 8, 2019). This study was approved by the institutional review board (Reference ID 0568–19-TLV), and all participating patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—General.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cohen, D., Kesler, M., Muchnik Kurash, M. et al. A lesson in humility: the added values of PET-MRI over PET-CT in detecting malignant hepatic lesions. Eur J Nucl Med Mol Imaging 50, 1423–1433 (2023). https://doi.org/10.1007/s00259-022-06099-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06099-8