Abstract
Background
In radioembolization, response is achieved through the irradiation and damaging of tumor DNA. For hepatic metastases of neuroendocrine tumors, a dose–response relationship has not been established yet. This study assesses whether increasing tumor-absorbed doses lead to increased response rates.
Methods
We included all patients who underwent yttrium-90 (90Y) glass microspheres radioembolization in our center if both pre- and post-treatment contrast-enhanced CT and post-injection PET/CT were available. Up to five hepatic tumors and the healthy hepatic tissue were delineated, and absorbed dose was quantified using post-injection PET/CT. Response was measured according to RECIST 1.1 on patient and tumor level. Linear mixed models were used to study the relationship between absorbed dose and response on tumor level. Logistic regression analysis was used on patient level to study dose–response and hepatic dose-toxicity relationships.
Results
A total of 128 tumors in 26 patients (31 procedures) were included in the response analysis. While correcting for confounding by tumor volume, a significant effect of response on dose was found (p = 0.0465). Geometric mean of absorbed dose for responding tumors was 170 Gy, for stable disease 101 Gy, and for progressive disease 67 Gy. No significant dose-toxicity relationship could be identified.
Conclusion
In patients with neuroendocrine tumor liver metastases, treated with 90Y-radioembolization, a clear dose–response relationship was found. We propose to perform 90Y-radioembolization with an absolute minimum planned tumor-absorbed dose of 150 Gy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yttrium-90 (90Y)-radioembolization is a locoregional therapy for treating primary or metastatic liver tumors in inoperable patients. Most of the evidence for the efficacy and safety of radioembolization resides with hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and colorectal cancer [1,2,3,4]. However, for neuroendocrine tumor (NET) liver metastases, evidence of its efficacy is still scarcely available [5, 6]. As the treatment effect of 90Y-radioembolization is achieved through irradiating tumor tissue by β-rays from 90Y-decay, thereby inducing DNA damage and subsequent apoptosis, a dose–response relationship is highly probable [7]. A dose–response relationship based on quantitative analysis of post-treatment 90Y PET/CT has already been demonstrated for colorectal cancer, intrahepatic cholangiocarcinoma, and hepatocellular carcinoma [1, 2, 8]. In intrahepatic cholangiocarcinoma, a threshold of 150 Gy in glass 90Y-radioembolization was suggested, as this predicted increased median OS [4]. However, these thresholds could not easily be reproduced in resin 90Y-radioembolization [9]. In hepatocellular carcinoma, a threshold of 205 Gy was suggested after analyzing prospective data in the DOSISPHERE-01 trial, as a significant difference in both response rate and median overall survival was found using this threshold [10]. Finally, in mCRC a cut-off of 189 Gy average tumor-absorbed dose was found to predict metabolic response 3 months after glass 90Y-radioembolization [2]. Thus, the importance of dosimetry to achieve high tumor-absorbed radiation doses cannot be underestimated. In neuroendocrine tumor liver metastases, a dose–response relationship was confirmed in resin 90Y-radioembolization, and a threshold for tumor-specific absorbed dose of 191 Gy was suggested [3]. A dose–response relationship assessment for glass 90Y-radioembolization has not been performed yet. This study analyzes the dose–response relationship and correlates radiation absorbed dose to survival and toxicity in patients with neuroendocrine tumor liver metastases treated with glass 90Y-radioembolization.
Methods
Patients
All patients who were referred to the nuclear medicine department for 90Y-radioembolization of well or moderately differentiated grade I-III NET in our center were included. Inclusion criteria for analyses included radioembolization with glass 90Y-microspheres (TheraSphere™, Boston Scientific), availability of contrast-enhanced computed tomography (CECT) of the liver at baseline, at least 3 months follow-up, and presence of at least one measurable target lesion in the liver according to the Response Evaluation Criteria In Solid Tumors (RECIST 1.1) [11]. If patients were treated with multiple sessions of 90Y-radioembolization, those sessions were considered separate treatments if response could be assessed prior to initiation of the sequential 90Y-radioembolization procedure. Patients in whom response could not be assessed before the next treatment sessions were excluded. Other exclusion criteria included absence of 90Y-PET/CT or contrast-enhanced CT at baseline or follow-up.
Treatment procedures
Patients were considered a candidate for 90Y-radioembolization after evaluation with multiphasic CECT and discussion in a multidisciplinary tumor board. Patients were included if they had progressive liver-dominant disease, maximum Common Terminology Criteria for Adverse Events (CTCAE) grade 1 elevated bilirubin level, and inoperable disease and were in a good clinical condition (i.e., World Health Organization Performance Score 0–2). In our institution, 90Y-radioembolization is generally proposed in patients who show progression after systemic therapy (i.e., PRRT) or who have disease only limited to the liver, even if no systemic therapy was given prior.
Before a 90Y-radioembolization procedure was initiated, a planning angiography was performed in each patient. During planning angiography, technetium-99 m (99mTc)-macroaggregated albumin (99mTc-MAA; approx. 150 MBq) was infused in proposed injection positions in either the proper, right, or left hepatic arteries. SPECT/CT and planar imaging were then performed to evaluate the distribution of activity and ascertain the absence of extrahepatic depositions. Prescribed activity (GBq) was calculated using the MIRD method:
where Aprescribed is the prescribed activity at time of administration, D is the desired average delivered absorbed dose to the target volume, and Mtarget is the mass of the perfused liver volume. Depending on visual assessment of tumor load and distribution of 99mTc-MAA, or in case of a radiation segmentectomy, the desired absorbed dose was adjusted by the treating physician at their discretion: the desired absorbed dose could be either increased (e.g., when superselective targeting was possible or a relatively high tumor-to-normal ratio on 99mTc-MAA was seen) or decreased (e.g., in case of extensive liver involvement or pre-existing decreased liver function). The desired average dose was adjusted to limit the healthy liver dose in the targeted liver lobe as approximated by 99mTc-MAA visually. The morning after treatment, a post-treatment 90Y-PET/CT was acquired to assess microsphere distribution.
Follow-up occurred 1 month (patient examination and laboratory testing) and 3 months (patient examination, laboratory testing, and imaging) after 90Y-radioembolization. Routine laboratory testing was targeted at monitoring hepatic toxicity and consisted of bilirubin (Bili), alkaline phosphatase (AP), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alanine transaminase (ALT), and albumin (Alb).
Outcomes
Primary endpoint in the study was per-tumor response (i.e., tumor level) according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) on CECT 3 months post-treatment for up to five hepatic tumors per patient [11]. Per-tumor response was defined as the quantified reduction in tumor size as measured according to RECIST 1.1. To facilitate a per-tumor analysis of dose and response, response was assessed in up to five tumors in the treated liver volume, thereby differing from the original RECIST 1.1 criteria. In contrast, overall response (i.e., patient level) was assessed using at maximum two tumors per organ (max 2 hepatic target lesions), in total 5 lesions, including extrahepatic disease. A nuclear medicine physician and a radiologist evaluated response independently after 3-month follow-up, each of them evaluating response in one part of the dataset. Complete response (CR) was defined as complete resolution of the tumor, partial response (PR) was defined as a reduction of at least 30% in the largest diameter, progressive disease (PD) was defined as an increase of at least 20%, and stable disease (SD) was chosen if no other category was applicable. Objective response was the combination of both CR and PR categories. As secondary endpoints, overall response on patient level (i.e., including non-hepatic tumor burden), overall survival, and hepatic toxicity were evaluated. Both clinical and biochemical hepatic toxicity were analyzed. Hepatic toxicity was scored according to CTCAE version 5.0, and for pooled hepatotoxicity, the modified scale was used, as proposed by Braat et al. [12]. Toxicity that occurred during follow-up was excluded if toxicity was present at baseline and CTCAE grade had not worsened. If toxicity was already present at baseline, the relative increase after treatment was used to grade toxicity, as proposed by CTCAE 5.0.
Dose evaluation
In order to assess the per-tumor dose–response relationship and per-patient dose–response and dose-toxicity relationship, the absorbed dose in multiple hepatic lesions was calculated using the post-treatment 90Y-PET/CT. The PET-images were acquired on a Biograph mCT time-of-flight PET/CT scanner (Siemens Healthcare GmbH), using one or two bed positions in order to fit the entire liver in the field of view (FOV). Acquisition time was 15 min per bed position, and consecutive bed positions overlapped approximately 43%. A CT scan was made for attenuation correction. Images were reconstructed using 4 iterations with 21 subsets and a 5 mm full width at half maximum (FWHM) Gaussian post-reconstruction filter. The reconstructed voxel size was 4.1 × 4.1 × 3.0 mm3.
Tumors were delineated using pre-treatment CECT. Manual rigid image registration and manual tumor delineations were performed in Simplicit90Y (Mirada Medical Ltd.). Tumor volumes of interest (VOIs) were transferred from the co-registered CECT to the 90Y-PET/CT, and the activity at the time of image acquisition was quantified and decay-corrected to the time of administration. Equation 1 was used to calculate the per-tumor-absorbed dose. A density of 1.06 kg/L was used to convert between volume and mass of liver/tumor tissue. In order to establish the per-tumor dose–response relationship, the absorbed dose and response for all five tumors were recorded individually. On patient level, the mean tumor-absorbed dose per patient was calculated as the weighted average of all tumor-absorbed doses (max. five target lesions plus all other remaining tumors in the treated volume), and overall response (including extrahepatic disease) was determined.
Statistical analysis
Patient characteristics and demographics were described using mean or median and their appropriate range estimates. Before toxicity analysis, CTCAE grading was dichotomized, grouping grade 0–II and grade III–V. In order to balance outcome groups, alternative grouping options were also considered. Response categories were also dichotomized before analysis, resulting in objective response (i.e., partial or complete response vs stable disease or disease progression) or disease control (i.e., response or stable disease vs disease progression). Tumor dose was log-transformed in all analyses due to skewness of the dataset.
For dose-toxicity analysis, logistic regression was used, only incorporating biochemical hepatic toxicity. The following variables were added and excluded based on the AIC (Akaike information criterion): dose on the treated healthy liver tissue, whole liver treatment (yes/no), planned average dose to the perfused volume, dose on all healthy liver tissue, mean tumor dose, liver volume, and healthy liver tissue volume. Descriptive statistics were calculated for clinical toxicity.
Dose–response relationships were analyzed on tumor level and on patient level. Tumor-level dose–response relationships were modeled using linear mixed-effects regression models, in order to incorporate any correlation between tumor doses within a patient. A random intercept for absorbed dose was included, if appropriate and if it improved the model’s performance. The tumor dose was used as the dependent variable, and tumor volume was included to adjust for confounding, as tumor volume may be related to both the injected activity in a perfused volume and with response.
ROC curves were constructed using logistic regression analysis, to model the discriminatory power of tumor dose on response, while adjusting for tumor volume. Objective response (i.e., complete or partial response per tumor according to RECIST 1.1) was defined as the outcome. Backward selection based on the AIC was used to exclude several variables from the model. Bootstrapping was used to correct for overfitting and to extract confidence intervals around the c-statistic, using 10,000 bootstrap samples. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated for different cut-off points for tumor dose. Logistic regression analysis was also used to model the relationship between healthy liver dose and biochemical toxicity (dichotomized toxicity grades as dependent variable). Initial variables tested in models included treatment volume, age, tumor dose, and infused activity. Directed acyclic graphs (DAGs) were constructed, after which the variable infused activity was ignored as it precedes tumor dose in the causal chain.
On patient level, overall survival was assessed as the time between start of the first radioembolization treatment and death due to any cause. Only the first treatment of patients who underwent multiple treatments was considered. Patients were censored if they were still alive at the time of analysis or were lost to follow-up. The relationship between mean tumor dose and survival was tested as a continuous variable in the Cox proportional hazard models, adjusting for tumor volume. Furthermore, dichotomized mean tumor dose (cut-off point 150 Gy), disease control, tumor grade, planned MIRD dose, and whole liver treatment were tested using log-rank test.
Statistical analysis was performed in R (R Core Team 2020, version 4.1.0). All statistical tests were performed two-sided. A p-value of < 0.05 was considered significant.
Results
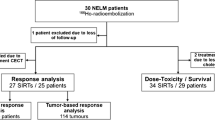
Thirty-five treatments with glass 90Y-radioembolization in 30 patients were analyzed individually (Table 1). Twenty-six patients underwent 90Y-radioembolization once, three patients were treated twice (two patients received left and right lobe treatment sequentially, one patient received two whole liver treatments), and one patient received three treatments (right liver lobe once and left liver lobe twice). All radioembolization sessions were analyzed as separate cases, as response and toxicity data was available for each individual treatment. Median prescribed absorbed dose in the perfused liver volume was 120 Gy (range 30–200 Gy, Table 2).
Dose–response
A total of 128 tumors treated in 31 angiography sessions were included in the mixed-effects model analysis. Median absorbed dose per tumor was 134 Gy (range 0.1–1879.1 Gy). A low tumor dose (< 30 Gy) was calculated in twelve tumors, mostly due to image registration mismatch. In four cases, radiological response could not be assessed due to unavailable CECT imaging during follow-up (Fig. 1). PR occurred after 4/31 (13%) procedures, SD after 17/31 (55%), and PD after 10/31 (32%). On tumor level, 3/128 (2%) tumors showed CR, 35/128 (27%) showed PR, 79/128 (62%) showed SD, and 11/128 (9%) showed PD (Table 3).
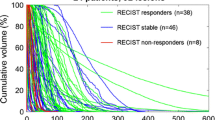
In the final model (i.e., after testing for random and fixed effects), a significant difference in dose per response category (p = 0.0465) was found while correcting for confounding by tumor volume. Therefore, mean tumor-absorbed dose was significantly related to response, irrespective of tumor size. The random intercept only partially explained the variance in tumor dose (SD of intercept = 0.54, SD of residual = 1.11). Tumors showing partial or complete response were found to have received a significantly higher absorbed dose, compared to tumors showing progression (Fig. 2). In tumors showing stable disease, the absorbed dose was 50% higher compared to tumors showing progression (p = 0.335, 95% CI [− 34%; 242%]), and in tumors showing objective response, the absorbed dose was 153% higher compared to tumors showing progression (p = 0.043, 95% CI [3.2%; 522%]). Geometric mean absorbed dose corrected for confounding was 67.3 Gy (95% CI [29.7; 153]), 101 Gy (95% CI [71.8; 142]), and 170 Gy (95% CI [108; 270]), in tumors showing progression, stable disease, and objective response, respectively.
In logistic regression analysis, objective response on tumor level was defined as the outcome. Thirty-eight of 128 included tumors showed objective response, while 90 did not. Tumor dose (p = 0.004) and total infused activity (p = 0.0498) were significant predictors of response. While correcting for confounding by tumor volume, the log-transformed tumor dose was significantly associated with tumor response (OR = 1.84, 95% CI [1.15; 2.96]). The bootstrapped AUC for predicting response while correcting for confounding was 0.71 (bootstrapped 95% CI [0.61; 0.80]); however, calibration was poor (Fig. 3). The median dose for responding tumors was 194 Gy (IQR [136; 291]), while the median absorbed dose for non-responding tumors was 114 Gy (IQR [62; 178]). The true difference in absorbed dose between responding and non-responding tumors was significant (Mann–Whitney test p < 0.001).
Specificity and sensitivity for different cut-off values were calculated (Supplementary Table 1). Consequently, an optimal cut-off point of 135 Gy was identified, with a Youden’s J equal to 0.385, and a more desirable dose of 200 Gy, as the specificity then exceeds 80%.
Dose-toxicity
Thirty treatment sessions and their respective 3-month follow-up were included in the toxicity analysis, including the four sessions in which no follow-up imaging was available. Only first-time radioembolization sessions were considered for toxicity analysis. When aggregating hepatic biochemical toxicity, eight patients had grade 3–4 toxicity, 15 patients had grade 1–2 toxicity, and seven patients showed no toxicity during 3-month follow-up. Biochemical toxicity was mostly GGT and ASAT elevation (Table 4). Clinical toxicity occurred in all but three patients, with grade 1–2 toxicity occurring in 24 patients, grade 3–4 toxicity in two patients (one case of radioembolization-induced liver disease (REILD, as defined by Braat et al.) who received 110 Gy on total healthy liver tissue and 180 Gy on tumor tissue, and one case of liver abscess who received 83 Gy on total healthy liver tissue and 693 Gy on tumor tissue), and grade 5 in one patient (fatal REILD, who received 128 Gy on total healthy liver tissue and 211 Gy on tumor tissue) [12].
The relationship between absorbed dose in treated healthy liver tissue and any biochemical toxicity of grade 3 or 4 was not significant in logistic regression analysis (OR = 1.01, 95% CI [0.985–1.038]; p = 0.409). For biochemical toxicity of grade 2 or higher, the relationship was also not significant (OR = 1.02, 95% CI [0.995–1.047]; p = 0.121). From both models, all tested variables were excluded. The bootstrap-corrected AUC for predicting toxicity grade 3 or 4 was 0.55 (bootstrapped 95% CI [0.46–0.82]), and the bootstrap-corrected AUC for predicting toxicity of grade 2 or higher was 0.68 (bootstrapped 95% CI [0.49–0.88]).
Survival
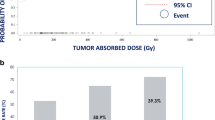
In survival analysis, 30 patients were included, and five patients were censored. Median overall follow-up time in all patients was 18 months (IQR [7; 33]). Median overall survival was 19.3 months (95% CI [12; 34.3]), and 1-year survival probability was 0.64 (95% CI [0.49; 0.85]).
Mean tumor dose was not significantly associated with overall survival in Cox-PH analysis as a continuous variable after correcting for confounding by tumor volume and age (HR per 100 Gy = 0.76, 95% CI [0.43; 1.33]). Using the cut-off value of 150 Gy (determined based on the Youden’s index in logistic regression and the median tumor-absorbed dose for responding tumors), median overall survival differed significantly (OS<150 Gy = 12.0 months, OS>150 Gy = 29.9 months) according to log-rank test (p = 0.018). However, after correcting for confounding by tumor volume and age in Cox regression analysis, the HR for the cut-off of 150 Gy on overall survival was 0.42 (95% CI [0.16; 1.12], p = 0.082; Fig. 4). Disease progression according to RECIST 1.1 was also significantly negatively associated with overall survival while correcting for tumor volume (HR 3.64, 95% CI [1.01; 13.1], p = 0.048; Fig. 4). Objective response at patient level occurred in four patients, and was therefore not tested. Tumor grade was a significant predictor of survival (p = 0.025), but tumor volume (p = 0.084), liver tumor burden > 25% (p = 0.182), planned MIRD dose of > 100 Gy (p = 0.330), and whole liver treatment (p = 0.163) did not impact overall survival (Table 5).
Discussion
The currently presented data show that response of NET liver tumors is dependent on tumor-absorbed dose after glass 90Y-radioembolization in patients with metastatic NET, in line with the AHARA concept (“as high as reasonably achievable”). A geometric mean absorbed dose of 170 Gy was found in tumors that showed response, as opposed to a geometric mean dose of 67 Gy found in tumors that showed disease progression and 101 Gy in tumors that remained stable in size. Based on the logistic regression analysis, we propose to perform 90Y-glass radioembolization with an absolute minimum planned tumor-absorbed dose of 150 Gy, which according to the presented data leads to increased response and survival benefit. A cut-off of > 200 Gy is even more desirable, as true negative rate for response then increases to more than 80%.
Radioembolization has improved over the years in terms of patient selection, targeted dose selection, tumor targeting, and prevention of liver tissue damage, and now evident dose–response relationship may be seen as both the result from and the incentive for these improvements. In improving efficacy of radioembolization, optimizing dosimetry is of utmost importance. This has already been demonstrated in earlier studies on dose–response relationship after glass 90Y-radioembolization for hepatocellular carcinoma (HCC) and colorectal cancer [2, 4, 8, 10]. In NET patients, a dose–response relationship after radioembolization has been demonstrated for resin 90Y-radioembolization [3]. In the study by Chansanti et al., tumor dose was significantly associated with both short-term and long-term response according to mRECIST, and an optimal cut-off of 193 Gy was proposed. However, their cohort was rather small (15 patients and 55 tumors). Furthermore, response was assessed using mRECIST, while in their cohort, 20% of tumors were hypovascular. Most notably, their results were based on dose estimation on pre-treatment 99mTc-MAA SPECT/CT, while the currently reported results were extracted from 90Y PET/CT. It is well known that absorbed dose estimation using 99mTc-MAA SPECT/CT is unreliable [13,14,15]. The room for improvement in estimating the tumor-absorbed dose using 99mTc-MAA is underlined by the significantly better performance of 166Ho-scout as planning procedure [16, 17]. It is therefore frequently debated whether patient dosimetry should be based on efficacy thresholds or on toxicity thresholds [18].
Considering other types of tumors treated with glass 90Y-radioembolization, dose–response and dose-survival correlation was confirmed in the dedicated DOSISPHERE-1 study (phase 3 RCT on glass 90Y-radioembolization in HCC, randomizing between standard MIRD-based treatment and personalized dosimetry) [10]. In the study, 28 patients were treated with glass 90Y-radioembolization using personalized dosimetry, and 28 patients using MIRD. A significant difference in objective response rate was found (71% vs 36%, p = 0.0074). Median overall survival also differed significantly (26.6 months vs 10.7 months, p = 0.0096). In mCRC, the relationship between radioembolization and metabolic response based on 18F-FDG PET/CT using all commercially available particles has been found; however, minimally effective tumor dose differs per particle used [2, 19, 20]. In the study by Alsultan et al., 85 tumors in 31 patients with colorectal liver metastases were treated with glass 90Y-radioembolization. They found a significant dose–response relationship with a 94% higher tumor-absorbed dose in tumor with complete metabolic response, and a 74% higher tumor-absorbed dose in patients with partial metabolic response, compared to tumors that progressed after three months. Furthermore, tumor-absorbed dose was a good predictor for response, with a c-statistic of 0.88. Concerning dose-toxicity analysis, no relationship of healthy liver dose and clinical toxicity was found, but linear regression showed that biochemical toxicity was related to healthy liver-absorbed dose. To date, no prospective studies using personalized dosimetry in mCRC have been conducted yet. Finally, Cheng et al. recently showed a dose–response relationship in cholangiocarcinoma patients treated with either resin or glass 90Y-radioembolization. A minimum tumor-absorbed dose of 150 Gy to achieve tumor response with a specificity of 80% was found for glass microspheres, but a considerably lower minimal dose of 54 Gy was found for resin microspheres [4]. Comparing studies on different types of tumors imposes problems. Dose thresholds for response can likely not be translated to neuroendocrine tumor patients, as response rates to radiation and tumor aggressiveness are important factors that account for differences in outcome.
From the presented data, a relation between healthy liver tissue dose and toxicity was not identified. However, because short-term hepatic toxicity did frequently occur in our study, and previously reported dose-toxicity relationships have been identified, be it in colorectal cancer and hepatocellular carcinoma patients, a positive relationship is still likely [19]. It is therefore difficult to propose a healthy tissue dose limit that physicians should adhere to, based on the current data. Overall, grade 3/4 hepatic toxicity was rather limited (8/35 procedures in total). Concerns have been raised on the application of radioembolization in low-grade NET patients by several small reports, discussing late cirrhotic changes years after treatment [21, 22]. However, scientific evidence on occurrence of long-term effects of radioembolization is sparse, and current available studies lack important clinical and dosimetric parameters to really understand and reliably interpret these concerns [23]. Additionally, morphological cirrhotic changes are often described on imaging studies, whether this negatively affects patient’s clinical condition and subsequent treatments remain largely unknown. In a large retrospective series on radioembolization in NET patients, many subsequent treatments following radioembolization have been safely applied, so this particular issue needs additional clarification [6].
In NET, peptide receptor radionuclide therapy (PRRT) using 177Lu-DOTATATE is often considered the first choice in treating inoperable metastasized NET after progression under somatostatin analogues. The NETTER-1 study (phase 3 RCT randomizing between long-acting octreotide or long-acting octreotide and 177Lu-DOTATATE) showed an objective response rate after PRRT of 18%, compared to reported response rates of up to 50% after resin 90Y-radioembolization [6, 24, 25]. This statistic raises the question whether it is justifiable to prioritize liver-directed therapies in patients with liver-only or liver-dominant disease. The benefits of an increased response rate may potentially outweigh the drawbacks of limited damage to the liver by radioembolization in these cases, even more so when performing personalized dosimetry. As PRRT has several systemic side effects (mainly bone marrow depression) that are not present after radioembolization, utilizing PRRT may best be postponed until extra-hepatic metastases develop or progression is observed after radioembolization. Moreover, there is currently no data advocating against early utilization of radioembolization, and radioembolization is no contra-indication for PRRT. Radioembolization is mostly advocated as one of multiple locoregional therapy options to be used in patients with predominant liver disease or stable extrahepatic disease, optionally in conjunction with systemic treatment. In current European guidelines (i.e., 2016 ENETS Consensus Guidelines and the 2020 ESMO Clinical Practice Guidelines), radioembolization is briefly mentioned as viable treatment for hepatic metastases of NET [26, 27]. Both guidelines propose the use of locoregional therapies including radioembolization in early disease stages, but a more specific recommendation is not made due to the lack of evidence. The preference for bland embolization, chemo-embolization, or radioembolization remains subject of scientific debate [5, 28,29,30,31]. Toxicity profiles seem similar, while response rates are variable. One big advantage of radioembolization as opposed to bland embolization and chemo-embolization is the possibility to perform personalized dosimetry.
This study has some limitations. First, a relatively small sample size was used, due to absence of qualitatively adequate follow-up imaging in some patients and overall limited number of glass 90Y-radioembolization treatments carried out in NET patients. However, due to per-tumor analysis, a rather adequate sample size was achieved through mixed-model analysis, from which conclusions on patient level could be inferred. Secondly, a relationship between clinical toxicity and healthy liver tissue dose could not be studied due to infrequent occurrence of events and absence of clear measuring tools. Thirdly, RECIST 1.1 was used as the primary endpoint for response. mRECIST criteria would be more suitable for hypervascular tumors, but in the current dataset, many tumors could not reliably be measured according to mRECIST or due to missing multiphase contrast-enhanced CTs at baseline or follow-up. Finally, a relatively small follow-up time was chosen, so that long-term response and toxicity could not be evaluated.
Even though the data presented in this study shows a significant correlation between dose and response on tumor level in NET, a correlation on patient level between tumor dose and response and survival needs to be studied in larger cohorts. The power of such studies needs to be sufficiently large, to accommodate for large variance in patient and tumor characteristics, inherently present in NET patients. Given the clear dose–response relationship, attention needs to be primarily focused on personalized dosimetry, both in research and in clinical practice. However, in patients with extrahepatic disease and bulky intrahepatic disease, combination therapies (radioembolization + systemic treatments) are worth investigating. As shown by the post hoc analysis of the NETTER-1 study, bulky liver disease results in a poorer outcome, representing a large portion of patients in daily practice, whom in theory may benefit from an additional “treatment boost” to liver disease. Whether the combination should entail somatostatin analogs, everolimus, chemotherapy (e.g., CAPTEM) or PRRT, remains a matter of scientific debate in light of limited scientific evidence [32,33,34,35,36].
In conclusion, this study presents the first evidence of a dose–response relationship in neuroendocrine tumor liver metastases treated with glass 90Y-radioembolization. Both on tumor level and patient level, a higher tumor-absorbed dose resulted in higher response rates. We propose to perform 90Y-radioembolization procedures with an absolute minimum planned mean tumor-absorbed dose of 150 Gy.
Data availability
The data that support the findings of this study and the code used to perform analyses are available from the corresponding author upon reasonable request.
References
Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. Relationship of tumor radiation–absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. 2020;296:673–84. https://doi.org/10.1148/radiol.2020191606.
Alsultan AA, van Roekel C, Barentsz MW, Smits MLJ, Kunnen B, Koopman M, et al. Dose-response and dose-toxicity relationships for yttrium-90 glass radioembolization in patients with colorectal cancer liver metastases. J Nucl Med. 2021;jnumed.120.255745. https://doi.org/10.2967/jnumed.120.255745.
Chansanti O, Jahangiri Y, Matsui Y, Adachi A, Geeratikun Y, Kaufman JA, et al. Tumor dose response in yttrium-90 resin microsphere embolization for neuroendocrine liver metastases: a tumor-specific analysis with dose estimation using SPECT-CT. J Vasc Interv Radiol. 2017;28:1528–35. https://doi.org/10.1016/j.jvir.2017.07.008.
Cheng B, Villalobos A, Sethi I, Wagstaff W, Galt J, Brandon D, et al. Determination of tumor dose response thresholds in patients with chemorefractory intrahepatic cholangiocarcinoma treated with resin and glass-based Y90 radioembolization. Cardiovasc Intervent Radiol. 2021;44:1194–203. https://doi.org/10.1007/s00270-021-02834-0.
Barat M, Cottereau A-S, Kedra A, Dermine S, Palmieri L-J, Coriat R, et al. The role of interventional radiology for the treatment of hepatic metastases from neuroendocrine tumor: an updated review. J Clin Med. 2020;9:2302. https://doi.org/10.3390/jcm9072302.
Braat AJAT, Kappadath SC, Ahmadzadehfar H, Stothers CL, Frilling A, Deroose CM, et al. Radioembolization with 90Y resin microspheres of neuroendocrine liver metastases: international multicenter study on efficacy and toxicity. Cardiovasc Intervent Radiol. 2019;42:413–25. https://doi.org/10.1007/s00270-018-2148-0.
Parliament MB, Murray D. Single nucleotide polymorphisms of DNA repair genes as predictors of radioresponse. Semin Radiat Oncol. 2010;20:232–40. https://doi.org/10.1016/j.semradonc.2010.05.003.
Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after 90Y-radioembolization with glass microspheres using 90Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–61. https://doi.org/10.1016/j.ijrobp.2018.05.062.
Willowson KP, Eslick EM, Bailey DL. Individualised dosimetry and safety of SIRT for intrahepatic cholangiocarcinoma. EJNMMI Phys. 2021;8:65. https://doi.org/10.1186/s40658-021-00406-2.
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, De Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. https://doi.org/10.1016/S2468-1253(20)30290-9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Braat MNGJA, Van Erpecum KJ, Zonnenberg BA, Van Den Bosch MAJ, Lam MGEH. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol. 2017;29:144–52. https://doi.org/10.1097/MEG.0000000000000772.
Haste P, Tann M, Persohn S, LaRoche T, Aaron V, Mauxion T, et al. Correlation of technetium-99m macroaggregated albumin and yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol. 2017;28:722–730.e1. https://doi.org/10.1016/j.jvir.2016.12.1221.
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, et al. Comparative dosimetry between 99mTc-MAA SPECT/CT and 90Y PET/CT in primary and metastatic liver tumors. Eur J Nucl Med Mol Imaging. 2020;47:828–37. https://doi.org/10.1007/s00259-019-04465-7.
Gnesin S, Canetti L, Adib S, Cherbuin N, Silva Monteiro M, Bize P, et al. Partition model-based 99mTc-MAA SPECT/CT Predictive dosimetry compared with 90Y TOF PET/CT posttreatment dosimetry in radioembolization of hepatocellular carcinoma: a quantitative agreement comparison. J Nucl Med. 2016;57:1672–8. https://doi.org/10.2967/jnumed.116.173104.
Smits MLJ, Dassen MG, Prince JF, Braat AJAT, Beijst C, Bruijnen RCG, et al. The superior predictive value of Ho-scout compared with mTc-macroaggregated albumin prior to Ho-microspheres radioembolization in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2019;47:798–806. https://doi.org/10.1007/S00259-019-04460-Y.
Chiesa C, Maccauro M. 166Ho microsphere scout dose for more accurate radioembolization treatment planning. Eur J Nucl Med Mol Imaging. 2019;47:744–7. https://doi.org/10.1007/S00259-019-04617-9.
Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imaging. 2020;47:3018–32. https://doi.org/10.1007/S00259-020-04845-4.
van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat AJAT, de Jong HWAM, et al. Dose-effect relationships of 166Ho radioembolization in colorectal cancer. J Nucl Med. 2021;62:272–9. https://doi.org/10.2967/jnumed.120.243832.
Van Den Hoven AF, Rosenbaum CENM, Elias SG, De Jong HWAM, Koopman M, Verkooijen HM, et al. Insights into the dose-response relationship of radioembolization with resin Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57:1014–9. https://doi.org/10.2967/jnumed.115.166942.
Tomozawa Y, Jahangiri Y, Pathak P, Kolbeck KJ, Schenning RC, Kaufman JA, et al. long-term toxicity after transarterial radioembolization with yttrium-90 using resin microspheres for neuroendocrine tumor liver metastases. J Vasc Interv Radiol. 2018;29:858–65. https://doi.org/10.1016/j.jvir.2018.02.002.
Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol. 2019;30:1915–23. https://doi.org/10.1016/j.jvir.2019.06.003.
Braat MNGJA, Braat AJAT, Lam MGEH. Personalized dosimetry: the way to limit hepatotoxicity. J Vasc Interv Radiol. 2020;31:515–6. https://doi.org/10.1016/j.jvir.2019.11.038.
Devcic Z, Rosenberg J, Braat AJA, Techasith T, Banerjee A, Sze DY, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55:1404–10. https://doi.org/10.2967/jnumed.113.135855.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. https://doi.org/10.1056/NEJMoa1607427.
Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–85. https://doi.org/10.1159/000443167.
Pavel M, Öberg K, Falconi M, Krenning EPP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:844–60. https://doi.org/10.1016/j.annonc.2020.03.304.
Egger ME, Armstrong E, Martin RC, Scoggins CR, Philips P, Shah M, et al. Transarterial chemoembolization vs radioembolization for neuroendocrine liver metastases: a multi-institutional analysis. J Am Coll Surg. 2020;230:363–70. https://doi.org/10.1016/j.jamcollsurg.2019.12.026.
Currie BM, Nadolski G, Mondschein J, Dagli M, Sudheendra D, Stavropoulos SW, et al. Chronic hepatotoxicity in patients with metastatic neuroendocrine tumor: transarterial chemoembolization versus transarterial radioembolization. J Vasc Interv Radiol. 2020;31:1627–35. https://doi.org/10.1016/j.jvir.2020.05.019.
Currie BM, Nadolski G, Soulen MC. Response Letter to Correspondence Regarding “Chronic Hepatotoxicity in Patients with Metastatic Neuroendocrine Tumor: transarterial chemoembolization versus transarterial radioembolization.” J Vasc Interv Radiol. 2021;32:483–4. https://doi.org/10.1016/j.jvir.2020.11.019.
Padia SA. Radioembolization versus chemoembolization for neuroendocrine metastases. J Vasc Interv Radiol. 2021;32:482–3. https://doi.org/10.1016/j.jvir.2020.11.017.
Braat AJAT, Bruijnen RCG, van Rooij R, Braat MNGJA, Wessels FJ, van Leeuwaarde RS, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020;21:561–70. https://doi.org/10.1016/S1470-2045(20)30027-9.
Soulen MC, van Houten D, Teitelbaum UR, Damjanov N, Cengel KA, Metz DC. Safety and feasibility of integrating yttrium-90 radioembolization with capecitabine-temozolomide for grade 2 liver-dominant metastatic neuroendocrine tumors. Pancreas. 2018;47:980–4. https://doi.org/10.1097/MPA.0000000000001115.
Kim HS, Shaib WL, Zhang C, Nagaraju GP, Wu C, Alese OB, et al. Phase 1b study of pasireotide, everolimus, and selective internal radioembolization therapy for unresectable neuroendocrine tumors with hepatic metastases. Cancer. 2018;124:1992–2000. https://doi.org/10.1002/cncr.31192.
Study of lanreotide in patients with metastatic gastrointestinal neuroendocrine tumors who are undergoing liver-directed radioembolization with yttrium-90 microspheres - full text view - ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT02859064. Accessed 4 Aug 2021.
Strosberg J, Kunz PL, Hendifar A, Yao J, Bushnell D, Kulke MH, et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: an analysis of the NETTER-1 study. Eur J Nucl Med Mol Imaging. 2020;1–11. https://doi.org/10.1007/s00259-020-04709-x.
Author information
Authors and Affiliations
Contributions
ML and AB were in charge of medical care of the treated patients. AB and SE were responsible for data collection. SE and CR performed data analysis. Data analysis was supervised by MWB and AB. MNB reviewed patient’s imaging. SE is the main author of this manuscript; all authors reviewed the manuscript and gave consent for publication of the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
By the retrospective and anonymous nature of the study, the need for approval by an ethics committee was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebbers, S.C., van Roekel, C., Braat, M.N.G.J.A. et al. Dose–response relationship after yttrium-90-radioembolization with glass microspheres in patients with neuroendocrine tumor liver metastases. Eur J Nucl Med Mol Imaging 49, 1700–1710 (2022). https://doi.org/10.1007/s00259-021-05642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05642-3