Abstract
Purpose
We compared the diagnostic accuracy of detecting distant metastases for baseline rectal cancer staging between PET/MRI and conventional staging (CS).
Materials and methods
This prospective study from November 2016 to April 2018 included 101 rectal adenocarcinoma patients for primary staging. These patients underwent whole-body PET/MRI in addition to CS (pelvic MRI and thoracic and abdominal contrast-enhanced CT). Different readers analyzed CS and PET/MRI findings for primary tumor, nodal, and metastatic staging. The presence, number, and location of metastases were recorded according to the organ involved (non-regional lymph nodes (LNs), liver, lungs, or others). Lesions were defined as positive, negative, or indeterminate. The number of lesions per organ was limited to 10. The McNemar test was used to compare the accuracies.
Results
PET/MRI exhibited a higher accuracy in detecting metastatic disease than CS in all patients (88.4% vs. 82.6%, p = 0.003) and in patients with extramural vascular invasion (EMVI) (88.9% vs. 85.5%, p = 0.013). The detection rate of PET/MRI was superior to that of CS for all lesions [84.1% vs. 68.9%, p = 0.001], as well as those in the liver (89.2% vs. 84.2%), non-regional LNs (90.0% vs. 36.7%), and lungs (76.4% vs. 66.9%). PET/MRI correctly classified 19/33 (57.5%) patients with indeterminate lesions on CS.
Conclusion
PET/MRI yields higher accuracy than CS for detecting distant synchronous metastases in the baseline staging of patients with rectal cancer and EMVI. PET/MRI exhibited a higher detection rate than CS for identifying non-regional LNs, hepatic lesions, and pulmonary lesions as well as correctly classifying patients with indeterminate lesions.
Trial registration
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Baseline staging of rectal cancer requires a multidisciplinary team to define the best treatment approach. The imaging work-up for the primary staging of rectal cancer consists of pelvic magnetic resonance imaging (MRI) for locoregional evaluation and thoracic and abdominal contrast-enhanced computed tomography (ceCT) for detection of distant metastases, as recommended by ESMO and NCCN guidelines [1, 2]. Alternatively, positron emission tomography/CT (PET/CT) can be used, especially for the following: (1) to characterize indeterminate lesions on ceCT, (2) to evaluate potentially curable metastatic disease (and exclude occult sites of metastases), and (3) to stage patients at high risk for metastases, i.e., with extensive extramural vascular invasion (EMVI) or high levels of carcinoembryonic antigen [1, 2]. Additionally, a liver MRI might be considered to assess indeterminate liver lesions on ceCT [1, 2]. The T and N stages of primary rectal tumors are defined according to pelvic MRI, which identifies locally advanced rectal tumors and facilitates in guiding the treatment plan [3]. The M stage is defined according to thoracic and abdominal ceCT, which might be followed by complementary PET/CT and liver MRI to clarify indeterminate lesions, as both methods exhibit higher accuracy for the detection of distant metastases [4, 5]. Moreover, the prognosis of patients with rectal cancer depends on the stage of the disease at the time of diagnosis, with overall survival in 5 years decreasing from 89.9% for localized tumors to 14.2% for systemic disease [6].
In this context, the use of PET/MRI for primary rectal cancer staging combines the standard imaging modality for the T and N stages with PET as well as both PET and liver MRI for the M stage. To date, data comparing PET/MRI with other imaging modalities for colorectal cancer is scarce and limited due to small patient numbers, heterogeneous populations (including both colon and rectal cancer patients for primary staging and restaging), and study designs that are mostly retrospective [7,8,9,10,11]. Nevertheless, these studies suggest that PET/MRI yields a diagnostic performance that is superior to both CT and PET/CT, which could have a robust clinical impact on patient management.
This study aimed to compare the diagnostic accuracy of PET/MRI compared to conventional staging (CS) (that consists of pelvic MRI and thoracic and abdominal ceCT) for the detection of distant metastases during the primary staging of rectal cancer patients. We hypothesized that, compared to combined MRI and CT, PET/MRI might exhibit higher accuracy with a lower prevalence of indeterminate lesions in detecting metastatic disease.
Materials and methods
Study population
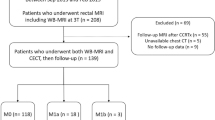
This prospective study was conducted from November 2016 to April 2018 and recruited 203 consecutive patients with biopsy-proven rectal adenocarcinomas (assessed by rigid proctoscopy to be up to 15 cm from the anal verge) to undergo [18F]fluorodeoxyglucose (FDG)-PET/MRI. The inclusion criteria were as follows: (1) age more than 18 years, (2) ready to sign the informed consent form, and (3) able to undergo the staging examinations (PET/MRI and ceCT) at a maximum interval of 2 weeks. The exclusion criteria were as follows: (1) previous treatment for rectal cancer, including endoscopic resection, and diagnosed synchronous non-colorectal neoplasia and (2) PET/MRI contraindications. The patients’ selection flowchart is presented in Fig. 1. The institutional review board approval was obtained, and this study was registered at clinicaltrial.gov under the identification number NCT02537340.
Imaging procedures
Thoracic and abdominal ceCT
The CT scans were performed within 2 weeks before or after PET/MRI was performed. Thoracic and abdominal CT scans were obtained from the lung apices to the superior iliac crest with a 16–256-multidetector CT (Brilliance CT, Philips, Netherlands). The scans were acquired during a breath hold after intravenous injection of contrast media (Iopamiron 300, Bracco, Italy) based on body weight (1.2 mL/kg) at a rate between 2 and 3 mL/s during the portal venous phase (70 s after injection). CT parameters were as follows: field of view of 300 mm, 90–120 kV (depending on patient body mass index), variable mAs, rotation between 0.5–0.75 s, pitch between 0.7 and 1.2, matrix of 512 × 512, slice thickness of 2 mm, and increment of 1 mm.
Whole-body PET/MRI (including pelvic and liver MRI)
Whole-body FDG-PET/MRI followed the guidelines of the European Association of Nuclear Medicine for oncological imaging in adults [12], adapted for a PET/MRI workflow. The injected activity of FDG was calculated according to the patient’s weight (4.5 MBq/kg, mean radiotracer dose of 296 MBq, and range of 197–414 MBq). After FDG injection, the patient rested for 20 min and was then transferred to the PET/MRI scanner for positioning and hyoscine butylbromide injection (20 mg, intravenous). The scanning was initiated 30 min after FDG injection and comprised three parts: (1) dedicated pelvic MRI (without PET acquisition), which was performed for locoregional staging of primary rectal cancer and followed the guidelines of the European Society of Gastroenterology and Abdominal Radiology [13]; (2) whole-body PET/MRI (acquisition started 60 min after FDG injection, mean uptake time of 64 min), which included 3–5 bed positions per patient and used a 3-min/bed position acquisition time under 3D image acquisition and standard reconstruction protocols; and (3) dedicated abbreviated (5 min) liver MRI (without PET acquisition). The MRI sequences and parameters are shown in Table 1.
Imaging analysis
The imaging readers were only aware of the indication of the examination (primary staging of rectal cancer), but blinded to other clinical data and imaging modalities. A step-by-step read was performed for each imaging modality.
Conventional staging
Pelvic MRI
A radiologist with 10 years of experience in reading MRI (C.D.O.) analyzed the dedicated pelvic MRI for locoregional staging. The following parameters were assessed for the primary tumor: (a) morphology (annular, semi-annular, ulcerated, or polypoid); (b) longitudinal (distance in cm from anal verge) and circumferential (clockwise) location; (c) extension (in cm); (d) presence of mucinous component (positive high signal within the tumor in the T2w sequence); (e) mesorectal fascia status (positive or negative if the circumferential resection margin was ≤ 1 mm or > 1 mm from the tumor, EMVI, or deposit, respectively; for low rectal tumors, a positive margin was considered if the distance between the tumor and the levator ani muscle was ≤ 1 mm or the intersphincteric plane was compromised); and (f) the presence of EMVI (positive if medium and large vessels were involved, according to Smith et al. [11]). For regional nodal staging, a positive lymph node (LN) was considered when at least one of the morphologically suspicious characteristics (irregular border and/or heterogeneous signal) was present.
Thoracic and abdominal ceCT
Another radiologist with 5 years of experience in reading CT (F.R.F.) analyzed the thoracic and abdominal ceCT for systemic staging and detection of distant metastases. The metastases were categorized as positive, negative, or indeterminate and listed according to the involved organ, i.e., non-regional nodes, liver, lungs, and others. Malignant criteria were defined based on size and morphology. A positive lesion was defined by a) a short axis diameter of ≥10 mm and/or b) ≥ 2 morphologically suspicious characteristics (round shape, irregular border, and/or hypovascular in the portal venous phase). An indeterminate lesion was defined by (a) a short axis diameter of < 10 mm and (b) only one morphologically suspicious characteristic (round shape, irregular border, and/or hypovascular in the portal venous phase). The maximum number of lesions per organ was 10.
Whole-body PET/MRI
A board-certified radiologist with 8 years of experience in reading MRI and PET (M.A.Q.) and a nuclear medicine physician with 16 years of experience in reading PET (C.A.B.) analyzed the PET/MRI images by using a dedicated workstation (Advantage Workstation version 4.6, GE Healthcare). The readers were kept blinded as the MRI component of the PET/MRI (comprised of the axial T2w of the pelvis, axial T1 DIXON, and coronal T2 SSFSE) did not include the dedicated pelvic MRI (high-resolution T2w sequences oriented to the tumor). For the primary tumor, PET semi-quantitative data was obtained by semi-automatically drawing a volume of interest over the primary tumor using the application PETVCAR (GE Healthcare), which was supported by a visual adaptation of the isocontouring to avoid the inclusion of structures not related to the tumor, such as the bladder. Maximum standard uptake value (SUVmax), mean SUV (SUVmean), metabolic tumor volume, and total lesion glycolysis (defined as the product of metabolic tumor volume and SUVmean) were recorded. For regional nodal staging, in addition to the MRI criteria, the following metabolic criterion was applied: all LNs with FDG uptake > 2.5 were considered positive, regardless of size or morphology. For metastatic disease staging, a combination of morphology and metabolism was used and varied depending on the organ (retroperitoneal LNs, lungs, liver, and others). For retroperitoneal LNs, a positive lesion was defined by one of the following criteria: (a) a short axis diameter of ≥ 10 mm, (b) ≥ 2 morphologically suspicious characteristics (round shape, irregular border, and/or heterogeneous signal), (c) all mucinous LNs (any size), and (d) the LN was FDG-positive (SUVmax > 2.5). For the lungs, a positive lesion was defined by one of the following criteria: (a) a size of > 1 cm and (b) focal FDG uptake higher than that of the surrounding parenchyma with or without a morphological correlation. For the liver and other organs (peritoneum, ovaries, and adrenal organs), a positive lesion presented at least two of the following three criteria: (a) intermediate intensity on T2w images, (b) high signal intensity impeding diffusion on images acquired with a high b value, and (c) focal FDG uptake higher than that of the liver parenchyma. A maximum of 10 lesions per organ was also defined.
Standard of reference
The standard of reference (SOR) for metastatic disease consisted of clinical/imaging follow-ups performed at 3, 6, and 12 months after initial staging and/or histopathological confirmation (when discrepancy between PET/MRI findings and other imaging modalities was present). A lesion was considered positive if it exhibited progression or a response after chemotherapy or if at least one new lesion appeared within 6 months after initial evaluation. Lesions that were stable under treatment were considered negative.
Statistical analysis
IBM SPSS Statistics (version 25) and RStudio (version 1.1.463) were used for statistical analysis. A two-tailed P value of < 0.05 was considered statistically significant. For the calculation of diagnostic accuracy, the indeterminate lesions were considered negative and the McNemar test was applied. The McNemar test was also used to calculate the diagnostic accuracy according to the status of EMVI detected by MRI. The numbers needed to treat were also calculated, i.e., the count of how many people need to be scanned in order for one person to benefit. The primary outcome was the presence of metastases, and the comparison was between the metastatic patients observed with CS and the metastatic patients observed with PET/MRI.
Results
Patient characteristics
This study included 101 patients (mean age, 62 years; range, 33–87 years; male-to-female ratio, 51:50). SOR detected 334 synchronous metastases in 36 patients (35.6%), predominantly in the liver (69.4%, 25/36 patients), followed by the lungs (47.2%, 17/36 patients) and non-regional LNs (41.2%, 15/36 patients). Of the 36 metastatic patients, 17 (47.2%) exhibited progressive or responsive lesions at imaging follow-up, 16 (44.4%) had histopathological confirmation, and 3 (8.3%) presented new lesions at imaging follow-up, all of which were confirmed negative on PET/MRI after a second analysis.
According to the dedicated pelvic MRI, most patients exhibited locally advanced rectal cancer, with 80.2% (81/101) of patients exhibiting at least T3b and 50.5% (51/101) of patients exhibiting positive regional LNs. A positive EMVI was observed in 62.7% (63/101) of patients, and an involved mesorectal fascia was observed in 49.5% (50/101) of patients. Table 2 summarizes the patients’ characteristics.
Diagnostic accuracy of PET/MRI vs. CS
From the 36 metastatic patients, CS and PET/MRI classified 23 and 31 patients as metastatic, respectively. With this difference, the calculated numbers needed to treat were 5 for the metastatic patients and 12 for all the rectal cancer patients. This indicates that for every 5 metastatic or 12 rectal cancer patients, one presents metastases only on PET/MRI. The patient-based analysis revealed that PET/MRI exhibited a significantly higher accuracy (88.4% vs. 82.6%, p = 0.003) and an especially higher specificity in detecting metastatic disease than CS (Table 3). The diagnostic accuracy of PET/MRI for detecting distant metastases was also significantly higher than that of CS (88.9% vs. 85.5%, p = 0.013) in patients with EMVI, but was not different (85.4% vs. 68.5%, p = 0.22) among patients without EMVI (Table 4; Figs. 2 and 3).
PET/MRI is superior to CS for staging primary rectal cancer with EMVI. The patient was an 85-year-old woman. Thoracic CT (a; long red arrows) and PET/MRI (d; long yellow arrows) revealed lung metastases. Abdominal CT (b) detected an indeterminate liver nodule (short red arrow). The PET/MRI findings (e) indicated that this nodule was suspicious (restricted diffusion and focal FDG uptake) and additionally revealed liver metastases (yellow short arrows). These lesions were confirmed by imaging follow-up
PET/MRI is similar to CS in a 63-year-old female patient with primary rectal cancer without EMVI. A single metastasis was observed on abdominal ceCT (a; red arrow) as well as PET/MRI with intermediate T2 signal (c), restricted diffusion (d), and high FDG uptake (e). No additional lesions were observed on PET/MRI. This lesion was confirmed by histology
From all of the 334 metastatic lesions, PET/MRI detected 281 in 31 patients, while CS detected 230 lesions in 23 patients (84.1% vs. 68.9%, p = 0.001). The organ-based analysis revealed that the detection rate of PET/MRI was significantly superior to that of CS for liver (89.2% (124/139 in 23 patients) vs. 84.2% (117/139 in 20 patients), p = 0.023), non-regional LNs (90.0% (54/60 in 18 patients) vs. 36.7% (22/60 in 8 patients), p = 0.001), and lungs (76.4% (97/127 in 13 patients) vs. 66.9% (85/127 in 13 patients), p = 0.019). No difference was observed for other lesions, and both methods presented a diagnostic rate of 75% (6/8 in 6 patients) (Figs. 4 and 5).
PET/MRI is superior to CS for upstaging from M0 to M1 in the liver and for clarifying indeterminate lesions detected by CS. The patient was a 61-year-old woman, and abdominal ceCT revealed one indeterminate liver lesion (a; red arrow). PET/MRI revealed that this lesion was positive with restricted diffusion and high FDG uptake (c, d; yellow arrows). PET/MRI also revealed two other liver lesions with similar characteristics (c, d; yellow arrows). These lesions were confirmed by imaging follow-up
On a patient-based analysis, PET/MRI and CS were congruent in 23 metastatic patients and PET/MRI alone identified 8 more patients. There was any metastatic patient positive on CS only. Five patients were considered positive for metastases during imaging follow-up and were considered false negative on both methods. On a lesion-based comparison, PET/MRI and CS detected 223/334 metastatic lesions (66.8%); PET/MR alone detected 58/334 lesions (17.4%), mainly non-regional lymph nodes; and CS alone detected 7/334 lesions (2.1%), especially lung lesions. Both methods missed 46/334 lesions (13.8%), especially lung and liver nodules. See Fig. 6.
Characterization of indeterminate lesions
Both PET/MRI and CS were indeterminate in six patients, of which all proved to be negative for metastases on imaging follow-up. PET/MRI detected five more indeterminate patients (accounting for 16 lesions—11 non-regional LNs, 0 in the liver, 1 in the lungs, and 4 in other sites), of which CS was negative in four (one false negative and three true negatives) and positive in one (a false positive). On the other hand, CS detected twenty-seven more indeterminate patients (accounting for 68 lesions—20 non-regional LNs, 29 in the liver, 16 in the lungs, and 3 in other sites), of which PET/MRI was negative in 18 (4 false negatives and 14 true negatives) and positive in 9 (4 false positives and 5 true positives). PET/MRI correctly classified 19/33 (57.5%) patients with indeterminate lesions on CS, while CS correctly classified 3/11 (27.2%) patients with indeterminate lesions on PET/MRI (Fig. 7).
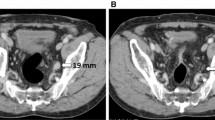
PET/MRI is superior to CS for upstaging from M0 to M1 in non-regional lymph nodes and for clarifying indeterminate lesions detected by CS. The patient was a 64-year-old man, and PET/MRI revealed two small (< 1 cm) indeterminate retroperitoneal lymph nodes (a; red arrows) that were considered positive due to high FDG uptake. The interaortocaval lesion was biopsied and confirmed as metastatic adenocarcinoma
Discussion
Our study demonstrated that PET/MRI has a higher accuracy than CS for the detection of distant synchronous metastases in baseline staging of patients with rectal cancer and of patients that presented EMVI within primary tumors. PET/MRI exhibited a higher detection rate than CS for detecting non-regional LNs as well as hepatic and pulmonary lesions. Moreover, PET/MRI exhibited a more correct classification of patients with indeterminate lesions than CS.
The accuracy for detecting metastatic disease was higher for PET/MRI than pelvic MRI and thoracic and abdominal CT. Recently, a Swedish study assessed the additional value of PET/MRI (and PET/CT) over CS of rectal cancer. Although only 24 patients were included, the PET component presented a disease upstaging from M0 to M1 in 3 out of 24 patients (12.5%) [11]. In our study, PET/MRI also enabled upstaging from M0 to M1 in eight patients. A meta-analysis of the role of PET and PET/CT in the primary staging of both colon and rectal cancer demonstrated a sensitivity and specificity of 91% and 95%, respectively, compared to a diagnostic accuracy of 80% for CT [14]. This is consistent with the results of our study in which we demonstrated a sensitivity of 90.8% and a specificity of 86.1% for PET/MRI and an accuracy of 82.6% for CS.
The distribution of the distant metastases of this study was more prevalent in the liver and lungs, consistent with the spreading pattern of rectal tumors [15]. Additionally, our study demonstrated a high incidence (15%) of non-regional LNs, similar to a study that performed retroperitoneal lymphadenectomy (17%) [16]. The characterization of metastatic LNs is a limitation of CT and MRI that rely on morphology (especially size and borders), whereas PET/MRI contributes a metabolic aspect, enhancing the sensitivity and specificity of this technique.
PET/MRI exhibited a superior detection of liver metastases compared to CS, in line with the literature that demonstrates that PET/CT and especially MRI exhibit a higher sensitivity than ceCT [17, 18]. Sivesgaard et al. [19] compared the diagnostic accuracy of ceCT, MRI, and FDG-PET/CT for the detection of liver metastases and, by analyzing 260 lesions, demonstrated that the sensitivity of MRI was superior to that of FDG-PET/CT, which was superior to ceCT (85.9% vs. 72.0% vs. 62.3%). Thus, we postulated that the combination of FDG-PET and MRI presents a diagnostic performance superior to that of CT for the detection of rectal liver metastases. Our study employed T2-weighted MRI sequence and diffusion-weighted imaging for assessment of liver lesions, but not contrast-enhanced sequences (e.g., gadolinium or Primovist). This was due to a time limitation, as our protocol was set to last 60 min without the dynamic phase of MRI. Lee et al. [20] demonstrated that the diagnostic performance of PET/MRI was superior to that of multidetector CT or PET for the detection of colorectal cancer liver metastases; however, PET/MRI did not differ from liver-specific contrast-enhanced MRI. Reiner et al. [21] also demonstrated similar diagnostic accuracies for the detection of liver metastases when PET/MRI was read with DWI (99%), contrast-enhanced MRI (98%), and both (99%). This reinforces the idea that the use of contrast in the context of PET/MRI may not be essential.
For lung lesions, however, it was expected that thoracic CT would exhibit a higher detection rate than PET/MRI, as MRI is limited to identifying lung nodules smaller than 1 cm [22]. Nevertheless, in our study, PET/MRI was superior to CT in detecting pulmonary metastases. This might be related to the defined criteria of malignancy with CT (a size > 1.0 cm) compared to the metabolic criteria with PET/MRI (focal FDG uptake higher than surrounding background). Thus, several small lesions (< 1.0 cm) that were considered indeterminate on CT were considered suspicious on PET/MRI due to the focal FDG uptake. Only few papers have compared PET/MRI and CT for the detection of pulmonary metastases. Rauscher et al. [23] demonstrated that PET/MRI exhibited an inferior detection rate for small lung lesions compared to PET/CT with diagnostic chest CT. Another study that included 51 patients with colorectal lung metastases demonstrated that PET/MRI exhibited a superior diagnostic rate (90%) compared to CT when evaluating lesions larger than 0.5 cm [7].
We also compared the accuracy of PET/MRI and CS in patients with and without EMVI, and PET/MRI exhibited superior detection of metastatic lesions only for EMVI within the primary tumor. Patients with EMVI exhibit an increased risk (OR, 5.68) of metastatic disease [24], which suggests that PET/MRI would detect lesions at a higher rate. So, a patient with positive EMVI and negative conventional staging could benefit from a more careful investigation. Nevertheless, these results should be interpreted with caution, as the number of metastatic patients without EMVI was too low (5 out of 38 patients) and thus cannot yield significant statistical results.
PET/MRI reduced the number of indeterminate findings observed on CS in more than half of our patient population. As demonstrated by Fraum et al. [25], PET/MRI facilitates better tumor staging, FDG activity localization, and lesion characterization. Other studies have shown that PET imaging may better characterize both lung [26] and liver lesions [27] in colorectal cancer patients.
A limitation of our study is that histological confirmation was not available for all distant metastases; nevertheless, almost half of our patient population was biopsied and all others had imaging follow-ups for at least 6 months. Second, despite the prospective study design, case selection bias might have been present, as most of the included patients presented advanced tumors and a high number of recruited patients was not included, especially because almost half of them had promptly initiated the treatment before performing the PET/MRI; however, this is a particular characteristic of our tertiary public cancer center. Third, only one radiologist assessed the pelvic MRI and thoracic and abdominal ceCT, which might have influenced the findings. Fourth, reaching a common interpretation of the PET/MRI results could have presented another limitation; therefore, we included both a nuclear physician and radiologist in this study.
This study has demonstrated that PET/MRI yields a higher accuracy than CS for the detection of distant synchronous metastases in patients undergoing rectal cancer staging. The diagnostic performance of PET/MRI was superior to that of CS in patients with EMVI within primary rectal tumors as well as for detecting non-regional LNs, hepatic lesions, and pulmonary lesions. Furthermore, PET/MRI reduced the number of indeterminate findings observed on CS. These results indicate that PET/MRI is a more appropriate diagnostic method for staging rectal cancer, but future studies should evaluate whether this incremental value of PET/MRI may change patient management and especially outcome.
References
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2017;28:iv22–40.
Benson AB, Venook AP, Bekaii-Saab T, Chan E, Chen Y-J, Cooper HS, et al. Rectal cancer, version 2.2015. J Natl Compr Cancer Netw. 2015;13:719–28.
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. RadioGraphics. 2019;39:367–87.
Seo HJ, Kim M, Lee JD, Chung W, Kim Y-E. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Investig Radiol. 2011;46:548–55.
Ozis SE, Soydal C, Akyol C, Can N, Kucuk ON, Yagcı C, et al. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer. World J Surg Oncol. 2014;12:26.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ CK (eds). SEER cancer statistics review, 1975–2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Internet]. 2019. Available from: https://seer.cancer.gov/statfacts/html/colorect.html
Kang B, Lee JM, Song YS, Woo S, Hur BY, Jeon JH, et al. Added value of integrated whole-body PET/MRI for evaluation of colorectal cancer: comparison with contrast-enhanced MDCT. Am J Roentgenol. 2016;206:W10–20.
Brendle C, Schwenzer NF, Rempp H, Schmidt H, Pfannenberg C, la Fougère C, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43:123–32.
Paspulati RM, Partovi S, Herrmann KA, Krishnamurthi S, Delaney CP, Nguyen NC. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging. 2015;40:1415–25.
Catalano OA, Coutinho AM, Sahani DV, Vangel MG, Gee MS, Hahn PF, et al. Colorectal cancer staging: comparison of whole-body PET/CT and PET/MR. Abdom Radiol. 2017;42:1141–51.
Rutegård MK, Båtsman M, Axelsson J, Brynolfsson P, Brännström F, Rutegård J, et al. PET/MRI and PET/CT hybrid imaging of rectal cancer – description and initial observations from the RECTOPET (REctal Cancer trial on PET/MRI/CT) study. Cancer Imaging. 2019;19:1–9.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2014.
Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–75.
Ye Y, Liu T, Lu L, Wang G, Wang M, Li J, et al. F-FDG PET-CT or PET: a meta-analysis including 2283 patients. Int J Clin Exp Med. 2015;8:21773–85.
Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765 Nature Publishing Group.
Quadros CA, Falcão MF, Carvalho ME, Ladeia PA, Lopes A. Metastases to retroperitoneal or lateral pelvic lymph nodes indicated unfavorable survival and high pelvic recurrence rates in a cohort of 102 patients with low rectal adenocarcinoma. J Surg Oncol. 2012;106:653–8.
Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–84.
Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of 18F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2015;42:152–63.
Sivesgaard K, Larsen LP, Sørensen M, Kramer S, Schlander S, Amanavicius N, et al. Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol. 2018;28:4735–47.
Lee DH, Lee JM, Hur BY, Joo I, Yi N-J, Suh K-S, et al. Colorectal cancer liver metastases: diagnostic performance and prognostic value of PET/MR imaging. Radiology. 2016;280:782–92.
Reiner CS, Stolzmann P, Husmann L, Burger IA, Hüllner MW, Schaefer NG, et al. Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection. Eur J Nucl Med Mol Imaging. 2014;41:649–58.
Heye T, Ley S, Heussel CP, Dienemann H, Kauczor HU, Hosch W, et al. Detection and size of pulmonary lesions: how accurate is MRI? A prospective comparison of CT and MRI. Acta Radiol. 2012;53:153–60.
Rauscher I, Eiber M, Furst S, Souvatzoglou M, Nekolla SG, Ziegler SI, et al. PET/MR imaging in the detection and characterization of pulmonary lesions: technical and diagnostic evaluation in comparison to PET/CT. J Nucl Med. 2014;55:724–9.
Siddiqui MRS, Simillis C, Hunter C, Chand M, Bhoday J, Garant A, et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer. 2017;116:1513–9.
Fraum TJ, Fowler KJ, McConathy J, Dehdashti F. Indeterminate findings on oncologic PET/CT: what difference does PET/MRI make? Nucl Med Mol Imaging. 2016;50:292–9.
Jess P, Seiersen M, Ovesen H, Sandstrøm H, Maltbæk N, Buhl AA, et al. Has PET/CT a role in the characterization of indeterminate lung lesions on staging CT in colorectal cancer? A prospective study. Eur J Surg Oncol. 2014;40:719–22.
Parsai A, Miquel ME, Jan H, Kastler A, Szyszko T, Zerizer I. Improving liver lesion characterisation using retrospective fusion of FDG PET/CT and MRI. Clin Imaging. 2019;55:23–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Digestive tract
Electronic supplementary material
ESM 1
(DOCX 16 kb).
Rights and permissions
About this article
Cite this article
Queiroz, M.A., Ortega, C.D., Ferreira, F.R. et al. Diagnostic accuracy of FDG-PET/MRI versus pelvic MRI and thoracic and abdominal CT for detecting synchronous distant metastases in rectal cancer patients. Eur J Nucl Med Mol Imaging 48, 186–195 (2021). https://doi.org/10.1007/s00259-020-04911-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04911-x