Abstract
Purpose
Radioligand therapy (RLT) with 177Lu-PSMA-617 is a promising option for patients with metastatic castration-resistant prostate cancer (mCRPC). The present study was designed to define the safety and initial response to a minimal effective injected activity/cycle of 177Lu-PSMA-617 in mCRPC patients. New protective agents for salivary glands and kidney were co-administered and dosimetry was carried out.
Patients and methods
A prospective single-arm, open label phase II study on mCRPC was activated at our institute in April 2017. Patients with histologically confirmed advanced mCRPC previously treated with standard life-prolonging agents were enrolled. Folic polyglutamate tablets were orally administered as parotid gland protectors and 500 mL of a 10% mannitol solution was intravenously infused to reduce kidney uptake before the injection of 3.7–5.5 GBq of 177Lu-PSMA-617 repeated four times at interval of 8 weeks. The adsorbed dose calculation was performed with MIRD formalism (OLINDA/EXM software). The Bryant and Day design was used to estimate the sample size taking account of both activity and toxicity.
Results
Forty-three eligible patients were evaluated for toxicity and initial response. Dosimetry was carried out in 13 patients. Two (4.8%) patients had G3 and 8 (19.5%) had G2 hematological toxicity. Only 3 (6.9%) patients had mild G1 salivary gland toxicity and 8 (19.5%) had G1 renal toxicity. A decrease of ≥ 30% in prostate-specific antigen (PSA) was achieved after the first cycle in 17 (40.5%) patients, of whom 13 had a PSA decline of >50% after the second cycle. The median adsorbed doses were 0.65 mGy/MBq (range 0.33–2.63) for parotid glands, 0.42 mGy/MBq (0.14–0.81) for kidneys, 0.036 mGy/MBq (0.023–0.067) for red marrow, and 0.038 mGy/MBq (0.018–0.135) for the whole body.
Conclusion
In advanced, heavily pre-treated mCRPC patients, 3.7 GBq/cycle of 177Lu-PSMA-617 was safe and produced early biochemical and imaging responses at PSMA whole-body scan post injection. Dosimetry of salivary glands suggests that the co-administration of polyglutamate tablets may reduce salivary gland uptake.
Clinical trial registration
EU Clinical Trials Register No.: 2016-002732-32; NCT03454750. Collection and assembly of data: April 2017 and February 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common cancer worldwide in terms of incidence and the third cause of death from cancer in males [1]. Standard care for patients with advanced or metastatic disease is androgen deprivation therapy (ADT) including luteinizing hormone-releasing agonists or antagonists and anti-androgen agents. However, the disease may progress despite castration levels of testosterone to become metastatic castration-resistant prostate cancer (mCRPC), which is currently treated with life-prolonging agents such as novel anti-androgens (abiraterone/enzalutamide), taxanes (docetaxel/cabazitaxel), and radium 223 [1].
PSMA (prostate-specific membrane antigen) is an attractive target for diagnosis and therapy of metastasized PCa as its expression levels are directly correlated with androgen independence, metastases, and progression [2]. PSMA, also known as glutamate carboxypeptidase II (GCPII), is a membrane-type zinc protease anchored in the cell membrane of prostate epithelial cells. PSMA expression increases as tumor de-differentiation increases, in metastatic and hormone-refractory cancers. In addition to being expressed by prostate cells, it is also expressed at lower levels by non-prostate tissues such as small intestine, proximal renal tubules, and salivary glands, which are considered critical organs in radioligand therapy (RLT) with radiolabeled PSMA [2,3,4].
A DOTA version of a PSMA inhibitor has been synthesized and has shown promising properties when labeled with 177lutetium [5]. This novel theranostic agent, 177Lu-DKFZ-PSMA-617 (177Lu PSMA–617), has been administered to patients with mCRPC who show strong PSMA expression at pre-therapy diagnostic PET/CT scan [6,7,8,9]. Several retrospective studies, mainly carried out in Germany where the ligand was developed [5, 10,11,12,13,14,15,16,17,18,19,20], have reported favorable biochemical and objective response rates in patients with far advanced mCRPC.
In May 2018, the results from the first prospective phase II study on 30 mCRPC patients were published in Australia, showing a high response rate and low toxicity [19]. In this particular study, all patients received 7500 MBq PSMA-RLT per cycle at 6-week intervals, whereas other trials did not have a standardized protocol for PSMA-RLT and treatment plans were also heterogeneous in terms of activity of [177Lu]Lu-PSMA, which ranged from about 1.1 to 9.3 GBq/cycle [21]. Thus, there is no single established therapeutic protocol for PSMA-RLT in mCRPC patients or best (minimal-maximal) activity dosage of [177Lu] Lu-PSMA according to different clinical situations.
Here we report, in a prospective study, dosimetry and acute side effect of 177Lu PSMA–617 co-administered, for the first time, with polyglutamate as protective agents for salivary glands. Moreover, a minimal effective activity per cycle was investigated.
Patients and methods
Patients
From April 2017 to February 2019, 43 heavily pre-treated patients with advanced prostate cancer were enrolled in this European phase II RLT prospective trial (Eudract no. 2016-002732-32, NCT03454750). Patients were eligible if they had histologically or cytologically confirmed prostate cancer, mCRPC defined according to PCWG3 criteria, and measurable disease according to RECIST 1.1. Criteria. Patients with only bone lesions could also be enrolled. Patients with documented radiological progression (in soft tissue and/or bone) and/or biochemical progression (sequence of 3 PSA increased values from a screening PSA value ≥ 2 ng/mL) according to PCWG3 in the pre-study period, refractory to or unfit for conventional life-prolonging new anti-androgen treatments (abiraterone and enzalutamide), chemotherapeutic agents (docetaxel and cabazitaxel), and radium-223 were admitted. All patients performed diagnostic PET/CT 68Ga-PSMA showing an evident uptake equal to or higher than that of salivary glands at metastatic tumor sites. Concomitant treatment with LH-RH analogs was needed. Inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status < 2, adequate hematological, liver and renal function, hemoglobin > 9 g/dL, absolute neutrophil count (ANC) > 1.5 × 109/L, platelets > 100 × 109/L, bilirubin ≤ 1.5 UNL (upper normal limit), ALT and AST < 2.5 UNL (< 5 UNL in presence of liver metastases), and creatinine < 2 mg/dL). Patients treated with chemotherapy and 223radium radiotherapy < 4 weeks previously or those receiving palliative radiotherapy < 2 weeks previously were excluded. The protocol was approved by the Ethics Committee of Area Vasta Romagna and IRST and by the competent Italian regulatory authorities. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice (GCP) guidelines. All patients gave written informed consent.
Study design
This was a single-center, prospective, non-controlled, open label, and phase II trial. The radiopharmaceutical 177Lu-PSMA 617 was injected four times every 8–12 weeks at a dosage ranging from 3.7 to 5.5 GBq. Patients < 75 years old who were unfit to be treated with docetaxel received 5.5 GBq of 177Lu PSMA-617. In a specific cohort, patients already treated with abiraterone/enzalutamide and docetaxel and/or aged > 75 years mainly received lower activities ranging from 3.7 to 4.4 GBq of 177Lu PSMA-617. Patients received up to two additional cycles if this could prolong clinical benefit according to the clinical investigator. A total cumulative activity (TCA) up to 33 GBq was envisaged, respecting dose constraints for normal organs after repeated intravenous (iv) injection of 177Lu PSMA-617 [12, 16].
Preparation and administration of Lu-PSMA and new protective agents
National Good Preparation standards (NBP MN) for pharmaceutical products were followed for 177Lu-PSMA-617 production, as required by current Italian legislation. DOTA-PSMA-617 was kindly provided by Endocyte Inc. (West Lafayette, IN, 47906, USA) and 177Lu was purchased from AAA (LuMark®, Baarle-Nassau, Netherlands) or ITG (Endolucinbeta®, Isotope Technologies Garching GmbH, Garching, Germany). The labeling procedure and quality control of 177Lu-DOTA-PSMA-617 compound was performed in the Radiopharmacy Laboratory of our institute (Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy).
The radiopharmaceutical was slowly infused intravenously over 15–30′ in a dedicated room using a dedicated delivery system (Paganelli–Chinol; patent US 7,842,023 B2). Before therapy, patients received iv administration of 500 mL mannitol 10%, 250 mL half an hour before therapy and 250 mL after therapy. Two polyglutamate folate tablets of plant origin (Ferramina, Morganpharma, Monteviale, Italy) were orally administrated to patients half an hour before, during, and 4 h after treatment. These tablets contain 200 μg of folic acid that are rich in glutamate groups engaging proteolytic activity of PSMA expressed in the salivary glands. Moreover, ice packs were positioned on parotid glands.
Dosimetry and imaging
Dosimetric analysis were performed in 13 patients, 7 injected with 4.4 GBq and 6 with 5.5 GBq. Pharmacokinetics, biodistribution, and absorbed dose to parotid (PGs), submandibular (SGs) and lacrimal glands (LGs), and kidneys, liver, red marrow (RM), whole body, and tumor (target lesions) were evaluated. Serial whole-body images were acquired at 0.5–1, 16–24, 36–48, and 120 h post injection. Imaging was performed on a Discovery NM/CT 670 scanner (International General Electric, General Electric Medical System, Haifa, Israel). Blood sampling for RM dosimetry evaluation was performed at the same time points as imaging acquisitions. In addition, a SPECT/CT scan, centered on the kidney region, was acquired 16–24 h post injection.
Image analysis was carried out on a Xeleris 3.0 workstation to allow the co-registration of serial post therapy whole-body scans (WBS) and the segmentation of the target organs and corresponding background regions. Dosimetry evaluations were performed with the MIRD formalism [22,23,24]. Contouring on SPECT/CT 3D images was performed on the MimVista software. Attenuation (based on transmission scan), scatter, and background corrections were applied [25, 26]. OLINDA/EXM software (v 1.0, [27, 28]) was used to assess effective half-life and absorbed dose with the inclusion of the patient-specific masses evaluated by CT scan. For each considered lesion, the volume was evaluated on pre-therapy 68Ga-PSMA PET/CT using the MimVista software. An adult male phantom was used for kidney, liver, RM, and whole-body evaluation, while a unit-density sphere model was used for SG, LG, and tumor lesion evaluation.
Relative kidney uptake was evaluated on SPECT/CT scan 16–24 h post injection. It was calculated by multiplying total counts per second by calibration factor ([cps/MBq] measured with the same SPCET/CT acquisition parameters [29]) and normalized to the total injected activity. Time-activity curves derived from planar images were then rescaled according to the ratio of relative uptake observed on post injection WBS and SPECT/CT at 16–24 h. The hybrid dosimetry evaluation was then performed for kidneys with the OLINDA/EXM software as well as for all other structures [26].
Statistical analysis
In this phase II prospective study, eligible patients were assigned to the following treatments:
A—patients treated with docetaxel and/or ≥ 75 years 3.7–4.4 GBq × 4 cycles every 8–12 weeks.
B—patients not treated with docetaxel or < 75 years 5.5 GBq × 4 cycles every 8–12 weeks.
Patients could receive up to two additional cycles (3.7–4.4 or 5.5 GBq, depending on the dosage assigned) based on the clinical benefit assessed by the investigators. The Bryant and Day design was used to estimate the sample size and takes in account both therapeutic activity and toxicity [30]. A sample size of 84 patients (42 per cohort) was required in the first step. The protocol envisaged a total of 210 patients.
The primary aim was to evaluate the safety and efficacy as co-primary objectives of two different administration schedules of 177Lu-PSMA-617 in two specific cohorts. Secondary objectives were dosimetry and late toxicity. In this preliminary analysis, acute toxicity and safety was evaluated according to Common Terminology Criteria for Adverse Events (CTC-AE) version 4.03. Frequency tables were performed for all categorical variables while continuous variables were presented using median and range or interquartile range (IQR). Dosimetric comparisons with external data have been performed via the distribution-free Kolmogorov–Smirnov test at a significance level of 0.10 due to low sample sizes. In this way, we have been able to statistically evaluate the null hypothesis that two different sets of data come from the same probability distribution. Statistical analyses were carried out with STATA/MP 15.1 (Stata Corp LP, USA) and R 3.6.1 for Windows.
Results
Between April 2017 and February 2019, 43 consecutive patients (IQR 66–77 years old, median 73 years) with PET/CT 68Ga-PSMA-positive mCRPC were enrolled in the present study. Baseline characteristics of the cohort are summarized in Table 1. Median Gleason score at diagnosis was 8 (IQR range 7–9). Twenty (46.5%) patients had undergone previous surgery on the prostate, while 5 (11.6%) had had previous radiotherapy. Fourteen (32.5%) patients had visceral metastases, 25 (58.1%) lymph node metastases, and 41 (95.3%) bone metastases. Twenty-three patients (53.5%) had previously received three treatment lines (abiraterone/enzalutamide or both and docetaxel and cabazitaxel), while 6 (13.9%) patients had only undergone palliative-intent radiotherapy. Fourteen (32.5%) patients had moderate pain (median NRS scale 4–7) at study enrolment. ECOG performance status was 1 in 20 (46.5%) patients, 2 in 4 (9.3%), and 0 in the remaining 19 (44.2%).
At the time of the analysis, all 43 patients had received at least one cycle of 177Lu-PSMA-617, 10 had received 2 (23%), 8 had received 3 (19%), 11 had received 4 (25%), 9 had received 5 (21%), and 3 had received 6 (7%) cycles. Thirty-one (72%) patients received a low dosage ranging from 3.7 to 4–4 GBq/cycle as they were at risk of side effects because of older age or amount of previous therapies administered, and the remaining 11 (28%) received 5.5 GBq. The median number of cycles was 3 (IQR 2–5). Median cumulative activity was 13.6 GBq (IQR 8.8–22.0 GBq).
Dosimetric evaluation
Dosimetry was performed for 13 patients (9 during first cycle, 4 during second cycle) (Table 2). A wash-in and wash-out trend was observed for all patients for PGs and a bi-exponential curve fitting was used. For kidneys, a combined wash-in/wash-out phase (7 patients) and pure wash-outs (6 patients) were observed (Fig. 1a). In cases of combined wash-in and wash-out phases, a maximum uptake was observed 16–24 h post infusion (Fig. 1a). A pure wash-out trend was observed for liver, RM, and whole body time-activity curves and fitted with mono-exponential (liver) or bi-exponential (RM and whole body) curves. A total of 46 bony lesions and 14 lymph nodal target lesions were evaluated. A wash-in and wash-out trend was observed for all tumor lesions and fitted with bi- or tri-exponential curves (Fig. 1a).
Median values of dosimetry evaluation for 177Lu-PSMA-617 (13 patients). Considered structures: tumors (bony and lymph node lesions), lacrimal glands, parotid glands, submandibular glands, kidneys (hybrid method), liver, red marrow, and whole body. a Time-activity curves, %IA = activity at time of image acquisition/injected activity. %IA was normalized to the single mass of the considered structure (%/g). b Mean absorbed doses (mGy/MBq)
Using a 2D planar imaging method, median values of mean absorbed dose were 0.65 mGy/MBq (range 0.33–2.63) for PGs, 0.59 mGy/MBq (0.23–1.51) for submandibular glands, 2.26 mGy/MBq (0.48–3.59) for LGs, 0.13 mGy/MBq (0.05–0.53) for liver, 0.036 mGy/MBq (0.023–0.067) for RM, and 0.038 mGy/MBq (0.018–0.135) for the whole body (Table 2; Figs. 1b and 2a). With regard to the kidneys, high intestine uptake was observed from day 2 to day 6 and with high overlapping of kidneys. Median value of kidney absorbed dose evaluated with a hybrid method was 0.42 mGy/MBq (range 0.14–0.81) (Table 2; Fig. 2b).
The median mass value of tumor lesions was 23.6 g (range 2.3–574.4) for bone and 7.5 g (2.1–56.7) for lymph nodes. The median values of corresponding mean absorbed doses were 4.70 mGy/MBq (range 0.74–55.86) for bony lesions and 3.64 mGy/MBq (0.25–15.10) for lymph node lesions (Table 3; Fig. 1b; Table S1 for detailed dosimetry).
Safety profile
The toxicity profile of 177Lu-PSMA-617 is shown in Table 4. There were no cases of grade (G) 4 toxicity requiring treatment suspension in either group. G3 toxicities were observed in 3 (6.9%) patients. The most commonly reported G3 toxicity was anemia (4.6%). G1 PG toxicity occurred in only 3 (6.9%) patients.
Preliminary efficacy
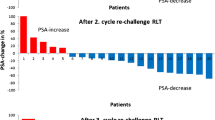
PSA decreased ≥ 30% in 17 (40.5%) patients after the first cycle and > 50% in 13 (30.8%) patients after the second cycle. All 43 patients treated with 177Lu-PSMA-617 demonstrated a high uptake in all disseminated lesions at the WBS post injection consistent with that of pre-therapy 68Ga PSMA PET/CT. Forty (93.0%) patients, including those treated with the lowest activity of 3.7 GBq (Fig. 3), showed a substantial decrease in tumor uptake at WBS after the second or third cycle of 177Lu-PSMA-617. The majority of these patients showed a clinical benefit with pain reduction, opioid suspension, and weight gain.
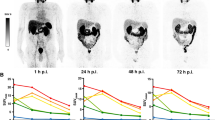
An 83-year-old male patient underwent 4 cycles of 4.4GBq 177Lu-PSMA for castration-resistant prostate cancer with diffuse bone metastases previously treated with surgery (radical prostatectomy in 2001; Gleason score 8[4 + 4]; stage pT4N0M0), multiple hormone manipulations (total androgen blockade, abiraterone, and enzalutamide), chemotherapy (taxotere and cabazitaxel), and 223RaCl2 (5 cycles in 2015). A right nefrectomy was performed in 2012 for renal clear cell cancer. a 68GaPSMA PET maximum intensity projection, fusion PET/CT, and CT views performed in September 2017 showing high 68GaPSMA uptake in diffuse bone metastatic disease prior to 177Lu-PSMA treatment. b Anterior and posterior planar views of whole-body scan performed after the first cycle of 177Lu-PSMA (November 2017) demonstrating intense 177Lu-PSMA uptake in bone metastases. c 68GaPSMA PET maximum intensity projection, fusion PET/CT, and CT views performed in June 2018 after the fourth cycle of 177Lu-PSMA showing an overall decrease in extent and degree of 68GaPSMA uptake in bone disease and increase in bone sclerosis. d Anterior and posterior planar views of whole-body scan performed after the fourth cycle of 177Lu-PSMA (May 2018) demonstrating similar marked decrease in the extent and degree of 177Lu-PSMA uptake in bone metastases
Discussion
In this report, we analyzed the preliminary results of 177Lu-PSMA-617 in terms of safety, initial response, and dosimetry in a cohort of frail mCRPC patients treated with low injected activity/cycle. In this heavily pre-treated patient population in progression after standard life-prolonging treatments, even a low activity of 3.7 GBq/cycle was sufficient to produce initial activity, with a clear clinical benefit. Overall, the 177Lu-PSMA-617 treatment was extremely well tolerated. The percentage of biochemical responses was < 50%, but it should be underlined that 31 (72%) of these patients received a low dosage ranging from 3.7 to 4–4 GBq/cycle as they were at risk of side effects because of older age or amount of prior therapies received. A comparison between the 3.7 and 5.5 GBq activity groups is not feasible at present, but when all 84 planned patients have been recruited and completed treatment, it will be interesting to see whether a higher injected activity corresponds to a better outcome in terms of PSA decline, objective response and OS. In addition to injected activity and the relative adsorbed dose, it is possible that other factors related to genetics, androgen receptor status, numbers of previous therapies, tumor load, and localization (bone or parenchymal) may influence the outcome of 177Lu-PSMA-617 RLT, not only in terms of disease control rate but also in terms of progression-free survival and overall survival. In this regard, RLT, being a target therapy, would be more suited to earlier stages of disease provided that toxicity is minimal and long-term side effects are acceptable. We believe that minimal activity capable of producing an objective response is an important step to be assessed prospectively. Although 7.4 GBq or higher dosages, as used in other studies [31], may produce better outcomes, the search for a minimal effective and nontoxic dosage is worth pursuing, especially in the prospect of using RLT in combination with other treatments or in an adjuvant setting. In our opinion, the ultimate goal of 177Lu-PSMA-617 therapy is not only to treat advanced mCRPC patients but also to try to reach the use of RLT in very early stages of the disease, avoiding side effects as much as possible and delaying total castration as well. For this reason, it is essential to establish a minimum effective dosage of 177Lu-PSMA-617.
Another important issue relates to PG uptake, which is the major limiting factor in RLT with radiolabeled PSMA, especially when 225Ac PSMA-RLT is used. Accordingly, we performed dosimetry calculations after the administration of folic polyglutamate tablets of plant origin to reduce PG uptake. PSMA possess two predominant enzymatic activities: the hydrolytic cleavage and liberation of glutamate from γ-glutamyl derivatives of folic acid and the proteolysis of the neuropeptide N-acetylaspartylglutamate (NAAG); due to this enzymatic activity, folic acid γ-Glu and NAAG are endogenous PSMA substrate [32, 33]. These various functions and the tissue distribution of PSMA result in different designations. In the proximal small intestine, PSMA removes γ-linked glutamates from poly-g-glutamated folate, including dietary derivatives [33], which is reflected in its name, folate hydrolase FOLH1 [34]. Our tablets contain plant-based folates that are rich in glutamate groups; these poly-γ-glutamated folates can engage proteolitic activity of PSMA expressed in the salivary glands and small intestine reducing 177Lu-PSMA-617 uptake at these levels. Polyglutamate tablet administration can reduce tumor uptake too. However, the tablets are given orally and left to sit in the mouth assuming at least a partial absorption, almost topical, through the oral mucosa and in the small intestine. Lutetium is administered intravenously soon after the tablets and therefore it is unlikely that the folic acid administered orally reaches the tumor sites before 177Lu-PSMA-617 and consequently inhibits tumor uptake. Post therapy WBS images and dosimetry evaluation of tumor lesions (see Table 3 and Table S1) would confirm our hypothesis. Certainly, these preliminary data need to be further investigated to understand the rate of competitive inhibition that might reduce tumor uptake. In this regard, a PG locoregional administration of folates should also be explored. A limitation of our protocol is the lack of a control group without PG protectors. However, other dosimetry studies reported data comparable with our findings, both providing dosimetry of normal organs and tissues. Kabasakal et al. and Kratochwil et al. [11, 15] suggested that the PGs are the main dose-limiting organ rather than the kidneys or bone marrow, as for peptide receptor radionuclide therapy (PRRT). Delker et al. [5] reported a high dose to SGs and kidneys, which was not, however, critical after administration of 3.6 GBq of 177Lu-PSMA-617 (range 3.4–3.9 GBq). In our protocol, we pre-medicated patients with folate tablets and mannitol 10%. The effect of mannitol on the proximal tubuli has been reported elsewhere [24], confirming that the reduction in kidney uptake is not significant. Conversely, in the present study, the folate tablets significantly reduced PG uptake compared with Kabasakal and Delker’s studies, where no protectors for PGs were administered. Figure 4 shows a graphical comparison between the absorbed doses for PGs reported in our study and the dosimetric data reported in the literature. The difference between our data and that of Delker [5], Kratochwil [15], and Kabasakal [11] was statistical significance (p values = 0.08, 0.05, and 0.06, respectively; Kolmogorov- Smirnov test, with a significance level equal to 10%). A statistical comparison with Baum et al.’s data [16] was not feasible as single-patient data were not available. A statistical comparison between the absorbed doses to tumor lesions was not possible too. However, data comparing our outcomes with those of Delker and Baum are reported in the supplementary material (Fig. S1).
For LGs, a small volume strongly influences the accuracy of structure definition in both planar and PET/CT images (median volume 1.66 cm3, range 0.73–3.20; volume as sum of left and right lacrimal glands). We did not protect LGs and the absorbed dose in our patients was similar to that of other studies [35]. This observation is consistent with the hypothesis that folate tablets reduce PG uptake in these patients. A substrate of N-acetyl aspartyl glutamate (NAAG) that could compete with 177Lu-PSMA-617 at LG level is spanglumic acid. Spanglumic acid is commercially available as anti-inflammatory eye drops and is currently under investigation at our institute for its potential to protect LGs. It would be interesting to see whether premedication with spanglumic acid is capable of reducing LG uptake. It should be underlined, however, that other glands of the conjunctiva also produce tears, i.e., accessory lacrimal glands (Krause’s glands). Another group of accessory glands (Wolfring glands) are located along the orbital margin of the tarsi, both above and below. It is probable that accessory lacrimal glands show low PSMA expression, which might explain the fact that, despite the high adsorbed dose, there are no cases reported of severe xerophthalmia even in patients treated with 225Ac PSMA.
Conclusion
Although preliminary, the data presented in this report indicate that 3.7 GBq/cycle could be considered as a minimum dosage capable of producing a response in patients with mCRPC. In our opinion, this is the minimal recommended dosage given the extremely limited toxicity even in older and/or frail patients. Folic acid tablets may reduce the absorbed dose of 177Lu PSMA to the SGs, indicating its potentially high impact on clinical practice for RLT with 177Lu PSMA and even more so for 225Ac PSMA.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82.
Wright GL, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81.
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39.
Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun. 2015;36:582–7.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37.
Ahmadzadehfar H, Rahbar K, Kurpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castration-resistant metastatic prostate cancer: a two centre study. EJNMMI Res. 2015;5:114.
Kabasakal L, AbuQbeitah M, Aygün A, Yeyin N, Ocak M, Demirci E, et al. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–83.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: bio-distribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–705.
Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benešová M, Mier W, et al. [(177)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:987–8.
Kratochwil C, Giesel FL, Stefanova M, Benesovà M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–13.
Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–9.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. 177Lu-DKFZPSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38:91–8.
Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with (177)Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Stabin MG.2008 Fundamentals of nuclear medicine dosimetry. Springer
Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37S–61S.
Snyder WS, Ford MR, Warner GG, Watson SB.1975 MIRD Pamphlet No. 11: “S” absorbed dose per unit cumulate activity for selected radionuclides and organs. http://snmmi.files.cms-plus.com/docs/hpra/MIRD Pamphlet 11.pdf.
Sarnelli A, Belli ML, Di Iorio V, Mezzenga E, Celli M, Severi S, et al. Dosimetry of 177 Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: preliminary results of EUDRACT/RSO 2016–002732-32 IRST protocol. Molecules. 2019;24:E621.
Belli ML, Mezzenga E, Di Iorio V, Celli M, Caroli P, Canali E, et al.2019 A whole body dosimetry protocol for peptide-receptor radionuclide therapy (PRRT): 2D planar image and hybrid 2D+3D SPECT/CT image methods. JoVE. , in press.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Bolch WE, Bouchet LG, Robertson JS, Wessels BW, Siegel JA, Howell RW, et al. MIRD Pamphlet No. 17: the dosimetry of nonuniform activity distributions—radionuclide S values at the voxel level. J Nucl Med. 1998;40:11s–36s.
Mezzenga E, D’Errico V, D’Arienzo M, Strigari L, Panagiota K, Matteucci F, et al. Quantitative accuracy of 177Lu SPECT imaging for molecular radiotherapy. PLoS One. 2017;12:e0182888.
Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51:1372–83.
Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmuller B, Kramer G, et al. Clinical outcome of standardized (177)Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur J Nucl Med Mol Imaging. 2020;47:713–20.
Anderson MO, Wu LY, Santiago NM, Moser JM, Rowley JA, Bolstad ES, et al. Substrate specificity of prostate-specific membrane antigen. Bioorg Med Chem. 2007;15:6678–86.
Kiess AP, Banerjee SR, Mease RC, Rowe SP, Rao A, Foss CA, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015;59:241–68.
Gourni E, Henriksen G. Metal-based PSMA radioligands. Molecules. 2017;22:E523.
Hohberg M, Eschner W, Schmidt M, Dietlein M, Kobe C, Fischer T, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with [(177)Lu]DKFZ-PSMA-617. Mol Imaging Biol. 2016;18:437–45.
Acknowledgments
The authors thank Gràinne Tierney and Cristiano Verna for the editorial assistance.
Funding
This work is partially supported by AIRC (project code IG20476) and Italian Ministry of Health (Ricerca Finalizzata, code RF-2016-02364230).
Author information
Authors and Affiliations
Contributions
Study concept and design: Giovanni Paganelli. Provision of study materials or patients: Stefano Severi, Maddalena Sansovini, Silvia Nicolini, Elisa Tardelli, Irene Marini, and Ugo De Giorgi. Diagnostic and therapeutic imaging: Monica Celli, Federica Matteucci, and Melchiore Giganti. Dosimetric study, quality control, and gamma camera calibration: Anna Sarnelli and Maria Luisa Belli. Radiopharmaceutical synthesis and quality control: Valentina Di Iorio. Data management: Manuela Monti. Analysis and interpretation of data: Flavia Foca, Giovanni Paganelli, and Ugo De Giorgi. Drafting of the manuscript: Giovanni Paganelli, Anna Sarnelli, and Ugo De Giorgi. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol was approved by the Ethics Committee of Area Vasta Romagna and by the competent Italian regulatory authorities. The study was conducted in accordance with the Declaration of Helsinki (and its later amendments) and good clinical practice (GCP) guidelines.
Informed consent
Informed consent was obtained from all individuals participating in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry
Electronic supplementary material
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Paganelli, G., Sarnelli, A., Severi, S. et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging 47, 3008–3017 (2020). https://doi.org/10.1007/s00259-020-04856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04856-1