Abstract
Background
Data are sparse regarding the feasibility of radioligand therapy (RLT) with [177Lu]Lu-PSMA-617 as a retreatment. We aimed to assess the outcome and safety of rechallenge PSMA-RLT in patients with progressive prostatic cancer who previously benefited from this therapy.
Materials and methods
Patients who received rechallenge therapy at our department from January 2015 to March 2018 were assessed. Non-haematological and haematological adverse events were evaluated from laboratory data and clinical reports and were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v. 5.0). Time to prostate-specific-antigen (PSA) progression and the overall survival (OS) rate of the study patients were calculated from the date of the first rechallenge cycle. Furthermore, the OS calculated from the first cycle baseline PSMA-RLT was compared with the survival of patients who received only baseline PSMA-RLT. The response data were determined using [68Ga]Ga-PSMA-PET/CT and measurements of the tumour marker PSA.
Results
Included in this retrospective study were 30 patients who were initially treated with a median of 3 cycles (range 1–5) of PSMA-RLT and were eventually retreated after a median of 6 months (range 2–26). Each patient received a median of 3 (range 1–6) rechallenge cycles. None of the patients experienced a disabling or life-threatening grade 4 adverse event according to the Common Toxicity Criteria (CTC). Grade 3 toxicity occurred in 8 patients (27%). Serious adverse events included leucopoenia (n = 2), neutropoenia (n = 1), anaemia (n = 4), thrombopenia (n = 4) and elevated renal parameters (n = 1). Irreversible adverse events occurred in 21 patients (70%). The permanent adverse events were mild/moderate (CTC grade 1/2) in 19 patients and serious (CTC grade 3) in two patients, respectively. According to PSA measurements, 75–90% of patients showed a benefit (response/stable) from the first 4 rechallenge cycles. The median OS was 12 months calculated from the first rechallenge cycle and 25 months calculated from the first cycle baseline PSMA-RLT. For comparison, the median OS in patients who received only baseline PSMA-RLT was 9 months. The difference according to the logrank test was significant: p value <0.001. Patients with a PSA decrease after the first cycle of rechallenge PSMA-RLT survived a median of 19 months, while patients with a PSA increase survived only 6 months.
Conclusion
Rechallenge prostate-specific membrane antigen (PSMA) therapy has an acceptable safety profile. The majority of the retreated patients benefited from the rechallenge therapy. Patients who showed a biochemical response achieved a longer OS compared to patients who did not respond. The median OS was significantly longer in patients after rechallenge PSMA-RLT than in patients who received only baseline PSMA-RLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The prostate-specific membrane antigen (PSMA) is highly expressed, especially in androgen-independent prostate cancer (PC), which makes it a promising target for the treatment of patients with castration-resistant prostate cancer (CRPC) [1,2,3,4,5,6].
The novel ligand PSMA-617 labelled with the beta emitter Lutetium-177 binds specifically to the PSMA and is being used for PSMA-radioligand therapy (PSMA-RLT). The internalisation of the radioligand enables the accumulation of radioactivity in the tumour tissue and irradiation from the inside [7,8,9,10].
In recent years, PSMA-RLT has rapidly gained interest worldwide for the treatment of patients with CRPC [11,12,13]. Unfortunately, data from randomised phase III prospective trials are lacking; therefore, PSMA-RLT remains a last-line option in advanced PC [14, 15]. This means that such patients are already castration-resistant, metastatic and, in most of the cases, symptomatic before they start the treatment. But even in this late disease stage, results from several studies have indicated that PSMA-RLT is effective and safe in patients with PC [11, 16,17,18,19,20,21,22,23,24]. Grade-3/4 haematologic toxicities occur in 3–11% of patients. To date, there have been no reports of severe non-haematologic toxicities [16, 17, 19,20,21,22, 25,26,27]. In the literature, the estimated progression-free survival (PFS) rate in treated patients ranges between 4.5 and 13.7 months, and the overall survival (OS) rate ranges between 7.5 and 15 months. These data are very encouraging, bearing in mind the fact that most PC patients undergo various therapies and have a history of multiple relapses before they receive the PSMA-RLT therapy [19,20,21, 24, 28, 29]. However, in this late stage of disease, treatment opportunities for patients who relapse after PSMA-RLT are very limited.
Re-administration of PSMA-RLT in patients who previously benefited from the therapy and experienced no relevant toxicity seems to be a promising option. The main focus of this article is to investigate survival, response and adverse events in patients who underwent rechallenge PSMA-RLT therapy.
Materials and methods
Patients
Included in the analyses were patients with initial disease control after baseline treatment with PSMA-RLT who eventually progressed and received rechallenge PSMA-RLT. The presence of PSMA-positive metastases should have been confirmed via [68Ga]Ga-PSMA-11 PET/CT. Patients’ data were retrieved from clinical records. Baseline patients’ characteristics, such as tumour spread and prior therapies, are summarised in Table 1. The protocol of this retrospective study was in accordance with the requirements of the local ethics committee, the Declaration of Helsinki or comparable ethical standards. All patients agreed to the scientific analysis of their data and gave written informed consent prior to the therapy.
Treatment
ABX GmbH (Radeberg, Germany) provided the PSMA-617 ligand, and IDB (Holland, Bearle-Nassau, Netherlands) provided the nuclide Lutetium-177. The radiolabelling proceeded locally. PSMA-RLT was injected intravenously as a slow bolus injection. The administered activity was adapted to the tumour load, renal parameters and bone marrow reserve. The median injected activity was 6.1 GBq per cycle (range 3.8–6.7 GBq), followed by a 1000-ml infusion of 0.9% NaCl solution. To avoid xerostomia, patients had to cool their salivary glands with cool packages 30 min before administration of the radiopharmaceutical. The distribution and tumour-uptake of the radionuclide was recorded using planar whole body scans and SPECT/CT 24 h after administration.
Toxicity assessment
Clinical data and laboratory profiles, including renal function parameters and haematological results, were recorded for each patient. Blood tests were performed before and after each PSMA-RLT cycle and were repeated every 2 weeks for a period of 2 months after the treatment and at each follow-up visit. All patients underwent a baseline [99mTc]Tc-MAG3-renography before starting PSMA-RLT. Additionally, patients exhibiting renal toxicity or a history of renal impairment underwent a [99mTc]Tc-MAG3-renography before each cycle, including quantitative measurements of the tubular extraction rate. Clinical reports included information about patients’ subjective health complaints, amount of analgesic drugs administered, weight loss and Eastern Cooperative Oncology Group (ECOG) performance status. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0) [30].
Response assessment
Response was demonstrated morphologically by [68Ga]Ga-PSMA-11-PET/CT and biochemically by PSA measurements. The imaging response was classified according to adapted PERSIST criteria as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Additionally, we defined a mixed response (MixR) as a partial response of the known metastases and, at the same time, the occurrence of new lesions. However, for the MixR, the overall tumour load should be either stable or less in comparison to the pre-therapeutic imaging. The biochemical response was classified according to the recommendations of the Prostate Cancer Working Group 3 (PCWG3): partial response (PR) if there is a PSA-decrease ≥50% and progressive disease (PD) if PSA increases ≥25%. As stable disease (SD) was regarded either PSA-increase <25% or PSA-decrease <50% [31]. Time to prostate-specific-antigen (PSA) progression was calculated from the date of the 1st rechallenge cycle.
Statistical analysis
Patients’ data were summarised in a database. Frequency analyses, descriptive statistics and statistical comparisons were carried out using SPSS software (IBM SPSS Statistics 24.0, New York). A chi-square test was performed to compare responses, and log-rank was used to compare survival rates. The significance level was set at p < 0.05. We also used Excel (Microsoft Office 2010) for water-flow analyses of the PSA changes. Overall survival was estimated with the Kaplan-Meier method (censored data) and calculated from the date of the initiation of the rechallenge treatment.
Results
Patients and treatment
We screened 216 patients who received PSMA-RLT in the period from November 2014 to March 2018 at our department. Of them, 30 individuals were included in the analyses. Table 1 represents the patients’ characteristics before PSMA-RLT. In summary, it can be stated that the patient population consisted mainly of elderly males with extensive disease but who were otherwise in good general condition. Nearly 73% of the patients had undergone prior chemotherapy, and about one-half of the patients had concomitant anti-hormonal therapy, such as abiraterone or enzalutamide. Most of the patients’ characteristics listed in Table 1 did not significantly differ from the characteristics of the initial screened population. The only difference was that the patients who eventually received rechallenge cycles RLT had a higher incidence of lymph node metastases than the patients who received only baseline PSMA-RLT (90% vs. 71%, p = 0.04).
Patients were initially treated with a median of 3 cycles (range 1 to 5 cycles) of PSMA-RLT and were eventually retreated with another 3 cycles (range 1 to 6 cycles). The period between the last cycle of the baseline therapy and the first rechallenge cycle varied between 2 and 26 months, with a median of 6 months. One of the patients had only a 2-month gap between the first course of PSMA-RLT and rechallenge treatment. This patient showed a PSA-decrease from 1350 to 254 ng/ml after the baseline treatment, which was a very good response. Unfortunately, 6 weeks afterwards followed a PSA-increase to 541 ng/ml, and for that reason we performed a rechallenge PSMA-RLT. After the rechallenge cycle the PSA increased again (855 ng/ml), eventually the patient died. The overall survival was 12 months after the first cycle of baseline therapy with PSMA-RLT and 6 months after the rechallenge treatment, respectively.
The median cumulative administered activity was 17.9 GBq at baseline therapy and 37.4 GBq after the rechallenge treatment. The median activity of the rechallenge treatment was 19.6 GBq, and the administered activity per cycle was 6.1 GBq. If response or stable disease occurred after the first rechallenge PSMA-RLT cycle according to the PSA values or [68Ga]Ga-PSMA-11-PET/CT, the patients continued the retreatment (1 to 2 additional cycles) and received follow-ups until subsequent progression.
Safety results
The assessed toxicity according to the CTC is visualised in Table 2. Encouragingly, there were no cases of life-threatening (CTC grade 4) adverse events after the retreatment. However, compared to the baseline data of this patient cohort (3% CTC grade-3 anaemia), there were more grade-3 adverse events. In total, 23% of patients experienced serious impairment of bone marrow function, such as thrombopenia (10%), anaemia (10%), leucopoenia (6%) or neutropoenia (3%). One patient developed serious renal impairment during the treatment; however, this might be disease-related because of the rapid progression of the retroperitoneal lymph node metastases.
Seventy percent of patients developed irreversible adverse events: 90% low-grade (N = 19) and 10% grade 3 (N = 2) toxicity. All patients with low-grade irreversible adverse events had anemia. One patient developed irreversible impairment of the renal function (CTC grade 3) and another patient developed permanent severe anemia (CTC grade 3). In 13% of cases, no irreversible toxicity was observed. The remaining 17% of patients had an unknown outcome regarding the course of the laboratory parameters after the occurrence of the adverse event.
Response and survival
The patients received follow-ups for a median of 3 months (range 1–15 months). Three patients received a follow-up of only 1 month. Two of these patients came from abroad and were lost to follow-up after initial email contact 1 month after the last treatment. Another patient died 1 month after the last therapy.
Table 3 shows an overview of the response data after the first, second, third and fourth cycles of retreatment according to PSA-level measurements and [68Ga]Ga-PSMA-PET/CT. Interestingly, in the PET/CT, we often observed MixR with new metastases, although the PSA level was stable or had even decreased. The incidence of MixR rose after each re-cycle. The median PSA-values decreased from 235 to 110 ng/ml after the first cycle and from 242 to 87 ng/ml after the second cycle, respectively. There was a slight increase of the median PSA-level from 222 to 268 ng/ml after the third cycle, but after the fourth cycle the values decreased again, from 461 to 293 ng/ml.
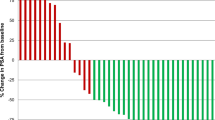
The waterfall plots in Fig. 1 represent the PSA change after the first to third cycles. Although some patients had previously benefited from the therapy, others experienced rapid progression and did not respond to the retreatment. Thus, the majority of patients benefited from additional cycles of PSMA-RLT: stable PSA-levels (increase <25%, decrease <50%) had 50–60% of patients. A PSA decrease ≥50% was seen in 26, 40 and 20% from the first, second and third rechallenge cycles, respectively. Only 10–22% of patients experienced a significant PSA increase (≥ 25%) after each of the first three rechallenge cycles.
Waterfall plots. Stable PSA-levels (increase <25%, decrease <50%) had 50–60% of patients. A PSA decrease ≥50% was seen in 26, 40 and 20% from the first, second and third rechallenge cycles, respectively. Only 10–22% of patients experienced a significant PSA increase (≥ 25%) after each of the first three rechallenge cycles
The median time to PSA progression, calculated from the date of the first rechallenge cycle, was 2.8 months (range 1–11 months). The median OS was 12 months after beginning the retreatment and 25 months after beginning the baseline PSMA-RLT. Patients with a PSA decrease of ≥50% after the first rechallenge PSMA-RLT cycle survived for a median of 19 months, which was longer than the survival rate of patients with a PSA increase ≥25%: only 6 months (not significant according to the logrank test). Patients who had stable PSA levels according to PCWG3 (PSA-increase <25% and PSA-decrease <50%) had a median OS of 12 months. The imaging response via [68Ga]Ga-PSMA-11-PET/CT also did not significantly correlate with the survival rate.
Figure 2 shows the median survival in the group of patients who received rechallenge PSMA-RLT, compared to the initial screened population. The difference in the overall survival was significant: 25 months after rechallenge PSMA-RLT vs. 9 months in the patients who received only baseline PSMA-RLT.
Survival analysis (Kaplan-Meier Curves) calculated from the 1. cycle PSMA-RLT. From the 216 screened patients, 30 received rechallenge PSMA-RLT. The difference in the median overall survival was significant: 25 months after rechallenge PSMA-RLT vs. 9 months in the remaining patients, who received only a baseline PSMA-RLT (logrank p < 0.001)
Discussion
PSMA-RLT therapy is a novel therapy option, primarily in patients with CRPC. Currently, a phase III randomised trial (VISION-trial) is recruiting patients with advanced progressive cancer to compare the effect of PSMA-RLT with the best supportive care. However, the estimated study completion date is in May 2021; until then, PSMA-RLT is expected to remain the last-line treatment option (ClinicalTrials.gov Identifier: NCT03511664).
Several retrospective studies have suggested that PSMA-RLT is effective and safe in patients with PC [11, 16,17,18,19,20,21,22,23,24]. These data are very encouraging but are also very heterogeneous. For example, in the literature, progression-free survival in treated patients ranges between 4.5 and 13.7 months, while overall survival ranges between 7.5 and 15 months [19,20,21, 24, 28, 29]. Recently, a phase II study from Melbourne confirmed the favourable safety profile of PSMA-RLT. The estimated PFS was 7 months, while the median overall survival rate was 13.5 months [32].
A current problem is the therapeutic approach taken when patients eventually progress after PSMA-RLT. Most of these patients will have had prior chemotherapy and novel anti-hormone therapies, such as abiterone and enzulatamide, and yet still relapsed. Therefore, in this late stage of disease with limited treatment options, it is difficult to find treatment opportunities for those patients who relapse after PSMA-RLT. Therefore, data from a larger group of patients about retreatments with PSMA-RLT seems to be important. In this analysis, we focused on the outcomes of 30 patients who underwent rechallenge PSMA-RLT.
Similar to other studies of PSMA-RLT, no life-threatening (CTC grade 4) adverse events after retreatment were observed in the current study. However, compared to the literature (3–11%) and the baseline data of our patient cohort (3% CTC grade-3 anaemia), grade-3 haematotoxicity was more common, occurring in 23% of patients [16, 17, 19,20,21,22, 25,26,27]. One patient developed renal impairment during the treatment; however, this might be disease-related, because of the rapid progression of the retroperitoneal lymph node metastases, and not necessarily drug-related.
[68Ga]Ga-PSMA-PET/CT performed after the first 4 rechallenge PSMA-RLT cycles showed an increasing incidence of MixR, with new metastases detected, although the PSA level was stable or had even decreased. According to RECIST criteria, the occurrence of new metastases is regarded as progressive disease [33]. However, because of a lack of alternative therapies, we continued the PSMA-RLT if the PSA level was at least stable and the overall tumour mass had not significantly increased under the rechallenge PSMA-RLT. At the end of the analyses, we discovered that the response via [68Ga]Ga-PSMA-PET/CT was not prognostic for survival in this patient cohort. A recent analysis from Cologne demonstrated that the therapy effects of PSMA-RLT shown via [68Ga]Ga-PSMA-PET/CT (SUV, affected bone volume and lymph node diameters) were mostly independent of PSA response [34]. These findings might also explain the discordance between PSA changes and findings via [68Ga]Ga-PSMA-PET/CT in our study. Thus, further studies are needed to evaluate the utility of [68Ga]Ga-PSMA-PET/CT in managing the treatment of patients undergoing PSMA-RLT.
Patients with a PSA-response according to PCWG3 survived a median of 19 months after the first rechallenge PSMA-RLT cycle, which was longer than the 6 months survival of patients with a PSA increase. However, maybe because of the limited number of patients, the difference was not significant according to the logrank test. Patients who had stable PSA levels achieved a median OS of 12 months.
The progression-free survival rate was defined as the median time to PSA progression and was calculated from the date of the first rechallenge cycle. The PFS in our patients was 2.8 months (range 1–11 months), which was shorter than the PFS reported in other studies after primary PSMA-RLT: 4.5–13.7 months [19,20,21, 28, 29, 32]. The reason for this discrepancy might be the increased aggressiveness of the disease in these multiply relapsed patients. However, the median overall survival calculated from the baseline PSMA-RLT was much longer in those patients who received rechallenge PSMA-RLT than in the patients who received only baseline PSMA-RLT: 25 months vs. 9 months (p < 0.001).
This was a single-centre study that included 30 patients who received a total of 181 cycles of PSMA-RLT; of these, 85 cycles involved rechallenge treatment. A possible limitation of this study is that since all the data were collected retrospectively, biases might have been introduced, such as the preselection of eminently eligible patients. Moreover, 2 patients were lost to follow-up 1 month after the last treatment. Another limiting factor is the small number of patients included. In sum, there is an increasing need for prospective studies with larger numbers of patients that can confirm the efficacy of PSMA-RLT as a rechallenge treatment.
Conclusion
The findings of this study suggest that rechallenge PSMA-RLT should be considered a therapeutic option for patients who previously responded to PSMA-RLT. Although there were more serious adverse events than expected, they might be disease-related and not necessarily drug-related. Patients who showed a biochemical response (PSA decrease) could achieve longer survival compared to patients who did not respond. Furthermore, the median overall survival was much longer in the patients after the rechallenge PSMA-RLT than in the patients who only received baseline PSMA-RLT. However, these data need to be considered cautiously because of the retrospective, non-randomised setting and the limited number of patients.
Retreatment with PSMA-RLT appears to be safe and effective, resulting in benefits for most patients. Given these encouraging results, further studies of salvage therapies with PSMA-RLT should be designed in a prospective setting.
References
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues [eng]. Clin Cancer Res. 1997;3(1):81–5.
Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises [eng]. J Biol Regul Homeost Agents. 2014;28(4):555–63.
Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique [eng]. Histopathology. 2007. https://doi.org/10.1111/j.1365-2559.2007.02635.x.
Ghosh A, Heston WDW. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer [eng]. J Cell Biochem. 2004. https://doi.org/10.1002/jcb.10661.
Wang X, Yin L, Rao P, Stein R, Harsch KM, Lee Z, et al. Targeted treatment of prostate cancer [ENG]. J Cell Biochem. 2007. https://doi.org/10.1002/jcb.21491.
Wright GL, Mayer Grob B, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996. https://doi.org/10.1016/S0090-4295(96)00184-7.
Jamous M, Haberkorn U, Mier W. Synthesis of peptide radiopharmaceuticals for the therapy and diagnosis of tumor diseases [ENG]. Molecules. 2013. https://doi.org/10.3390/molecules18033379.
Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer [ENG]. J Nucl Med. 2015. https://doi.org/10.2967/jnumed.114.147413.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions [eng]. J Nucl Med. 2015. https://doi.org/10.2967/jnumed.115.161299.
Rajasekaran SA, Anilkumar G, Oshima E, Bowie JU, Liu H, Heston W, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen [eng]. Mol Biol Cell. 2003. https://doi.org/10.1091/mbc.E02-11-0731.
Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA Theranostics: current status and future directions [eng]. Mol Imaging. 2018. https://doi.org/10.1177/1536012118776068.
Ahmadzadehfar H, Aryana K, Pirayesh E, Farzanehfar S, Assadi M, Fallahi B, et al. The Iranian Society of Nuclear Medicine practical guideline on radioligand therapy in metastatic castration-resistant prostate cancer using 177Lu-PSMA. Iranian Journal of Nuclear Medicine. 2018;26(1):2–8.
Wüstemann T, Haberkorn U, Babich J, Mier W. Targeting prostate cancer: prostate-specific membrane antigen based diagnosis and therapy [eng]. Med Res Rev. 2018. https://doi.org/10.1002/med.21508.
Ahmadzadehfar H, Albers P, Bockisch A, Boegemann M, Böhme C, Burchert W, et al. Lutetium-177-PSMA-Radioligandentherapie: Konsensus im Rahmen der GKV-finanzierten Versorgung zwischen den Hochschulkliniken in Aachen, Bonn, Düsseldorf, Essen und Köln und dem MDK Nordrhein. [Lutetium-177-PSMA radioligand therapy : consensus within the framework of GKV-funded care between the university hospitals in Aachen, Bonn, Düsseldorf, Essen, and Cologne and the MDK Nordrhein] [ger]. Urologe A. 2018. https://doi.org/10.1007/s00120-018-0642-2.
Fendler WP, Kratochwil C, Ahmadzadehfar H, Rahbar K, Baum RP, Schmidt M, et al. Therapie mit 177Lu-PSMA-617, Dosimetrie und Nachsorge beim metastasierten kastrationsresistenten Prostatakarzinom. [177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer] [ger]. Nuklearmedizin. 2016;55(3):123–8.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment [eng]. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-016-3481-7.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis [eng]. J Nucl Med. 2016. https://doi.org/10.2967/jnumed.116.173757.
Rahbar K, Bögeman M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer [eng]. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-017-3877-z.
Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer [eng]. Clin Nucl Med. 2016. https://doi.org/10.1097/RLU.0000000000001240.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 Radioligand therapy in advanced prostate cancer patients [eng]. J Nucl Med. 2017. https://doi.org/10.2967/jnumed.116.183194.
Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013 [eng]. J Nucl Med. 2016. https://doi.org/10.2967/jnumed.115.170167.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617 [eng]. J Nucl Med. 2016. https://doi.org/10.2967/jnumed.115.171397.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy [eng]. J Nucl Med. 2016. https://doi.org/10.2967/jnumed.115.168443.
Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using 177LuLu-PSMA-617 [eng]. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-017-3716-2.
Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-Centre study [eng]. EJNMMI Res. 2015. https://doi.org/10.1186/s13550-015-0114-2.
Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer [eng]. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.7245.
Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using 177LuLu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer [eng]. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-017-3681-9.
Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving 177LuLu-PSMA-617 radioligand therapy [eng]. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.21600.
Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer [eng]. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-017-3751-z.
References National Cancer Institute Guidelines For Investigators: Adverse event reporting requirements for DCTC DCTD (CTEP and CIP) and DCP INDs and IDEs. 2013. - Google-Suche. Available from: https://www.google.de/webhp?sourceid=chrome-instant&ion=1&espv=2&ie=UTF-8#q=references+National+Cancer+Institute+Guidelines+For+Investigators:+Adverse+event+reporting+requirements+for+DCTC+DCTD+(CTEP+and+CIP)+and+DCP+INDs+and+IDEs.+2013. Accessed 11 Oct 2018.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3 [eng]. J Clin Oncol. 2016. https://doi.org/10.1200/JCO.2015.64.2702.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-Centre, single-arm, phase 2 study. Lancet Oncol. 2018. https://doi.org/10.1016/S1470-2045(18)30198-0.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) [English]. Eur J Cancer. 2009. https://doi.org/10.1016/j.ejca.2008.10.026.
Taeger P, Hammes J, Hohberg M, Wild M, Schomaecker K, Kobe C, et al. Discrete evaluation of multi-cycle Lu-177-PSMA-617-therapy effects on bone versus lymph node metastases in patients with metastasized castration-resistant prostate cancer (mCRPC). J Nucl Med. 2018;59(supplement 1):523.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or non-financial competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yordanova, A., Linden, P., Hauser, S. et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging 46, 1073–1080 (2019). https://doi.org/10.1007/s00259-018-4222-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4222-x