Abstract
Purpose
Recently, the new hybrid chelator DATA (6-amino-1,4-diazepine-triacetate) has been introduced, which has the advantage of high yield and radiolabelling of DATA-based octreotide derivative (TOC) at room temperature in contrast to tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA) that needs 95 °C for effective labelling. However, the diagnostic potential of DATA-TOC has not been studied with other chelators in humans. The aim of this study was to compare the diagnostic efficacy of [68Ga]Ga-DATA-TOC with [68Ga]Ga-DOTA-NOC (which is the current standard for imaging neuroendocrine tumours (NET)) in patients of gastroenteropancreatic neuroendocrine tumours (GEP-NETs).
Methods
Fifty patients (thirty-one males and nineteen females) with biopsy-proven GEP-NETs were included in the study. Patients age ranged from 14 to 75 years (mean 46.11 years). All patients underwent two PET studies with [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC. Images were evaluated visually and semi-quantitatively using maximum standardized uptake values (SUVmax) of tumour, mediastinum and liver. Tumour-to-liver (T/L) and tumour-to-mediastinum (T/M) SUVmax ratios were computed. For the purpose of comparison, patient-wise as well as lesion-wise analysis was carried out. The nonparametric-related samples Wilcoxon signed-rank test was used for comparison of the SUVmax values and ratios.
Results
On visual evaluation, the biodistribution and image quality of [68Ga]Ga-DATA-TOC was similar to [68Ga]Ga-DOTA-NOC. Physiological liver uptake was lower in [68Ga]Ga-DATA-TOC as compared with [68Ga]Ga-DOTA-NOC, 7.65 ± 5.37 vs 8.94 ± 5.95 (p = 0.009), respectively. On a patient-wise analysis, both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were lesion-positive in the 44 patients (88%) and were negative in the 6 patients (12%). On a lesion-based analysis, [68Ga]Ga-DATA-TOC had 98.6% concordance with [68Ga]Ga-DOTA-NOC (232 out of 235 lesions detected). The target tumour SUVmax on [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 36.63 ± 32.24 and 40.82 ± 36.89, respectively (p = 0.097). The T/L SUVmax ratios were not significantly different (5.99 ± 5.52 vs 5.67 ± 4.96, p = 0.77).
Conclusion
[68Ga]Ga-DATA-TOC PET/CT imaging produced results that were comparable with [68Ga]Ga-DOTA-NOC. It, thus, has potential utility as an effective and safe alternative to 68Ga-DOTA-NOC with the added benefit of ease, cost-effective and improved yield of instant kit-type synthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Somatostatin receptor (SSTR) imaging uses radiolabelled somatostatin analogues which bind to the somatostatin receptors (SSTR1–5), commonly overexpressed in neuroendocrine tumours (NETs). NETs have shown a rising trend in incidence over the past four decades due to increasing awareness and better diagnostic tools [1]. Surveillance, Epidemiology and End Results (SEER) registry reports six-fold increase in incidence rates from 1973 (1.09/100,000) to 2012 (6.98/100,000) [2]. NETs arise most commonly in the gastroenteropancreatic (GEP) tract (75 %), followed by the lungs (25%) [1]. The 2010 WHO classification divides GEP-NETs into G1, G2 and G3 tumours based on the mitotic index and Ki67, cellular proliferation marker [3].
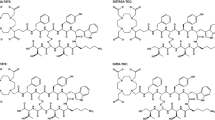
Currently, [68Ga]-DOTA-based peptides (Phe1-Tyr3-octreotide (TOC), NaI3-octreotide (NOC), and Tyr3-octreotate (TATE)), used as positron emission tomography (PET) tracers have proven utility in staging, restaging, treatment response assessment and detection of unknown primary in NETs [4, 5]. By demonstrating SSTR status in NETs in vivo, it determines the eligibility of patients for peptide receptor radionuclide therapy (PRRT), e.g. 177Lu-labelled DOTA-conjugated peptides, which is an effective and safe treatment option for advanced or progressive SSTR-positive NETs [6, 7]. Commonly used 68Ga radiopharmaceuticals are based on the octadentate bifunctional chelator DOTA (DOTA, tetraazacyclododecane-1,4,7,10-tetraacetate). The disadvantage of DOTA-based precursors is the requirement of relatively harsh conditions (heating at 80–95 °C for up to 30 min, at pH 4.6) for radiolabelling. This is because of mismatch between the small ionic radius of Ga(III) and the large cavity size of the macrocycle (DOTA), which fits larger metal ions (i.e. yttrium, the lanthanide ions and calcium) more efficiently [8]. The DATA chelators are a novel class of tri-anionic ligands based on 6-amino-1,4-diazepine-triacetic acid, which have smaller cavity size better suited for the chelation of 68Ga. Moreover, they represent a novel class of hybrid chelators due to the one nitrogen atom arranged in exo-position (Fig. 1). The remaining cyclic moiety is supposed to maintain high kinetic stability in vivo, while the acyclic moiety shall guarantee for easy labelling.
Indeed, compared with macrocyclic chelators based on the cyclen scaffold (i.e. DOTA derivatives), DATA (6-amino-1,4-diazepine-triacetate) chelators allow for quantitative radiolabelling more rapidly and at lowest precursor concentration and under milder conditions. Particularly, the 68Ga labelling procedures with DATA are carried out at room temperature (22–24 °C) with excellent labelling yield and reproducibility as well as kinetic and thermodynamic stability [9,10,11,12].
This facilitates preparation of ready-for-injection 68Ga radiopharmaceuticals in an instant kit-type protocol, similar to the procedures established for most of the 99mTc labelling protocols. The DATA-based octreotide derivative TOC allows radiolabelling with 68Ga at room temperature, making it more suitable for kit-type labelling, in contrast to [68Ga]Ga-DOTA-NOC that needs 95 °C for effective labelling [13, 14]. Pre-clinical studies have shown excellent results in terms of stability and specific binding [15]. The human studies for the biodistribution and dosimetry of the new tracer are being undertaken [16]. Currently, there is first evidence in one patient regarding the diagnostic efficacy of [68Ga]Ga-DATA-TOC. Therefore, we undertook this prospective and systematic study to compare [68Ga]Ga-DATA-TOC with the current standard [68Ga]Ga-DOTA-NOC in the patients of GEP-NETs.
Patients and methods
Patients and study design
Patients with suspected or histologically proven, primary or recurrent GEP-NETs, who were referred to our centre for staging, restaging or post-therapy follow-up, were consecutively enrolled in this prospective study between December 2015 and December 2017. Patients with GEP-NET grade 1 and grade 2 were included in our study, while excluding patients with poorly differentiated NET (grade 3). All patients included gave a written and informed consent to undergo both [68Ga]Ga-DOTA-NOC and [68Ga]Ga-DATA-TOC PET/CT imaging. Consent from parents was taken in case the age of the patient was less than 18 years. Short-acting octreotide was discontinued for 24 h prior to imaging, and in those on long-acting octreotide, imaging was performed in the week before the next dose, which was scheduled monthly. Ethical clearance was obtained by the institute ethical committee prior to initiation of the study (Ref. No. IECPG-101/30.12.2015).
Radiopharmaceutical synthesis and quality control
For [68Ga]Ga-DOTA-NOC synthesis, 68Ga (1110–1850 MBq [30–50 mCi]) was eluted from a 68Ge/68Ga generator (ITG) using 0.1 M HCl. The eluent was loaded on a miniaturised column of organic cation-exchanger STRATA-X C column to pre-concentrate (using 80% acetone/0.15 M HCl). The processed 68Ga (half-life, 68.3 min; positron branching fraction, 88%; effective positron energy maximum, 1.9 MeV) was directly eluted with 97.7% acetone/0.05 M HCl into the reaction vial containing 30–50 μg of DOTA-NOC and 0.4 M buffer at pH of 4. Synthesis was performed at approximately 126 °C for 10–15 min, followed by transferring of product from the reaction vessel on to the C-18 cartridge. The labelled product from the C-18 cartridge is eluted finally by 70% ethanol and further rinsed with 10 ml normal saline.
For [68Ga]Ga-DATA-TOC synthesis, 68Ga was directly eluted with 97.7% acetone/0.05 M HCl into the reaction vial containing 30 μg of DATA-TOC and 1 ml of buffer (0.4 M sodium acetate at pH 4). The labelling was performed at 23 °C (room temperature) for 10 min, followed by transferring of product from the reaction vessel on to the C-18 cartridge. The labelled product from the C-18 cartridge is eluted finally by 70% ethanol and further rinsed with 10 ml normal saline. The radiochemical purity every time was > 95% for [68Ga]Ga-DATA-TOC immediately after labelling and purification step was not needed.
The mean synthesised batch activity of [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 16.9 ± 7.2 mCi and 13.7 ± 4.9 mCi with a mean percentage yield of 70% and 63.1%, respectively. This reflects a ca. 25% higher overall batch yield for the DATA-based analogue, which is due to the improved initial yield when labelling at room temperature compared with nonquantitative yield for the DOTA analogue after heating at 95 °C, as well as due to the non-needed purification of the [68Ga]Ga-DATA-TOC, saving time. The time required for the synthesis of 68Ga-DOTA-NOC was approximately 30 min, of which the 10–15 min comprised the heating process. On the other hand, the entire [68Ga]Ga-DATA-TOC synthesis was completed in 15 min with a labelling time of 10 min in ambient temperature.
PET/CT acquisition protocol
After passing the quality control tests, patients were administered 110–185 MBq (mean 142.45 ± 29.23 MBq) of [68Ga]Ga-DATA-TOC and 110–185 MBq (mean 135.79 ± 19.98 MBq) (p = 0.186) of [68Ga]Ga-DOTA-NOC intravenously. Whole body images were acquired using an integrated PET/CT scanner (Siemens Biograph mCT 64), after 45–60 min of injection. CT topogram was acquired to define the range of the study, covering the area from skull to mid-thighs. Diagnostic CT without IV contrast enhancement was acquired on 64 slice spiral CT mode with low tube current (120 kVp, 48–76 mAs), slice thickness of 4 mm and a pitch of 1. Images were acquired using a matrix of 512 × 512 pixels and pixel size of about 1 mm. 3D PET emission scans were acquired for 120 s per bed position. PET data was acquired using matrix of 128 × 128 pixels with a slice thickness of 1.5 mm. PET images were reconstructed using ordered subset expectation maximization ((OSEM); 2 iterations and 8 subsets).
PET/CT image interpretation/analysis
PET images were visually evaluated for areas of abnormal radiotracer accumulation which were noted in terms of location in the corresponding CT images. Region of interest (ROI) was carefully drawn on each lesion corresponding to the site of abnormal [68Ga]Ga-DOTA-NOC/[68Ga]Ga-DATA-TOC accumulation. Metabolic activity of the lesion was assessed using maximum standardised uptake value (SUVmax). SUVmax was chosen for semi-quantitative assessment as it shows less inter-observer variability. Each lesion was then characterised as primary or metastatic based on location and CT changes. Patients were classified into positive and negative for SSTR expressing lesions; sites and number of lesions (maximum of five per organ) were counted. The SUVmax of the liver and mediastinum were also noted for each patient, and the tumour-to-liver (T/L) and tumour-to-mediastinum (T/M) SUVmax ratios were computed. For the purpose of comparison, patient-wise and lesion-wise analyses were carried out. A target lesion with highest SUVmax was selected on [68Ga]Ga-DOTA-NOC, and each lesion was compared with corresponding lesion on [68Ga]Ga-DATA-TOC.
Statistical analysis
68Ga DATATOC PET/CT scans were compared with 68Ga-DOTA-NOC PET/CT on a per patient and per lesion basis. McNemar test was used to estimate discordance between two tracer groups on patient-based comparison. Student’s t test was used to estimate level of significance in the difference in the number of lesions among two groups. The nonparametric-related samples Wilcoxon signed-rank test was used for comparison of the SUVmax values and ratios among two groups. All statistical analysis was performed using dedicated statistical software (SPSS 23.0, IBM), and p value lower than 0.05 was considered as significant.
Results
Demographics
Fifty-three patients with suspected or known GEP-NETs were enrolled in the study. Two patient were found to be grade III NET with Ki67 of > 20%, and one patient had initial diagnosis of NET but on review of histopathology in our institute, it turned out to be low grade sarcoma and thus, were subsequently excluded. Hence, a total of 50 patients (thirty-one males and nineteen females) with suspected or histologically proven GEP-NETs were enrolled in the study. The mean age of the patients was 46.1 ± 14.3 years (range, 14–75 years). All fifty patients underwent both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOCPET/CT imaging within a period of 1 to 10 days in a random fashion. Twenty-four patients (48 %) were referred for initial diagnosis and staging (prior to any treatment), 13 patients (26 %) for restaging and 13 patients (26%) had been referred for evaluation of treatment response. Out of total 50 patients, 24 patients (48%) had primary lesion in pancreas, 6 patients (12%) had gastric NET and 14 patients (28%) had intestinal NETs (Table 1). Five patients (10%) presented as unknown primary with liver metastases. Surgical histopathology reports were available in forty-eight patients. In two patients, surgery could not be performed as no lesion was localised on conventional and molecular imaging. Twenty-one patients (43.7 %) had well-differentiated grade 1 NET and 27 patients (56.2 %) had grade 2 NET.
Biodistribution
On visual evaluation, maximum intensity projection (MIP) images of both tracers demonstrated excellent contrast and similar biodistribution profile. The SUV mean values of the pituitary, mediastinum, spleen, kidneys, adrenals, bone marrow, liver and muscle (left thigh) are detailed in Table 2.
Patient-based comparison
On a patient-wise analysis, both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were lesion-positive in 44 patients (88%) and lesion negative in 6 patients (12%). No discordance was noted between the two tracers on patient-based comparison (McNemar, p = 1.00). On visual evaluation of lesions in 44 patients who had positive imaging, we observed comparable lesion uptake in both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC scans (Fig. 2). Target tumour SUVmax values (mean ± SD) of [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 36.63 ± 32.24 and 40.82 ± 36.89, respectively. Difference between SUVmax values was not significant (n = 44, p = 0.097). Target lesion to liver (T/L) SUVmax ratios for [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 5.99 ± 5.52 and 5.67 ± 4.96 (n = 44, p = 0.770), respectively. Target to mediastinal blood pool (T/M) SUVmax ratios for [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 19.85 ± 16.37 and 26.38 ± 27.93 (n = 44, p = 0.025), respectively. Median (IQR) SUVmax values of primary and metastatic lesion in various organs in [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC are described in Table 3. The absolute tumour uptake (Bq/mL) of [68Ga]Ga-DATA-TOC was significantly higher compared with [68Ga]Ga-DOTA-NOC (Median (IQR):, 7435 (9463) versus 6560 (10013); p = 0.012).
Maximum intensity projection image, transaxial fused PET/CT and CT images of [68Ga]Ga-DATA-TOC (a, b and c) and [68Ga]Ga-DOTA-NOC (d, e and f) show similar distribution of liver metastases and primary pancreatic lesion in a 42-year-old patient of grade I pancreatic NET. Transaxial fused PET/CT images (b and e) show radiotracer uptake in pancreatic lesion and liver lesions. The lower uptake of the normal liver background on [68Ga]Ga-DATA-TOC [b] better delineates liver lesions as compared with [68Ga]Ga-DOTA-NOC (e)
Out of 44 patients with positive scan findings, 17 patients had grade 1 GEP-NET and 27 patients with grade 2 GEP-NET. The median (IQR) SUVmax of target lesion in [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 27.31 (45.73) and 28.66 (47.79), with no significant difference (p = 0.492) in grade 1 patients. The median (IQR) SUVmax of target lesion in [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were 31.51 (36.20) and 27.01 (48.20), and showed no significant difference (p = 0.149) in grade 2 patients.
Out of the 6 patients who were negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC scans (n = 6), four patients had elevated serum chromogranin A levels and were referred for primary diagnosis. One patient with suspected insulinoma who was referred for staging was negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC imaging. He was subsequently confirmed to have insulinoma on histopathology. There was one patient who had isolated liver lesions on CT which were proven NET metastases on biopsy. He was referred for unknown primary NET but was negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC imaging. Two patients who were referred for diagnosis of NET with elevated serum chromogranin A levels and negative conventional imaging were negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC scans and negative on follow-up of 6 months. The other two patients underwent primary tumour resection and were referred for restaging with elevated chromogranin A, had negative imaging and were negative on follow-up as well. Among these patients with negative imaging, two were false negative and four were true negative. The sensitivity and specificity of the two tracers with histopathology as reference standard was 95.6 % and 100 %, respectively. The concordance of the two tracers was 100% on patient-based comparison.
Lesion-based comparison
On a lesion-wise analysis, there were 235 clearly defined lesions in [68Ga]Ga-DOTA-NOC images. While on the other hand, [68Ga]Ga-DATA-TOC images clearly defined 232 (98.7 %) lesions and 3 lesions (2 liver lesions and 1 lymph node) were missed because of lack of significant uptake (Table 4). The difference in the number of lesions detected between the two tracers was not statistically significant (Student’s t test, p = 0.182) and showed good correlation (r = 0.997, p < 0.05). [68Ga]Ga-DATA-TOC missed 3 lesions in two patients, while in all other positive patients (n = 42), the number of lesions detected with the two tracers was equal. One of these patients was a 35-year-old man with a diagnosis of well-differentiated NET of ileum grade 1. On histopathological evaluation, the proliferation index was found to be 2%. There were 5 lesions detected by [68Ga]Ga-DOTA-NOC imaging. However, only 3 of these lesions were positive on [68Ga]Ga-DATA-TOC imaging and the rest of the 2 liver lesions were negative (Fig. 3). The other patient was a 65-year-old man who presented with left lower limb swelling. The swelling was found to be due to lymph nodal mass in retroperitoneum. USG guided FNAC revealed well-differentiated NET of unknown origin grade 2. The proliferation index of the tumour was found to be 20% on histopathological evaluation. There were eight lymph node lesions on [68Ga]Ga-DOTA-NOC imaging, however only seven of them were positive on [68Ga]Ga-DATA-TOC imaging.
Maximum intensity projection image, transaxial fused PET/CT, CT of [68Ga]Ga-DATA-TOC (a, b and c) and [68Ga]Ga-DOTA-NOC (d, e and f) in a 35-year-old man with grade 1 NET of ileum (Ki67 = 2%), with hepatic metastases post-resection of primary lesion. Three focal areas of increased radiotracer uptake in the liver are seen in [68Ga]Ga-DOTA-NOC (d), while in [68Ga]Ga-DATA-TOC (a), two lesions in segment VI/VII of the liver are not well demarcated. Focal radiotracer accumulation in hypodense lesion in segment IVa of the liver is seen in both [68Ga]Ga-DATA-TOC (b, c) and [68Ga]Ga-DOTA-NOC (e, f)
Discussion
In the present study, we compared the novel PET tracer [68Ga]Ga-DATA-TOC, with the current standard [68Ga]Ga-DOTA-NOC for imaging of somatostatin receptor expression in patients with GEP-NETs. [68Ga]Ga-DATA-TOC with DATA as the chelator has proven advantageous over DOTA-conjugated somatostatin analogues in terms of radiolabelling with Ga-68 quickly at room temperature with high labelling efficiency in lesser time without the need for purification of the product, which facilitates instant kit-type radiopharmaceutical preparation [12,13,14]. [68Ga]Ga-DATA-TOC has exhibited high stability and excellent specific targeting in preclinical studies [15]. We could successfully reproduce those data from the literature in our clinical routine setting. Accordingly, radiolabelling yields are certainly higher than 95% even at room temperature, which avoids a heating step and a purification step. Due to the almost quantitative yields at labelling and the time saved when skipping a purification step, overall batch yields are about 25% higher for the DATA analogue compared with the DOTA analogue for identical starting activities.
We obtained excellent image quality with good target to background ratio with [68Ga]Ga-DATA-TOC, which was comparable to that obtained with the present standard [68Ga]Ga-DOTA-NOC (Fig. 2). Visual evaluation of [68Ga]Ga-DATA-TOC revealed a biodistribution pattern similar to [68Ga]Ga-DOTA-NOC [16], confirming the results of first human study in a single patient [12]. Our study is a head-to-head comparison of both tracers in the same subset of patients. We found lower values of median SUVmax of [68Ga]Ga-DATA-TOC in the pituitary, muscles and marrow, and higher in mediastinum, kidneys and spleen in comparison with [68Ga]Ga-DOTA-NOC, but this was not significant (Table 1). Both radiotracers were predominantly excreted by the kidneys, and a slightly higher physiological uptake in the kidneys was observed on [68Ga]Ga-DATA-TOC scan as compared with [68Ga]Ga-DOTA-NOC at 1-h post-injection (Table 2). In a preclinical biodistribution study by Nock et al., [67Ga]Ga-DATA-TOC showed a high uptake of 15.45 ± 2.71% ID/g at 1-h post-injection, declining to 6.35 ± 1.39% ID/g at 4-h post-injection in the HEK293-hsst2 xenografts. The corresponding values for [67Ga]Ga-DOTA-TOC were 12.47 ± 2.76 and 9.73 ± 1.82, respectively. The higher uptake at the initial 1 h and the lower uptake at 4-h post-injection of [67Ga]Ga-DATA-TOC clearly indicates the faster clearance from most of the physiological tissues than [67Ga]Ga-DOTA-NOC [15]. Our 1-h post-injection data were in agreement with the results of Nock et al. However, the delayed acquisition to revalidate the results of Nock et al. was out of scope of the current manuscript.
[68Ga]Ga-DATA-TOC demonstrated lower background uptake in the liver in comparison with [68Ga]Ga-DOTA-TOC (p = 0.009), while a higher background activity of [68Ga]Ga-DATA-TOC was observed in the mediastinal blood pool (p = 0.726). Interestingly, the T/L ratio of [68Ga]Ga-DATA-TOC was slightly higher compared with that of [68Ga]Ga-DOTA-NOC. On the other hand, the T/M blood pool ratios of [68Ga]Ga-DOTA-NOC were significantly superior to that of [68Ga]Ga-DATA-TOC. The higher background mediastinal blood pool activity of [68Ga]Ga-DATA-TOC, may possibly explain the lower liver background uptake. In line with our results, Schmidt-Kreppel et al. have demonstrated that normal liver uptake was significantly lower (3.3 vs 4.8) for [68Ga]Ga-DATA-TOC than [68Ga]Ga-DOTA-TOC [17]. Johnbeck et al., in their review, suggested that the amount of physiological uptake of a tracer in the liver might affect the performances of the different somatostatin receptor PET tracers, as the extent of liver metastasis is often a determinant for the choice of treatment [18]. Moreover, treatment options such as chemoembolization, surgical liver resection, radionuclide treatment or liver transplantation are highly dependent on the amount and localization of liver metastases. Hence, one could argue that the less prominent physiological uptake of [68Ga]Ga-DATA-TOC in the liver might help to better delineate and diagnose liver metastases. Interestingly, we did not observe the same in one of our patients with a diagnosis of well-differentiated NET of ileum, two liver lesions were positive on [68Ga]Ga-DOTA-NOC imaging, however, were negative on [68Ga]Ga-DATA-TOC. The fact that NOC binds to SSTR 2, 3 and 5, and TOC binds to only SSTR 2 and 5 could be the reason for the missing liver lesions [19, 20]. However, the binding affinities (mainly hSST2 affinity) of [natGa]Ga-DATA-TOC and [natGa]Ga-DOTA-TOC were found similar with only sub-nanomolar differences in the respective IC50 values, as demonstrated by Sinnes et al. [12]. [68Ga]Ga-DATA-TOC showed similar SUVs in tumour lesions as [68Ga]Ga-DOTA-TOC in preclinical studies [12]. Both DATA analogue and DOTA analogue were shown to be sst2-preferring and specifically internalised in HEK293-hsst2 cells, with [67Ga]Ga-DOTA-TOC internalising faster in both cell lines. Whereas in mice-bearing HEK293-hsst2 tumours, [67Ga]Ga-DOTA-TOC exhibited higher tumour values, and [67Ga]Ga-DATA-TOC cleared faster from background tissues [15]. Direct comparison of the two tracers, [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-TOC, in a 46-year-old male patient, NET patient revealed very similar tumour uptake for the two 68Ga-radiotracers, but with a higher tumour-to-liver contrast for [68Ga]Ga-DATA-TOC [12].
[68Ga]Ga-DATA-TOC has comparable diagnostic efficacy as compared with [68Ga]Ga-DOTA-NOC. No discordance was noted between [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC on the patient-based comparison (McNemar, p = 1.00). Out of the 6 patients who were negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC scans (n = 6), 4 patients had elevated serum chromogranin A levels and were referred for primary diagnosis. The other two patients underwent primary tumour resection and were referred for restaging with elevated chromogranin A. Two patients, one with insulinoma and one with hepatic metastases, with an unknown primary were negative on both [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC imaging. Insulinomas are known to have low SR expression (false negative) and more specific tracer-like [68Ga]Ga-exendin are recommended in these patients [19, 20]. The possible explanation for no accumulation of the two tracers in the liver metastases could be a higher grade NET (grade II, Ki67 = 17%) which led to no significant tracer uptake.
Target tumour lesion SUVmax values of [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC were comparable (Table 2). Target lesion to liver (T/L) SUVmax ratios for [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC did not differ significantly (Table 2). Target to mediastinal blood pool (T/M) SUVmax ratios for [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC showed a statistically significant difference with lower values for [68Ga]Ga-DATA-TOC (Table 2). Schmidt-Kreppel et al. also showed a significantly lower lesion-to-background ratio of [68Ga]Ga-DATA-TOC in lymph node metastases (4.9 vs 9.7) and hepatic metastases (2.6 vs 4.8) in comparison with [68Ga]Ga-DOTA-NOC [17]. The ratio between tumour and the normal liver tissue is a well-established index for diagnosing liver lesion in the literature. In one of the few direct comparison studies, when using [68Ga]Ga-DOTA-NOC, Wild et al. detected significantly more liver lesions than when using [68Ga]Ga-DOTATATE, and the tumour-to-background ratios were calculated to be 2.7 and 2.0, respectively [21]. But in our study, T/M was more accurate in diagnosing lymph nodal and liver metastases as well as primary tumour than T/L. This can be due to the inclusion of patients with extensive liver metastasis and due to lower physiological uptake in the liver. Hence, we can use mediastinal blood pool as background for such cases. Our study suggested T/M to be more accurate for characterising metastasis when there is extensive liver involvement.
On the basis of the number of lesions detected, [68Ga]Ga-DATA-TOC detected an almost identical number of lesions compared with [68Ga]Ga-DOTA-NOC (232 vs 235, p = 0.182). Overall discordance in detection of 3 lesions was observed in 2 patients, but this did not have a clinical impact on patient management as metastases to multiple sites were present. Our study has a limitation due to the use of TOC and NOC in direct comparison study. There are differences in affinity for SSTR in vitro among the most used tracers [68Ga]Ga-DOTA-TOC, [68Ga]Ga-DOTA-TATE and [68Ga]Ga-DOTA-NOC. However, the literature showed that there is no difference in diagnostic efficacy in TOC, NOC and TATE, and hence, they can be used in place of each other as per availability and experience [21,22,23,24,25,26,27,28,29,30]. However, the aim of the study was to perform a one-on-one comparison of the biological behaviour of two different tracers directly in each patient. To our knowledge, this is the first report which compares the diagnostic efficacy of [68Ga]Ga-DATA-TOC and [68Ga]Ga-DOTA-NOC in the same patient group.
No adverse reactions related to the intravenous [68Ga]Ga-DATA-TOC injection were observed in our patient population.
Lastly, regarding the cost effectiveness, the preparation of [68Ga]Ga-DATA-TOC is simple, fast and reveals high yields in a broad pH range. Due to the fact of the superior labelling efficiency of the DATA5m chelator, conjugated compounds can be simply prepared by a one vial kit approach similar like Tc-99 m. By this approach, users are able to renounce the expensive acquisition and maintenance of automated labelling modules including costly single use labelling cassettes. DATA5m compounds, like DATA-TOC open up the Ga-68 labelling chemistry towards the well-established Tc-99 m preparation technique. Saving process time, process costs and reducing radioactive waste in the clinic routine.
Conclusion
DATA is new bifunctional hybrid-type chelator with the advantage of radiolabelling at room temperature in lesser time with high radiochemical yield in an instant kit-type fashion. Batch activities are also superior to those of [68Ga]Ga-DOTA-NOC due to faster and quantitative initial labelling with the avoidance of a purification step. We demonstrated that the elegant labelling profile of [68Ga]Ga-DATA-TOC is accompanied by its effective pharmacological profile, maintaining the imaging profile of [68Ga]Ga-DOTA-NOC. In terms of diagnostic accuracy, the results of [68Ga]Ga-DATA-TOC PET/CT imaging were comparable with [68Ga]Ga-DOTA-NOC imaging, which is the current standard for imaging. Therefore, [68Ga]Ga-DATA-TOC seems promising as a PET tracer with the potential for cost-effective instant kit-type labelling, which allows a wide applicability.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
National Cancer Institute. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/
Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours of the Digestive System (4th Edition). International Agency for Research on Cancer (IARC); 2010; France, 13–14.
Kumar R, Sharma P, Garg P, Karunanithi S, Naswa N, Sharma R, et al. Role of (68)Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur Radiol. 2011;21:2408–14.
Jain TK, Karunanithi S, Dhull VS, Roy SG, Kumar R. Carcinoma of unknown primary of neuroendocrine origin: accurate detection of primary with (68)Ga-labelled [1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid]-1-NaI3-octreotide positron emission tomography/computed tomography enterography. Indian J Nucl Med. 2014;29(2):122–3.
Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabelled somatostatin analogues. Neuroendocrinology. 2017;105:295–309.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-DOTATE for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35.
Spang P, Herrmann C, Roesch F. Bifunctional gallium-68 chelators: past, present, and future. Semin Nucl Med. 2016;46(5):373–94.
Waldron BP, Parker D, Burchardt C, Yufit DS, Zimny M, Roesch F, et al. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem Commun (Camb). 2013;49(6):579–81.
Seemann J, Eppard E, Waldron BP, Ross TL, Roesch F. Cation exchange-based post-processing of 68Ga-eluate: a comparison of three solvent systems for labelling of DOTATOC, NO2AP(BP) and DATA(m). Appl Radiat Isot. 2015;98:54–9.
Farkas E, Nagel J, Waldron BP, Parker D, Tóth I, Brücher E, et al. Equilibrium, kinetic and structural properties of gallium(III) and some divalent metal complexes formed with the new DATAm and DATA5m ligands. Chem Eur J. 2017;23(43):10358–71.
Sinnes J-P, Nagel J, Waldron B, Maina T, Nock BA, Bergmann RK, et al. Instant kit-preparation of 68Ga-radiopharmaceuticals via the chimeric chelator DATA: proof-of-principle with [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019;9(1):48.
Seemann J, Waldron BP, Roesch F, Parker D. Approaching ‘kit-type’ labelling with 68Ga: the DATA chelators. ChemMedChem. 2015;10:1019–26.
Seemann J, Waldron BP, Parker D, Roesch F. DATATOC: a novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature. EJNMMI Radiopharmacy Chem. 2016;1:4.
Nock AB, Kaloudi A, Nagel J, Sinnes JP, Roesch F, Theodosia MT. Novel bifunctional DATA chelator for quick access to site-directed PET 68Ga-radiotracers: preclinical proof-of-principle with [Tyr3] octreotide. Dalton Trans. 2017;46:14584–90.
Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54:61–7.
Schmidt-Kreppel B, Plum T, Gaertner FC, Maina T, Nock BA, Bergmann RK, et al. Biodistribution of [68Ga]Ga-DATA-TOC in comparison with [68Ga]Ga-DOTATOC in normal tissues and neuroendocrine tumour lesions. Eur J Nucl Med Mol Imaging. 2017;44(2):119–956.
Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–77.
Bodei L, Ambrosini V, Herrmann K, Modlin I, et al. Current concepts in 68GA-DOTATATE NEN imaging: interpretation, biodistribution, dosimetry and molecular strategies. J Nucl Med. 2017;58(11):1718–26.
Ambrosini V, Campana D, Tomassetti P, Grassetto G, Rubello D, Fanti S. PET/CT with 68Gallium-DOTA-peptides in NET: an overview. Eur J Radiol. 2011;80(2):116–9.
Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, et al. Comparison of [68Ga]Ga-DOTA-NOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54(3):364–72.
Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42(1):80–7.
Kabasakal L, Demirci E, Ocak M, Decristoforo C, Araman A, Ozsoy Y, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:1271–7.
Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52:1864–70.
Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2010;55:389–98.
Breeman WA, de Blois E, Sze Chan H, Konijnenberg M, Kwekkeboom DJ, Krenning EP. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin Nucl Med. 2011;41:314–21.
Frilling A, Sotiropoulos GC, Radtke A, Malago M, Bockisch A, Kuehl H, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850–6.
Ambrosini V, Campana D, Bodei L, Nanni C, Castellucci P, Allegri V, et al. [68Ga]Ga-DOTA-NOCPET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med. 2010;51:669–73.
Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–8.
Waldron B, Roesch F, Parker D. Novel chelators for 68-Gallium PET. J Nucl Med. 2013;54(2):497–507.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical clearance
Received from Institute Ethics Committee (Ref. No. IECPG-101/30.12.2015).
Informed consent
Informed consent was obtained from all patients.
Disclaimer
This work has not been submitted elsewhere or has not under consideration to any other journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Digestive tract
Rights and permissions
About this article
Cite this article
Yadav, D., Ballal, S., Yadav, M.P. et al. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: comparison with [68Ga]Ga-DOTA-NOC PET/CT. Eur J Nucl Med Mol Imaging 47, 860–869 (2020). https://doi.org/10.1007/s00259-019-04611-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04611-1