Abstract
Purpose
We aimed to study brain metabolism and presence of beta-amyloid deposits using positron emission tomography (PET) in patients with amyotrophic lateral sclerosis (ALS).
Methods
This prospective cross-sectional study included 18 patients with definite or probable ALS according to the revised El Escorial diagnostic criteria, and 24 healthy controls. Patients underwent neurological and neuropsychological assessments, PET with 18F-fluorodeoxyglucose (FDG), and amyloid-PET with 18F-florbetaben.
Results
Patients with ALS showed hypometabolism in the frontal area and hypermetabolism in the cerebellum compared to healthy controls. Four patients (22 %) displayed cognitive impairment and decreased metabolism in the frontal area extending bilaterally to the parietal regions, and increased metabolism in the posterior area of the cerebellum. In patients with no cognitive impairment, metabolism was lower in the left superior frontal gyrus and higher in the anterior and posterior lobes of the cerebellum. In the individual analysis, six patients (35 %) displayed more anterior involvement with hypometabolism affecting the superior frontal, medial, and inferior gyri; six patients (35 %) exhibited a more posterior pattern with hypometabolism in the precentral and postcentral gyri and in the superior and inferior parietal lobules; two patients (11 %) showed a mixed pattern; and three patients (17 %) showed no alterations in brain metabolism. Three (16 %) showed increased 18F-florbetaben uptake compared to controls.
Conclusions
We have identified two main patterns of brain metabolism with an association to cognitive status. Only a subgroup of patients showed an increased uptake of the amyloid tracer. Our results suggest that ALS is heterogeneous from a clinical, metabolic, and molecular standpoint.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a disease with highly heterogeneous clinical and pathological traits. Post-mortem studies have shown that ALS affects a number of areas and structures in the brain, causing not only motor neuron degeneration, but also a wide range of alterations in extra-motor areas such as the dorsolateral prefrontal cortex, anterior cingulate, hippocampus, dentate gyrus, parietal lobe, substantia nigra, cerebellum, amygdala, and basal ganglia [1–5].
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is used to study brain metabolism as an indicator of synaptic dysfunction and neurodegeneration in neurodegenerative disorders. Frontal hypometabolism is the most frequent finding in 18F-FDG PET imaging in patients with ALS [6]. However, there is no consensus in the literature about the involvement of other areas or structures, such as the dorsolateral prefrontal cortex, the lateral and medial premotor cortex, the insula, the occipital cortex, the anterior temporal lobe, and the parietal lobe. It has been suggested that the different regions involved may be related to subtypes of the disorder (clinical, genetic, etc.) [6–11].
In recent years, cognitive alterations have also been reported among some patients. In fact, ALS has been associated with certain forms of frontotemporal dementia (FTD) [12–14], and some have suggested a link between ALS and Alzheimer’s disease (AD) [15]. In rodent models, a connection between amyloid precursor protein and ALS was observed, and an amyloid-like cascade has been suggested to explain certain aspects of the pathophysiology of ALS [16, 17]. Recent histological studies report amyloid plaques in 35–50 % of patients with ALS, even in patients without dementia or aphasia [4, 15]. Since these post-mortem studies are conducted in terminal stages of the disease, it may be difficult to interpret the pathophysiological role of beta-amyloid deposits in ALS.
Amyloid tracers enable in vivo studies of amyloid deposits; the validity of these studies is based on the correlation between PET imaging findings and histological results [18, 19]. To our knowledge, there are no published amyloid-PET studies in patients with ALS, and only three case reports of patients with ALS and dementia who were evaluated using amyloid-PET (PiB-PET in all cases) have been published to date [20–22].
Our purpose was to study brain metabolism and beta-amyloid deposition using 18F-FDG PET and 18F-florbetaben in a cohort of patients with ALS.
Material and methods
Study design and population
We conducted a prospective cross-sectional descriptive study in a cohort of 18 patients diagnosed with ALS (definite, probable, or probable laboratory-supported) according to the revised El Escorial diagnostic criteria [23]. Patients were recruited from the ALS unit of a university hospital. From 67 ALS patients on active follow-up in our unit, 24 were excluded due to advanced clinical status or criteria of “possible ALS”, and 25 cases rejected to participate.
The sample included 18 patients: 12 women and six men. Mean age at the time of inclusion in the study was 57.58 ± 11.16 years. Mean age at symptom onset was 55.44 ± 12.20 years, and mean progression time from symptom onset to inclusion in the study was 29.39 ± 27.94 months. Onset was spinal in 13 patients and bulbar in the remaining five. At the time of the study, 15 patients displayed motor weakness in the limbs, nine had bulbar symptoms, five required non-invasive ventilation, and one required gastrostomy feeding. The mean ALSFRS-R score was 35.06 ± 7.58 (Table 1). All patients were in treatment with riluzole and underwent a neurological examination, a neuropsychological assessment, and an amyloid-PET study. Medical histories were completed based on the information reported by both patients and their companions.
The control group included 24 healthy individuals. Controls had no neurological disorders or systemic diseases with the potential to cause neurological impairment. Mean age in the 24 controls was 60.50 ± 8.15 years; all controls completed cognitive tests and 18F-FDG PET studies, which yielded apparently normal results. Seven controls (mean age 59.14 ± 8.57 years) also underwent amyloid-PET studies. There were no age- or sex-related differences between controls and patients.
Subjects were recruited between January and July 2015. The study was approved by the Clinical Research Ethics Committee at our hospital. All participants signed informed consent forms.
Clinical and cognitive assessment
Medical histories were recorded for of all subjects. Additionally, all participants underwent a neurological examination and neuropsychological assessment, including the Mini-Mental State Examination (MMSE) [24], the Spanish-language version of Addenbrooke’s Cognitive Examination III (ACE-III) [25], memory span (forward and backward digit spans) [26], visuospatial span (Corsi block-tapping test) [26], Trail Making Test [27, 28], Rey-Osterrieth Complex Figure Test [29, 30], Free and Cued Selective Reminding Test [31, 32], Visual Object and Space Perception Battery (object decision, progressive silhouettes, position discrimination, and number location subtests) [33], Stroop Color-Word Interference Test [34, 35], letter verbal fluency (words beginning with ‘p’ in 1 min), category fluency test (names of animals in 1 min) [36, 37], and Tower of London (Drexel Edition) [38]. We used normative data from NEURONORMA, a normative data project conducted in our setting and similar to MOANS in the United States [39]. Participants also completed action fluency tests [40, 41], picture-sentence matching tasks, semantic association tests, the Hayling Test [42], Edinburgh Cognitive and Behavioural ALS Screen (ECAS) [43], Cambridge Behavioural Inventory revised (CBI-R) [44], and Anosognosia Questionnaire for Dementia [45]. We followed the DSM-IV diagnostic criteria for dementia [46]. The revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) was used to assess level of disability [47]. Five patients failed to complete all neuropsychological tests due to physical inability (severe tetraparesis/quadriplegia and/or severe dysarthria/anarthria).
In ALS patients, mean MMSE score was 26.94 ± 3.74 and mean ACE-III score was 83.44 ± 15.38. Fourteen patients with ALS displayed normal cognitive function for their age and education level, while four presented cognitive impairment. Of these four, two did not meet the criteria for dementia (one with executive dysfunction, one with executive dysfunction associated with hippocampal memory impairment), whereas the other two did (global deficit in all evaluated cognitive areas) (Table 1).
Positron emission tomography
Image acquisition
The clinical-cognitive assessment and imaging studies were conducted with an interval of less than 2 months between them. PET images were acquired using a PET-computed tomography (CT) Siemens Biograph True Point platform including a 6-slice detector with a latest-generation PET scanner featuring a lutetium oxyorthosilicate crystal array. CT parameters for both 18F-FDG and 18F-Florbetaben PET-CT studies were: 130 kVp, 40 mAs/rotation, and one rotation. Slice thickness was 3 mm, the reconstruction interval was 1.5 mm, and pitch was 0.75. Iterative 3D image reconstruction was performed with two iterations and 21 subsets. We used a 30 cm field of view and a Gaussian filter with full width at half maximum of 4 mm. FDG-PET and amyloid-PET studies were conducted on different days, but with an interval of less than 2 months between them.
FDG-PET studies were performed in 17 patients. Patients fasted a minimum of 6 h before undergoing the FDG-PET scan. 18F-FDG (mean dose of 185 MBq) was administered intravenously 30 min before image acquisition. During this time, patients rested in a dark room with their eyes closed. Static PET images were acquired for 15 min, at a single bed position.
We used 18F-florbetaben for the amyloid-PET study. Participants were given this radiotracer (mean dose of 300 MBq) as a slow intravenous injection (6 s/mL), followed by a 10 mL saline flush (0.9 % sodium chloride, 9 mg/mL). Static PET images at a single bed position were acquired for a period of 20 min starting at 90 min from the 18F-florbetaben injection.
Processing and statistical analysis of 18F-FDG PET images
Images were preprocessed using Statistical Parametric Mapping software version 8 (SPM8) (The Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College of London). Images were transformed from DICOM to NifTI-1 format using MRIConvert version 2.0 (Lewis Center for Neuroimaging, University of Oregon). Images were normalised to Montreal Neurological Institute (MNI) space using an FDG-PET template [48], and subsequently smoothed with a 12-mm full width at half maximum Gaussian kernel. The study groups were compared using the t-test for two independent samples. Analyses were conducted by group (for example, ALS group vs control group) and individually (each patient vs the control group, i.e. single-subject analysis) using proportional scaling [49]. Age and sex were added to the model as covariates. Uncorrected p values < 0.001 were considered statistically significant and an extent threshold > 50 voxels was used. We used an uncorrected p value < 0.005 and an extent threshold > 50 voxels for the individual analysis. MNI coordinates were converted to Talairach coordinates using the conversion applet included in Bioimage Suite 2.0 [50]. Additional data about voxel-based brain mapping analysis results are available in Online Resource 1.
Processing and statistical analysis of 18F-florbetaben-PET images
Images were preprocessed using SPM8. Indirect spatial normalisation to MNI was performed with a template created at our centre based on magnetic resonance (MR) images from a random sample of patients. We used controls, patients with ALS, and patients with AD to create the template. A region of interest (ROI) analysis of the Automated Anatomical Labeling Atlas (AAL) was conducted using MarsBaR software [51]. We used grey matter ROIs exclusively to avoid the effect of normal uptake in healthy white matter. The whole cerebellum and the pons served as regions of reference, and we calculated the standard uptake value relative (SUVR) from each brain region and each of the regions of reference (SUVRc, cerebellum; SUVRp, pons). The mean and standard deviation (SD) for each ROI were calculated based on the uptake displayed by the control group. We then estimated z scores for each region in each of the patients with ALS (single-subject analysis). Uptake in each ROI was assigned a z score, and presence of amyloid deposits was defined as a z score > 2 in at least 2 ROIs using both the cerebellum and the pons as regions of reference. Additionally, a composite SUVRc for AD patients (AD-SUVRc) was calculated based on the following regions: medial orbitofrontal area, anterior and posterior cingulate, superior parietal lobule, precuneus, and inferior temporal area. These regions are considered to have the greatest discriminant ability for diagnosing AD [52].
Statistical analysis
Data were analysed using IBM® SPSS statistical software, version 20 [53]. The descriptive analysis was performed using either absolute frequencies and percentages [n (%)] or means ± SD. Given the sample size, non-parametric tests were used for comparison of clinical and demographic characteristics between groups. Qualitative variables were compared using the Fisher exact test. To compare means between two groups we used the Mann–Whitney U test. p values ≤0.05 were considered statistically significant and led to rejection of the null hypothesis.
Results
FDG-PET
Analysis by group
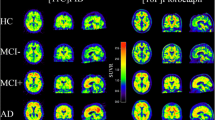
Compared to controls, patients with ALS (n = 17) displayed bilateral frontal hypometabolism in the superior, middle, and medial frontal gyri bilaterally; bilateral anterior cingulate, left precentral gyrus; right posterior cingulate; and left inferior parietal lobule. The ALS group also showed hypermetabolic areas in the cerebellum bilaterally (Fig. 1). Frontal hypometabolism in the superior, middle, and medial frontal gyri, anterior cingulate, thalamus (bilateral), left precentral gyrus, and left inferior parietal lobule was observed in ALS patients with cognitive impairment (n = 4). Metabolism was increased in the cerebellum (posterior lobe) and fusiform and middle occipital gyri (Fig. 2) in these patients. On the other hand, ALS patients with no cognitive impairment (n = 13) displayed hypometabolism in the left superior frontal gyrus and hypermetabolism in the anterior and posterior lobes of the cerebellum (Fig. 3).
Individual analysis
Each ALS patient was compared to the control group. Figure 4 shows the regions with lower metabolism in each case. Two different patterns were identified: predominantly anterior hypometabolism (n = 6) and predominantly posterior hypometabolism (n = 6). Two patients displayed a mixed pattern and three showed no alterations in brain metabolism. The patients with predominantly anterior hypometabolism showed metabolic alterations mainly in the superior, middle, and inferior frontal gyri, while patients with the predominantly posterior pattern displayed hypometabolism mostly in the precentral and postcentral gyri, precuneus, superior and inferior parietal lobules, and cingulate gyrus.
Table 2 shows the clinical and demographic characteristics of the groups with predominantly anterior and predominantly posterior hypometabolism in 18F-FDG PET images. We observed a trend to a higher prevalence of cognitive deficit in the group with the predominantly anterior pattern (50 vs. 0 %; p = 0.09).
Amyloid-PET
Analysis by group
We compared the SUVR for different brain regions between ALS group and controls using the Mann–Whitney U test and found no statistically significant differences. Likewise, composite AD-SUVRc was 1.29 ± 0.07 in the ALS group and 1.31 ± 0.08 in the control group (p = 0.856).
Individual analysis
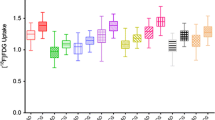
Following the previously established criterion for determining the presence of amyloid deposits from a single-subject analysis, we found three patients showing more pronounced tracer uptake than the control group’s in several regions: middle and superior frontal gyri, lingual gyrus, precentral gyrus, supplementary motor area, inferior and middle temporal gyri, parahippocampal gyrus, straight gyrus, hippocampus, fusiform gyrus, and cuneus. Table 3 compares the clinical and demographic characteristics of the group displaying increased 18F-florbetaben uptake and the group showing normal uptake.
The patients with increased 18F-florbetaben uptake showed reduced metabolism in the right middle frontal gyrus compared to controls. Patients with normal uptake displayed reduced metabolism in the superior and middle frontal gyri, precentral and postcentral gyri, and bilaterally in the anterior cingulate. There were no ALS patients in a single-subject analysis with a composite AD-SUVRc score 1.5 standard deviations above the mean values for the control group.
Discussion
This study analysed the 18F-FDG and amyloid-PET findings in a cohort of patients with ALS. We identified two main patterns of brain metabolism, which might be associated to some differences in clinical profiles, as has also been recently reported [11]. Furthermore, we detected an increased uptake of amyloid-tracer 18F-florbetaben in a subgroup of patients. Evaluating brain metabolism as a marker of neurodegeneration and synaptic dysfunction in vivo, as well as examining deposition of the proteins, such as amyloid, involved in the pathophysiology of neurodegenerative diseases, significantly contributes to our knowledge of those diseases.
Previous studies with 18F-FDG PET have demonstrated that ALS patients show lower metabolism than controls in prefrontal regions and several other areas. In this regard, different studies have pointed to the temporal, parietal, and occipital lobes, insula, putamen, globus pallidus, and thalamus [8–10]. Our group analysis confirmed frontal hypometabolism as the main finding in patients with ALS. However, the individual analysis showed relatively heterogeneous brain metabolism patterns with several different affected areas in the frontal, parietal, and temporal lobes. We attempted to distinguish between two patterns according to whether hypometabolism was predominantly anterior (frontal lobe) or posterior (precentral and poscentral gyri and extending to the parietal lobes). No differences were found in progression time or clinical severity between these patterns (as shown in Table 2), which suggests that neither of them evolves to become the other. Nevertheless, the cases with a predominantly anterior pattern displayed cognitive impairment more frequently (50 %) than the group with a predominantly posterior pattern (0 %). Likewise, extensive frontal hypometabolism was observed in the two patients with the most pronounced cognitive impairment and meeting criteria for dementia. This suggests that frontal hypometabolism and its extension to other areas is linked to the degree of cognitive impairment in patients with ALS [11]. 18F-FDG PET findings may therefore constitute a marker of cognitive impairment in cases showing hypometabolism in anterior regions, especially if it is extensive, although further studies with a larger sample size are necessary to confirm this issue. This is relevant, considering that cognitive assessment is of limited utility in advanced stages of the disease, although other factors such as cognitive reserve should also be taken into account [54]. The presence of different metabolic patterns may be explained by the clinical, genetic, and prognostic heterogeneity of ALS [55]. In this regard, several studies have revealed different 18F-FDG PET patterns in patients with ALS and FTD depending on causal mutations and clinical progression [56–58].
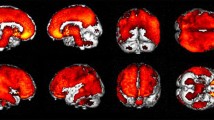
Another interesting finding from our study was brain hypermetabolism in patients with ALS, described in previous studies as a phenomenon mainly affecting the cerebellum and mesencephalon [10, 11]. Some researchers suggest that increased metabolism may be secondary to astrocytosis [10, 11, 59]. Our sample also displayed increased metabolism in the cerebellum, along with interesting topographic differences: hypermetabolism in the posterior lobe of the cerebellum in patients with cognitive impairment; and hypermetabolism in the anterior and posterior lobes (although more marked in the anterior lobe) in those with no cognitive impairment. Given that the anterior lobe of the cerebellum is mainly involved in motor function and the posterior lobe in cognitive function [60–63], our findings may support the assumption that hypermetabolism may be caused by a compensatory mechanism secondary to neuronal degeneration in functionally related regions. In Fig. 5, the regions with a lower and higher metabolism in our ALS patients are shown together with the main connections between the cerebellum and the neocortex. This supports the idea of a functional compensation as the explanation of the hypermetabolism observed in ALS.
Main cerebellar areas involved in cognitive function. Feedforward tracts (pons), in red, and feedback tracts (thalamus), in green, which connect the cerebellum and the cerebral cortex. Hypometabolic (yellow) and hypermetabolic (blue) areas in the cerebellum. dPMC dorsal premotor cortex, MD mediodorsal thalamic nuclei, aPFC anterior prefrontal cortex, SPL superior parietal lobule, RPN rostral pontine nuclei
Amyloid-PET imaging found that three patients (16.66 %) displayed greater 18F-florbetaben uptake than controls in multiple regions, which suggests presence of amyloid deposits. To our knowledge, no prior studies have addressed using amyloid-PET to evaluate ALS, except for individual case reports [20–22] describing two patients with ALS/dementia associated with amyloid deposits. One patient exhibited amyloid deposition in the frontal lobe, parietal lobe, both lateral temporal lobes, posterior cingulate gyrus, and precuneus, and the other patient in the prefrontal, posterior cingulate, and parietal cortices. Both displayed hypometabolism in 18F-FDG PET, one of them in both parietal lobes and the left temporolateral cortex, and the other in the left temporal and prefrontal cortices. These previously reported cases suggest that AD may be a comorbidity in patients with ALS, rather than implying that beta-amyloid could contribute to ALS pathophysiology.
The hypothesis that the amyloid protein plays a role in ALS is based on the high rate of amyloid deposits detected in these patients by post-mortem studies (35–50 % according to the main studies) [4, 15]. This percentage is higher than the prevalence rate of cerebral β-amyloidosis reported by studies in healthy volunteers [64]. Known pathogenic mechanisms of beta-amyloid include accumulation of aggregates, inflammatory cascade, neuronal oxidative damage, tau protein alterations, neurofibrillary tangle formation, synaptic defects, and neurotransmitter depletion [65]. Many of these events are common to a number of neurodegenerative diseases, such as ALS. Amyloid accumulation may not only be the cause, but also the consequence of some of those diseases. This study included patients in a non-end-stage of the disease, thus providing information about the amyloid deposition in early phases of the disease.
In our study, the percentage of increase in amyloid deposits is lower than those reported by series of histological studies, suggesting that amyloid accumulates at a late stage of the disease. In addition, amyloid deposits in ALS and AD are located in different areas, and there are no metabolic alterations in the areas linked to AD; these findings seem to indicate that these ALS cases are not preclinical AD. This observation, in turn, confirms that amyloid-PET is a useful tool for differential diagnosis of AD and other neurodegenerative diseases, especially when used to analyse the areas most affected by AD (posterior cingulate, precuneus, etc.) [52]. Likewise, the fact that a subgroup of patients displayed amyloid deposits at relatively early stages of the disease seems to indicate a connection between amyloid and ALS. Similarly, amyloid deposits are not very likely to be related to age, considering that 72.22 % of our patients were younger than 65, and that the patients displaying increased 18F-florbetaben uptake were 47, 54, and 68 years old. In population studies of healthy subjects evaluated with PiB-PET, the frequency of amyloid deposits in this age range is much lower and deposition is limited to the regions most linked to AD [64].
On the other hand, although amyloid tracer findings have been validated by several autopsy studies demonstrating the correlation between increased uptake and increased amyloid deposition, they may be less specific than expected. For example, amyloid tracers show non-specific uptake by normal white matter [66] and may bind to plaques or fibrils produced by other non-amyloid aggregates involved in neurodegenerative diseases [67].
Our study has a few limitations. Because its design called for thorough cognitive assessment and neuroimaging studies, we mainly included patients in relatively early clinical stages. Likewise, its cross-sectional design did not allow us to draw conclusions as to whether the metabolic patterns described or the subgroup of patients showing increased 18F-florbetaben uptake might be associated with different outcomes. Another limitation of our study concerns the analysis of PET images. We used two regions of reference for amyloid-PET images in order to avoid the effect caused by using only one region. It would be interesting to conduct longitudinal studies or even obtain histological confirmation in terminal stages in order to compare PET findings with the results from patient follow-up. We scaled 18F-FDG PET images to the global mean, since some regions (cerebellum, pons, etc., used in other studies on neurodegenerative diseases such as AD and FTD) may show metabolic alterations in patients with ALS because they participate in motor control. Given that most of our patients were in early stages, we feel that scaling to the mean should not have a significant impact on the main results [68].
In conclusion, our study reports findings from a cohort of patients assessed using 18F-FDG and 18F-florbetaben. Patients with ALS showed, in comparison to healthy controls, a lower metabolism, mainly in frontal lobe, anterior cingulate and parietal lobe, and higher metabolism in cerebellum. Using single-subject analysis, we have identified two main patterns of brain metabolism: one with predominantly anterior hypometabolism (especially in the superior, middle, and inferior frontal gyri), which is more often associated with cognitive impairment, and a second with more posterior hypometabolism (precentral and postcentral gyri, precuneus, parietal lobules). Only a low percentage of patients displayed abnormal tracer uptake in amyloid-PET images, and this finding did not seem to correlate with presence of AD. These results suggest that ALS presents heterogeneous clinical, metabolic, and molecular characteristics. While FDG-PET and amyloid-PET may help identify disease subgroups, further studies should analyze the connection between these findings and the prognosis and progression of patients with ALS.
References
Maekawa S, Al-Sarraj S, Kibble M, Landau S, Parnavelas J, Cotter D, et al. Cortical selective vulnerability in motor neuron disease: a morphometric study. Brain. 2004;127:1237–51.
Takeda T, Uchihara T, Arai N, Mizutani T, Iwata M. Progression of hippocampal degeneration in amyotrophic lateral sclerosis with or without memory impairment: distinction from Alzheimer disease. Acta Neuropathol. 2009;117:35–44.
Wilson CM, Grace GM, Munoz DG, He BP, Strong MJ. Cognitive impairment in sporadic ALS: a pathologic continuum underlying a multisystem disorder. Neurology. 2001;57:651–7.
Coan G, Mitchell CS. An assessment of possible neuropathology and clinical relationships in 46 sporadic amyotrophic lateral sclerosis patient autopsies. Neurodegener Dis. 2015;15:301–12.
Machts J, Loewe K, Kaufmann J, Jakubiczka S, Abdulla S, Petri S, et al. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology. 2015;85:1301–9.
Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand. 1992;85:81–9.
Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119:2105–20.
Jeong Y, Park KC, Cho SS, Kim EJ, Kang SJ, Kim SE, et al. Pattern of glucose hypometabolism in frontotemporal dementia with motor neuron disease. Neurology. 2005;64:734–6.
Renard D, Collombier L, Castelnovo G, Fourcade G, Kotzki PO, LaBauge P. Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurol Belg. 2011;111:306–9.
Pagani M, Chio A, Valentini MC, Oberg J, Nobili F, Calvo A, et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. 2014;83:1067–74.
Canosa A, Pagani M, Cistaro A, Montuschi A, Iazzolino B, Fania P, et al. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology. 2016;86:44–9.
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11.
Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114(1):63–70.
Neumann M, Kwong LK, Sampathu DM, Trojanowski JQ, Lee VM. TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: protein misfolding diseases without amyloidosis. Arch Neurol. 2007;64:1388–94.
Hamilton RL, Bowser R. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 2004;107:515–22.
Bryson JB, Hobbs C, Parsons MJ, Bosch KD, Pandraud A, Walsh FS, et al. Amyloid precursor protein (APP) contributes to pathology in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21:3871–82.
Edbauer D, Haass C. An amyloid-like cascade hypothesis for C9orf72 ALS/FTD. Curr Opin Neurobiol. 2016;36:99–106.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015;11:964–74.
Yamakawa Y, Shimada H, Ataka S, Tamura A, Masaki H, Naka H, et al. Two cases of dementias with motor neuron disease evaluated by Pittsburgh compound B-positron emission tomography. Neurol Sci. 2012;33:87–92.
Martikainen MH, Gardberg M, Jansson L, Roytta M, Rinne JO, Kaasinen V. Brain 18F-FDG and 11C-PiB PET findings in two siblings with FTD/ALS associated with the C9ORF72 repeat expansion. Neurocase. 2014;20:150–7.
Farid K, Carter SF, Rodriguez-Vieitez E, Almkvist O, Andersen P, Wall A, et al. Case report of complex amyotrophic lateral sclerosis with cognitive impairment and cortical amyloid deposition. J Alzheimers Dis. 2015;47:661–7.
Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Matias-Guiu JA, de Bobadilla FR, Escudero G, Perez-Perez J, Cortes A, Morenas-Rodriguez E, et al. Validation of the Spanish version of Addenbrooke’s Cognitive Examination III for diagnosing dementia. Neurologia. 2015;30:545–51.
Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a neuropsychological instrument. San Antonio: The Psychological Corporation; 1991.
Partington J, Leiter R. Partington’s pathways test. Psychol Serv Cent Bull. 1949;1:9–20.
Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Theory and clinical interpretation. 2nd ed. Tucson: Neuropsychology Press; 1993.
Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol. 1941;28:215–85.
Osterrieth PA. Le test de copie d’une figure complexe: contribution à l’etude de la perception et de la mémoire. Arch Psychol. 1944;30:206–356.
Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learn Verbal Behav. 1973;12:543–50.
Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–40.
Warrington EK, James M. Visual object and space perception battery. Suffolk: Thames Valley Test Company; 1991.
Stroop JR. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643–62.
Golden CJ. Stroop color and word test. Chicago: Stoelting; 1978.
Ramier AM, Hecaen H. Rôle respectif des atteintes frontales et de la latéralisation lésionnelle dans les déficits de la “fluence verbale”. Rev Neurol. 1970;123:17–22.
Crawford JR, Wright R, Bate A. Verbal, figural and ideational fluency in CHI. International Neuropsychological Society and Australian Society for the Study of Brain Impairment, 2nd Pacific Rim Conference. Cairns: Australia. 1995;1:321.
Culbertson WC, Zillmer EA. Tower of London: Drexel University (TOL DX). North Tonawanda: Multi-Health Systems; 2001.
Pena-Casanova J, Blesa R, Aguilar M, Gramunt-Fombuena N, Gomez-Anson B, Oliva R, et al. Spanish multicenter normative studies (NEURONORMA Project): methods and sample characteristics. Arch Clin Neuropsychol. 2009;24:307–19.
Burgess PW, Alderman N, Evans J, Emslie H, Wilson B. The ecological validity of tests of executive function. J Int Neuropsychol Soc. 1998;4:547–58.
Piatt AL, Fields JA, Paolo AM, Troster AI. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia. 1999;37:1499–503.
Burgess PW, Shallice T. The Hayling and Brixton Tests. Thames Valley Test Company: Thurston; 1997.
Niven E, Newton J, Foley J, Colville S, Swingler R, Chandran S, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): a cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:172–9.
Wear HJ, Wedderburn CJ, Mioshi E, Williams-Gray CH, Mason SL, Baker RA, et al. The Cambridge behavioural inventory revised. Dement Neuropsychol. 2008;2:102–7.
Migliorelli R, Teson A, Sabe L, Petracca G, Petracchi M, Leiguarda R, et al. Anosognosia in Alzheimer’s disease: a study of associated factors. J Neuropsychiatry Clin Neurosci. 1995;7:338–44.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Revised 4th ed.). Washington, DC; 2000.
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–93.
Matias-Guiu JA, Cabrera-Martin MN, Perez-Castejon MJ, Moreno-Ramos T, Rodriguez-Rey C, Garcia-Ramos R, et al. Visual and statistical analysis of 18F-FDG PET in primary progressive aphasia. Eur J Nucl Med Mol Imaging. 2015;42:916–27.
Lacadie CM, Fulbright RK, Todd Constable R, Papademetris X. More accurate Talairach coordinates for neuroimaging using nonlinear registration. Neuroimage. 2008;42:717–25.
Tzourio-Mazoyer N, Landeau D, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–11.
IBM Corp. Released 2010. IBM SPSS Statistics for Mac, Version 19.0. Armonk, NY: IBM Corp.
Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168–73.
Sabatelli M, Conte A, Zollino M. Clinical and genetic heterogeneity of amyotrophic lateral sclerosis. Clin Genet. 2013;83:408–16.
Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging. 2014;41:844–52.
Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806.
Matias-Guiu JA, Cabrera-Martin MN, Moreno-Ramos T, Garcia-Ramos R, Porta-Etessam J, Carreras JL, et al. Clinical course of primary progressive aphasia: clinical and FDG-PET patterns. J Neurol. 2015;262:570–7.
Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39:251–9.
Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, et al. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22:2663–76.
O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–65.
Riedel MC, Ray KL, Dick AS, Sutherland MT, Hernandez Z, Fox PM, et al. Meta-analytic connectivity and behavioral parcellation of the human cerebellum. Neuroimage. 2015;117:327–42.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–70.
Jack Jr CR, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005.
Skaper SD. Alzheimer’s disease and amyloid: culprit or coincidence? Int Rev Neurobiol. 2012;102:277–316.
Matias-Guiu JA, Cabrera-Martin MN, Matias-Guiu J, Oreja-Guevara C, Riola-Parada C, Moreno-Ramos T, et al. Amyloid PET imaging in multiple sclerosis: an (18)F-florbetaben study. BMC Neurol. 2015;15:243.
Kuang G, Murugan N, Tu Y, Nordberg A, Agren H. Investigation of the binding profiles of AZD2184 and thioflavin T with amyloid-β(1–42) fibril by molecular docking and molecular dynamics methods. J Phys Chem B. 2015;119:11560–7.
Dukart J, Perneczky R, Forster S, Barthel H, Diehl-Schmid J, Draganski B, et al. Reference cluster normalization improves detection of frontotemporal lobar degeneration by means of FDG-PET. PLoS ONE. 2013;8, e5541.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Funding
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Voxel-based brain mapping analysis results (DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Matías-Guiu, J.A., Pytel, V., Cabrera-Martín, M.N. et al. Amyloid- and FDG-PET imaging in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging 43, 2050–2060 (2016). https://doi.org/10.1007/s00259-016-3434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3434-1