Abstract
Purpose
This retrospective study investigated the usefulness of F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) after interventional therapy for hepatocellular carcinoma (HCC).
Methods
Between March 2007 and November 2010, 31 patients (24 men, 7 women; mean age, 61.8 ± 11.0 years) with 45 lesions underwent PET/CT within 1 month after interventional therapy for HCC. Twenty-six patients with 40 lesions underwent transcatheter arterial chemoembolization (TACE), two patients with 2 lesions underwent radiofrequency ablation (RFA), and three patients with 3 lesions underwent percutaneous ethanol injection therapy (PEIT). Patients with a history of previous interventional therapy were excluded. Visual analysis was graded as positive when FDG was observed as an eccentric, nodular, or infiltrative pattern, and negative in case of isometabolic, hypometabolic, or rim-shaped uptake. For quantitative analysis, the standardized uptake value (SUV) was measured by region of interest technique. Maximum SUV (SUVmax) was assessed, and the ratio of SUVmax of tumor to mean SUV of normal liver (TNR) was calculated. The patients were divided into two groups, with and without residual tumor, based on 6-month clinical follow-up with serum alpha-fetoprotein and contrast-enhanced abdominal CT.
Results
Of the 45 lesions, 24 were classified in the residual tumor group and the other 21 lesions in the no residual tumor group. No residual tumor was detected after RFA or PEIT. By visual analysis, the respective values for sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 87.5, 71.4, 77.8, 83.3, and 80.0 %. However, there were no significant differences in the SUVmax and TNR between the two groups.
Conclusions
It is suggested that FDG PET/CT may play a role in the evaluation of early treatment response after interventional therapy for HCC. The results indicate that FDG PET/CT visual analysis may be more useful than quantitative analysis. Further prospective studies with a large number of patients and established protocol are needed to substantiate our results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and ranks fifth globally in frequency [1, 2]. Hepatic resection and liver transplantation are the optimum treatments for patients with HCC. However, fewer than 20 % of patients are surgical candidates because of advanced or multifocal disease, poor functional hepatic reserve, or shortage of organ donors [3–5]. Therefore, interventional therapy has gained an increasing role in the treatment of HCC. This HCC therapy is based on radiofrequency ablation (RFA), percutaneous ethanol injection therapy (PEIT), and transcatheter arterial chemoembolization (TACE), with RFA and PEIT being effective in relatively small HCC and TACE being the main interventional therapy in large or advanced intrahepatic HCC [2, 6, 7].
Assessment of tumor response after interventional therapy is important to determine whether the tumor is completely treated or needs additional treatment. Conventional imaging modalities, such as computed tomography (CT) or magnetic resonance imaging (MRI), have been widely used for the evaluation of treatment response after interventional therapy for HCC. The determination of treatment response using size criteria, based on the Response Evaluation Criteria in Solid Tumors (RECIST), does not necessarily apply well to interventional therapy in HCC. Hence, most investigators have relied on the presence or absence of contrast enhancement for the evaluation of therapeutic response as well as changes in tumor size [4, 8, 9]. More recently, modified RECIST has been proposed. However, further studies are needed to confirm the accuracy of this method [10].
Positron emission tomography (PET) with F-18 fluorodeoxyglucose (FDG) is a functional imaging modality that reflects glucose metabolism of the region of interest. It is widely used for staging and restaging of a malignancy, monitoring therapeutic response, and detection of recurrent tumors of various cancers. Integrated PET/CT provides superior performance in the evaluation of the malignancy over PET or CT alone. However, FDG PET/CT is less suitable for the detection of primary HCC because of variable FDG uptake. The relatively low sensitivity (50–55 %) is attributable to the degree of glucose-6-phosphatase in HCCs [11–15]. Several studies have reported variable FDG accumulation in the diagnosis of primary HCC [11, 12, 14–17]. However, there are few reports on the efficacy of FDG PET/CT in follow-up of early therapeutic response after interventional therapy of HCC [2, 12, 18]. In addition, no reports have focused on the FDG uptake pattern.
The aim of the present study was to investigate retrospectively the usefulness of FDG PET/CT in the evaluation of early treatment response after interventional therapy for HCC using visual analysis including FDG uptake pattern as well as quantitative analysis.
Materials and Methods
Patients
Between March 2007 and November 2010, we retrospectively analyzed data of 108 patients with HCC who underwent interventional therapy and a follow-up FDG PET/CT scan at our institution. Inclusion criteria in the current study were the first use of interventional therapy (TACE, RFA, or PEIT) for HCC and FDG PET/CT within 1 month after interventional therapy. Patients with a history of the previous interventional therapy or an interval from interventional therapy to FDG PET/CT longer than 1 month were excluded.
Finally, 31 patients (24 men, 7 women; mean age, 61.8 ± 11.0 years) with 45 lesions were enrolled. All patients conformed to the diagnostic guidelines of HCC set by the Korean Liver Cancer Study Group [19]. The characteristics of the patients are shown in Table 1. Twenty-six patients with 40 lesions underwent TACE, two patients with 2 lesions underwent RFA, and three patients with 3 lesions underwent PEIT. The time interval between interventional therapy and FDG PET/CT was 0–31 days (mean 9.0 ± 8.8 days). None of these patients received pretreatment FDG PET/CT examination. All patients received follow-up contrast-enhanced CT (CECT) after interventional therapy to evaluate the treatment response. Serum alpha-fetoprotein (AFP) was evaluated before and after therapy. The study protocols were approved by the Institutional Review Board of our hospital (IRB number: 11-112).
PET/CT Study
FDG PET/CT was performed using an integrated PET/CT system (Discovery STE, GE Healthcare, Milwaukee, WI, USA). The patients fasted for more than 6 h before undergoing scanning. The blood glucose level of all patients was below 150 mg/dL prior to the FDG injection. Patients were encouraged to rest during the FDG uptake period. Images were acquired 60 min after intravenous injection of 7.0 MBq/kg of FDG. The data acquisition protocol was as follows: craniocaudal CT scan, with 60–150 mA adjusted to the patient’s body weight, 120 keV, and 3.75 mm section thickness (adjusted for PET section thickness) was performed for attenuation correction and localization. No oral or intravenous contrast was injected. Immediately after CT scanning, a caudocranial PET scan was obtained. Six to eight positions from the base of the skull or top of the brain to the proximal thigh were imaged for 3 min per table bed. The data were reconstructed iteratively by the ordered subset expectation maximization method. Finally, co-registered images were displayed on a workstation.
Image Interpretation
PET/CT images were retrospectively reviewed by two experienced nuclear medicine physicians with all available clinical and imaging information. These reviewers reached a consensus in cases of discrepancy. Dynamic CECT images were interpreted by an experienced radiologist. The PET data were initially visualized using arbitrary window and level values defined subjectively to ensure proper image interpretation. Visual analysis was graded as positive when FDG uptake was observed as an eccentric, nodular, or scattered pattern on both attenuation-corrected and noncorrected images (Fig. 1). If the lesion showed FDG uptake equal to or lower than that in the surrounding liver parenchyma or uniform and regular rim-shaped uptake pattern, the diagnosis was defined as negative (Fig. 2). For quantitative analysis, the standardized uptake value (SUV) was measured by the region of interest (ROI) technique. Maximum SUV (SUVmax) was assessed, and the ratio of SUVmax of tumor to mean SUV (SUVmean) of normal liver (TNR) was calculated. The contralateral hepatic lobe without tumor component was regarded as a normal liver regardless of cirrhotic liver disease. The normal liver ROI was drawn as a 2.8-cm-diameter spherical shape and located in the central portion of the lobe contralateral to the individual tumor location. ROI was drawn in the central portion of segment 7-8 in the case of HCCs of the left lobe, for example. In case of HCC in the right lobe, the normal liver ROI was drawn in the central portion of segment 2-3.
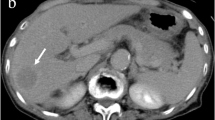
a–c Representative cases of residual tumor. a A 69-year-old man received transcatheter arterial chemoembolization (TACE). Coronal images of the PET and fused PET/CT obtained 1 day after TACE reveal eccentric FDG uptake along the margin of Lipiodol accumulation at the right hepatic lobe. b A 69-year-old man received TACE. Scattered pattern of FDG uptake is noted in segment 4 on the axial plane of PET and fused PET/CT images obtained 3 days after treatment. c A 42-year-old man with infiltrative hepatocellular carcinoma (HCC) in the left hepatic lobe. The PET and fused PET/CT axial images obtained 29 days after TACE show nodular and scattered increased FDG activity
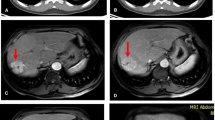
a–d Representative case of rim-shaped FDG uptake. A 63-year-old women who had hepatocellular carcinoma (HCC) in segment 8 received radiofrequency ablation (RFA) treatment. The fused PET/CT (a) and PET (b) images performed 1 day after RFA show rim-shaped increased FDG activity surrounding the ablation zone. The uniform peripheral enhancement is noted on contrast-enhanced arterial-phase CT 1 day after RFA (c). On follow-up, a CT image obtained 39 days after RFA (d) demonstrates loss of enhancement, which is indicative of benign periablational enhancement
Data Analyses
The patients were divided into two groups, with and without residual tumor, based on 6-month clinicoradiologic follow-up of CECT with/without serum AFP. When the lesion showed enhancement in the arterial phase of post-treatment CECT, it was considered to be residual tumor. If all tumors of a patient were diagnosed as negative on CECT, serum AFP was considered as a gold standard indicator as well as CECT. However, when all tumors of the patient were not fully treated and some still remained, were aggravated, or showed the presence of the newly developed metastasis, serum AFP level was not regarded as a reliable indicator because it can not reflect the individual tumor status. Thus, in these cases, CECT alone was used for follow-up of the individual primary lesions. Tumor size was measured by the longest diameter on the axial plane in the arterial phase of the pretreatment abdominal CT or MRI.
All data are expressed as mean ± SD. Statistical analysis was done using Predictive Analytics SoftWare (PASW) version 18.0 (IBM, Somers, NY). A p-value <0.05 was considered significant. The difference in quantitative parameters between the two groups was compared using Mann-Whitney U test. Wilcoxon signed rank test was used to compare serum AFP levels before and after interventional therapy. The average measure of the intraclass correlation coefficient (ICC) was assessed for the interobserver reliability. The PET/CT results were classified as true-positive, true-negative, false-positive, and false-negative based on clinicoradiologic evidence. Also, Mann-Whitney U test was used to compare the interval between interventional therapy and PET/CT for true and false results. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PET/CT were evaluated.
Results
Table 2 shows the diagnostic values of post-treatment FDG PET/CT using visual analysis. The respective values for sensitivity, specificity, PPV, NPV, and accuracy were 87.5, 71.4, 77.8, 83.3, and 80.0 %. The interobserver reliability was high, with an ICC value of 0.907, and the result was statistically significant (p < 0.001).
Two lesions after TACE revealed discrepancies between attenuation-corrected and noncorrected PET images. Both lesions showed increased FDG uptake at iodized oil (Lipiodol, Guerbet, Aulnay-sous-Bois, France) accumulation on attenuation-corrected PET/CT images, but no uptake on non-attenuation-corrected PET images, and so were defined as negative with overestimation artifact (Fig. 3).
a–c Attenuation-correction artifact due to chemoembolization agent. A 69-year-old man received transcatheter arterial chemoembolization (TACE). Attenuation-corrected PET/CT images (a, b) reveal increased FDG uptake within Lipiodol deposition in segment 5 (arrows), but non-attenuation-corrected PET image (c) shows no abnormal FDG uptake and is indistinguishable from normal liver parenchyma. These findings provide an example of an overestimation artifact from Lipiodol
Six tumors (four lesions with TACE and two lesions with PEIT) were positive on PET/CT images. However, all of these lesions were diagnosed as negative on clinicoradiologic evidence (Fig. 4). On the other hand, three tumors with TACE were negative on PET/CT and positive based on clinicoradiologic evidence. The clinical characteristics of these cases are summarized in Table 3.
a–c An example of a false-positive case on FDG PET/CT obtained immediately after PEIT. A 55-year-old man received percutaneous ethanol injection therapy (PEIT) in segment 8. The fused PET/CT (a) and PET (b) coronal images obtained 1 day after PEIT show increased FDG activity (arrow) along the medial aspect of the ablation zone (asterisk). No contrast enhancement is noted in the necrotic mass (asterisk) on a contrast-enhanced arterial-phase CT image 33 days after therapy (c), 3-month and 6-month follow-up CT (not shown)
Of the 45 lesions, 24 were classified in the residual tumor group and the other 21 lesions in the no residual tumor group. No residual tumor was detected after RFA or PEIT. The results of quantitative analysis between the two groups are summarized in Table 4. Tumor size of the residual tumor group was significantly higher than that of the no residual tumor group (64.8 ± 44.8 vs. 23.0 ± 12.8 mm, p = 0.001). No significant differences were noted in the SUVmax and TNR between the two groups (p > 0.05).
Table 5 shows the intervals between interventional therapy and PET/CT for true and false results. The intervals for true and false results were 8.8 ± 8.3 and 10.4 ± 9.8 days. There was no significant difference in the interval between the two groups (p = 0.689).
The differences in serum AFP level in patients with and without residual tumor are shown in Table 6. Of the 31 patients, 20 were classified in the residual tumor group and 11 were classified in the no residual tumor group. There were significant differences in post-therapeutic serum AFP between the two groups and in pre- and post-therapeutic serum AFP in the no residual tumor group (25,203.8 ± 84,153.7 vs. 24.5 ± 41.2 ng/ml, p = 0.022; 142.6 ± 220.4 vs. 24.5 ± 41.2 ng/ml, p = 0.025, respectively). There were no significant differences in pretherapeutic serum AFP between the two groups (p = 0.220),or in serum AFP over time in the residual tumor group (p = 0.528).
Discussion
The diagnostic success of FDG PET is based on the increased glycolytic rate seen in most tumors [20]. FDG is transported into cancer cells by glucose transporter proteins and then phosphorylated by hexokinase to FDG-6-phosphate. FDG-6-phosphate cannot be further metabolized in most cancer cells, and so it selectively accumulates in cancer tissues [16]. However, different degrees of glucose-6-phosphatase, which vary widely in different types of HCC, produce variable FDG accumulation in HCCs [2, 21]. Thus, the feasibility of PET in diagnosis of HCC is limited due to lower sensitivity [14, 15]. However, only a few studies have dealt with the role of FDG PET/CT in the evaluation of early treatment response after interventional therapy for HCC [2, 12, 18]. Furthermore, no report has focused on both FDG uptake pattern and intensity.
Increased FDG accumulation in inflammatory and infectious processes is well recognized [22, 23]. Reactive tissue changes, such as focal hyperemia and inflammation, are depicted in the periphery of the ablated lesion soon after RFA, PEIT, and TACE, and show a uniform and peripheral FDG uptake [9, 24–26]. Therefore, the authors regarded a rim-shaped FDG uptake surrounding a target lesion after interventional therapy as negative, despite the greater activity than normal liver.
Visual analysis of the present study showed high sensitivity but moderate specificity. Kim et al. reported that sensitivity, specificity, PPV, NPV, and accuracy of FDG PET/CT for viable HCC during early post-embolic period were 100, 63, 84, 100, and 88 %, respectively [11]. In a study of FDG PET/CT after TACE combined with RFA for HCC, Zhao et al. demonstrated the high efficacy of early post-therapeutic PET/CT with 90.9 % positive detection rate [27]. These findings are similar to our results. In the pattern-based analysis, 8 of the 10 rim-shaped uptake lesions were determined as true negative. This result strongly demonstrated that not all hypermetabolic lesions could be considered residual tumor, even if they showed greater FDG activity than the surrounding liver. It will be necessary to consider FDG uptake pattern to provide more accurate treatment response. Furthermore, of the 27 lesions with eccentric, nodular, or scattered patterns, 21 lesions were diagnosed as true positive.
There were six false-positive and three false-negative results in the present study. One false positive showed an eccentric FDG uptake in the lateral aspect of the ablation zone, but no evidence of residue on clinical follow-up. This may have been due to a benign inflammatory lesion in the needle track, as mentioned in an earlier study [28]. The other false-positives were also regarded as inflammatory changes during the early post-procedure period. When FDG PET is negative after the interventional therapy, although the causes are not clear, it means that tumors lie dormant with temporary dysfunction, but can survive [2] . Veit et al. reported that detection of residual tumors in the rim-like area remains challenging, as increased glucose metabolism due to tissue regeneration may superimpose on small areas of residual tumor, resulting in false-negative results [23]. Several studies have demonstrated that tissue regeneration and inflammation in the periphery of the necrotic zone can be seen as early as 2 days after ablation [21, 23, 29]. The observations support the recommendation to perform follow-up PET/CT early or at least within 2 days. However, three of the six patients with false-positives in our study underwent PET/CT within 24 h after treatment. Nevertheless, no statistically significant difference was noted in the time interval of therapy to PET/CT between true and false results. This indicates that a false result may not be associated with the interval from therapy to PET/CT scan. Further large population studies are required to define the time frame of tissue regeneration and to set the ideal time point of early follow-up PET/CT scan after interventional therapy.
In this study, we observed Lipiodol-induced attenuation-correction artifacts in two patients, which were positive on attenuation-corrected images and negative on noncorrected images. Chemoembolization materials cause an overestimation of PET activity when using CT information for PET attenuation correction [30]. Thus, non-attenuation-corrected PET images should be considered to avoid a misdiagnosis in patients treated with TACE.
All residual tumors, 24 lesions of 20 patients, were diagnosed after TACE. On the other hand, none of the patients showed residual tumor after RFA or PEIT. The patients with relatively small HCC may undergo RFA and PEIT as curative treatment. TACE, in contrast, can be used as a bridge to liver transplantation or as a palliative treatment in large or advanced intrahepatic HCC [5, 7]. From this perspective, our results are considered to be reasonable.
No significant differences were presently observed in the SUVmax and TNR between the residual and no residual tumor groups. True causes remain uncertain but may relate to the relatively higher background activity of the liver and inflammatory change during the early post-therapeutic period [31]. However, our results are in contrast to the previous study of Torizuka et al. [18]. These authors reported that the SUVs of viable tumors were significantly higher than those of nonviable tumors. This may have been due to different types of interventional therapy and/or the time interval from therapy to PET/CT.
Using serial AFP determinations to monitor treatment of HCC is well established in patients with increased AFP values prior to therapy, and several expert groups recommend serial follow-up to monitor efficacy of treatment [32–35]. Leung et al. found a marked reduction in serum AFP concentration in patients after effective combined systemic chemotherapy for inoperable HCC [36]. Lee et al. reported that patients with initial remission after TACE appeared to have lower serum AFP levels and smaller tumor size, compared to the patients with residual tumor [37]. In our analysis, serum AFP level and tumor size were correlated with treatment response. These findings are consistent with those in the literature.
The present study has several limitations. Because of the retrospective design, only a small number of patients were included and we adopted CECT as a gold standard due to the absence of histologic examination, although it is not a perfect method. It may limit the statistical power of our study, especially for quantitative analysis. Also, none of our patients received pretreatment PET/CT. Kim et al. reported that lipiodolized HCCs frequently show increased FDG uptake caused by increased glycolysis and the aggressive behavior of residual viable tumor [11]. Thus, increased FDG uptake seen on post-therapeutic PET/CT is considered as a reliable finding, despite the lack of a baseline examination.
Even if residual/recurrent tumor is found after interventional therapy, main treatment is usually another or the same interventional therapy such as TACE or PEIT. In this kind of therapy, the therapeutic effect extends more than 1 month. Further therapy for recurrent or residual tumor is usually performed at least several weeks after the previous therapy. In this setting, early detection of residual tumor by PET may be not valuable. However, sometimes TACE has been used as a theragnostic modality and in some cases clinicians are considering surgery or chemotherapy as well as subsequent interventional therapy if the first interventional therapy is unsatisfactory [11, 18, 34]. Under such conditions, early follow-up PET/CT may help clinicians to respond more quickly to the remaining tumor. With comprehensive knowledge of false-positive patterns, FDG PET/CT may play a role in the evaluation of early treatment response after interventional therapy for HCC. The results of the present study suggest that FDG PET/CT visual analysis may be more useful than quantitative analysis. Well-designed large prospective studies will be needed to substantiate the conclusion.
References
Röcken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis. 2001;19:269–78.
Higashi T, Hatano E, Ikai I, Nishii R, Nakamoto Y, Ishizu K, et al. FDG PET as a prognostic predictor in the early post-therapeutic evaluation for unresectable hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2010;37:468–82.
Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–86.
Thabet A, Kalva S, Gervais DA. Percutaneous image-guided therapy of intra-abdominal malignancy: imaging evaluation of treatment response. Abdom Imaging. 2009;34:593–609.
Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210–6.
Kwon JH. Is percutaneous ethanol injection therapy still effective for hepatocellular carcinoma in the era of radiofrequency ablation? Gut Liver. 2010;4:S105–12.
Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67–76.
Lim HK, Han JK. Hepatocellular carcinoma: evaluation of therapeutic response to interventional procedures. Abdom Imaging. 2002;27:168–79.
Kim KW, Lee JM, Choi BI. Assessment of the treatment response of HCC. Abdom Imaging. 2011;36:300–14.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Kim HO, Kim JS, Shin YM, Ryu JS, Lee YS, Lee SG. Evaluation of metabolic characteristics and viability of lipiodolized hepatocellular carcinomas using 18F-FDG PET/CT. J Nucl Med. 2010;51:1849–56.
Paudyal B, Oriuchi N, Paudyal P, Tsushima Y, Iida Y, Higuchi T, et al. Early diagnosis of recurrent hepatocellular carcinoma with 18F-FDG PET after radiofrequency ablation therapy. Oncol Rep. 2007;18:1469–73.
Kim YK, Lee KW, Cho SY, Han SS, Kim SH, Kim SK, et al. Usefulness 18F-FDG positron tomography/computed tomography for detecting recurrence of hepatocellular carcinoma in posttransplant patients. Liver Transpl. 2010;16:767–72.
Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–9.
Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–7.
Sun L, Wu H, Guan YS. Positron emission tomography/computer tomography: challenge to conventional imaging modalities in evaluating primary and metastatic liver malignancies. World J Gastroenterol. 2007;13:2775–83.
Sun L, Guan YS, Pan WM, Luo ZM, Wei JH, Zhao L, et al. Metabolic restaging of hepatocellular carcinoma using whole-body 18F-FDG PET/CT. World J Hepatol. 2009;31:90–7.
Torizuka T, Tamaki N, Inokuma T, Magata Y, Yonekura Y, Tanaka A, et al. Value of fluorine-18-FDG-PET to monitor hepatocellular carcinoma after interventional therapy. J Nucl Med. 1994;35:1965–9.
Kim JW. Diagnosis of hepatocellular carcinoma. J Kor Liver Cancer Study Group. 2010;10:1–5.
Lammertsma AA, Hoekstra CJ, Giaccone G, Hoekstra OS. How should we analyse FDG PET studies for monitoring tumour response? Eur J Nucl Med Mol Imaging. 2006;33:S16–21.
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33.
Imperiale A, Federici L, Lefebvre N, Braun JJ, Pfumio F, Kessler R, et al. F-18 FDG PET/CT as a valuable imaging tool for assessing treatment efficacy in inflammatory and infectious diseases. Clin Nucl Med. 2010;35:86–90.
Veit P, Antoch G, Stergar H, Bockisch A, Forsting M, Kuehl H. Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: initial results. Eur Radiol. 2006;16:80–7.
Purandare NC, Rangarajan V, Shah SA, Sharma AR, Kulkarni SS, Kulkarni AV, et al. Therapeutic response to radiofrequency ablation of neoplastic lesions: FDG PET/CT findings. Radiographics. 2011;31:201–13.
Becker CD, Grossholz M, Mentha G, Roth A, Giostra E, Schneider PA, et al. Ablation of hepatocellular carcinoma by percutaneous ethanol injection: imaging findings. Cardiovasc Intervent Radiol. 1997;20:204–10.
Jiang B, Lou Q, Ding XF, Sa XY, Chen LR, Yu SY, et al. Histopathological changes in rat transplanted hepatoma after lipiodol transarterial embolization. Zhonghua Zhong Liu Za Zhi. 2004;26:205–8.
Zhao M, Wu PH, Zeng YX, Zhang FJ, Huang JH, Fan WJ, et al. Evaluating efficacy of transcatheter arterial chemo-embolization combined with radiofrequency ablation on patients with hepatocellular carcinoma by 18FDG-PET/CT. Ai Zheng. 2005;24:1118–23.
Lokken RP, Gervais DA, Arellano RS, Tuncali K, Morrison PR, Tatli S, et al. Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol. 2007;189:845–8.
Khandani AH, Calvo BF, O'Neil BH, Jorgenson J, Mauro MA. A pilot study of early 18F-FDG PET to evaluate the effectiveness of radiofrequency ablation of liver metastases. AMJ Am J Roentgenol. 2007;189:1199–202.
Heusner TA, Fronz U, Jentzen W, Verhagen R, Forsting M, Bockisch A, et al. The effect of different chemoembolization materials on CT-based attenuation correction in PET/CT. Rofo. 2007;179:1159–65.
Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61–77.
Sturgeon CM, Duffy MJ, Hofmann BR, Lamerz R, Fritsche HA, Gaarenstroom K, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56:e1–48.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–30.
Benson 3rd AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–91.
Giovannini M, Elias D, Monges G, Raoul JL, Rougier P. Hepatocellular carcinoma. Br J Cancer. 2001;84:74–7.
Leung TWT, Patt YZ, Lau WY, Ho SKW, Yu SCH, Chan ATC, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–81.
Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52–8.
Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.H., Won, K.S., Choi, B.W. et al. Usefulness of F-18 FDG PET/CT in the Evaluation of Early Treatment Response After Interventional Therapy for Hepatocellular Carcinoma. Nucl Med Mol Imaging 46, 102–110 (2012). https://doi.org/10.1007/s13139-012-0138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-012-0138-8