Abstract
Purpose
To compare the accuracy of different MR sequences in simultaneous PET/MR imaging for T staging in non-small-cell lung cancer in relation to histopathology.
Methods
The study included 28 patients who underwent dedicated thoracic PET/MR imaging before tumour resection. Local tumour staging was performed separately by three readers with each of the following MR sequences together with PET: transverse T2 BLADE, transverse non-enhanced and contrast-enhanced T1 FLASH, T1 3D Dixon VIBE in transverse and coronal orientation, coronal T2 HASTE, and coronal TrueFISP. The staging results were compared with histopathology after resection as the reference standard. Differences in the accuracy of T staging among the MR sequences were evaluated using McNemar’s test. Due to multiple testing, Bonferroni correction was applied to prevent accumulation of α errors; p < 0.0024 was considered statistically significant.
Results
Compared with histopathology, T-staging accuracy was 69 % with T2 BLADE, 68 % with T2 HASTE, 59 % with contrast-enhanced T1 FLASH, 57 % with TrueFISP, 50 % with non-enhanced T1 FLASH, and 45 % and 48 % with T1 3D Dixon VIBE in transverse and coronal orientation, respectively. Staging accuracy with T2 BLADE was significantly higher than with non-enhanced T1 FLASH and with T1 3D Dixon VIBE in transverse and coronal orientations (p < 0.0024). T2 HASTE had a significantly higher T-staging accuracy than transverse T1 3D-Dixon-VIBE (p < 0.0024).
Conclusion
Transverse T2 BLADE images provide the highest accuracy for local tumour staging and should therefore be included in dedicated thoracic PET/MR protocols. As T1 3D Dixon VIBE images acquired for attenuation correction performed significantly worse, this sequence cannot be considered sufficiently accurate for local tumour staging in the thorax.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its high sensitivity, 18F-FDG PET has proven to be indispensable in lymph node (N) and distant metastasis (M) staging in patients with non-small-cell lung cancer (NSCLC) [1, 2]. However, local tumour extent can only be assessed poorly due to the low spatial and anatomical resolution of PET. As morphological images with a high spatial resolution are mandatory for local tumour (T) staging, an additional CT scan is necessary. Hence, the combination of both modalities (18F-FDG PET/CT) allows precise TNM staging [3–5] in one hybrid examination and has therefore become widely accepted in clinical practice and the latest guidelines [6].
Apart from the lower radiation dose of MR imaging compared with CT, optimized MR sequences allow high-resolution imaging of small pulmonary nodules [7–9] and due to its inherent soft-tissue contrast, pleural and mediastinal involvement may be diagnosed more precisely using MR imaging than CT [10, 11]. Although comparable results in staging accuracy were found in preliminary studies [12–14] evaluating PET/MR imaging in the setting of lung cancer assessment, the addition of functional MR imaging is promising. The simultaneous acquisition of diffusion-weighted imaging provides information about tumour biology complementary to metabolic information depicted by PET [15–17]. This information might be used to perform more precise tumour characterization and might serve as a tool for prognostic evaluation [18] that cannot be performed with PET/CT. Additionally, MR-assisted PET motion correction could reduce SUV measurement errors by reducing smearing [19] and improving attenuation correction [20] without the need for additional triggering devices or increased radiation exposure.
However, PET/MR imaging also poses new challenges. In contrast to PET/CT, the excellent soft-tissue contrast potentially requires a multitude of MR sequences, while the absence of established MR imaging protocols for PET/MR imaging can result in long examination times. This may hinder the broader dissemination of PET/MR imaging in clinical practice [21]. To increase the spectrum of clinical applications of this hybrid imaging method, some authors have promoted extensive MR imaging protocols to provide all required information in a single examination [22, 23]. Others, however, have promoted faster short PET/MR protocols [24–27]. According to these studies, MR information redundant to PET should be avoided and MR imaging should be used solely for anatomical correlation to achieve examination times comparable to those with low-dose PET/CT [27]. In this setting, even a rather nondiagnostic T1 3D Dixon volume-interpolated breath-hold examination (VIBE) sequence used for MR-based attenuation correction has been explored for diagnostic purposes as part of a “quick” PET/MR imaging examination. Although this single MR sequence protocol has a fast acquisition time, whether the inherent soft tissue contrast of MR imaging with this protocol has been sufficiently explored for diagnostic purposes may be questioned.
In lung cancer imaging, local tumour stage is determined by tumour size and potential invasion of adjacent organs. Therefore, the ideal MR sequence should be able to image the tumorous lesion with high contrast compared to its surroundings, robustly depict lesion size and possible local invasion, and provide accurate spatial registration with PET images. We analysed the diagnostic accuracy of different MR sequences acquired as part of a dedicated thoracic PET/MR protocol in order to optimize thoracic PET/MR protocols.
Material and methods
Patients
All 28 therapy-naive patients (13 women, 15 men; mean age 65.1 ± 8.2 years) who underwent a dedicated, thoracic PET/MR scan from August 2012 to February 2014 followed by NSCLC resection were included in this retrospective analysis. Tumour resection was performed within 5 weeks of the PET/MR examination. The resection specimens were investigated according to institutional standards in line with the WHO/IARC diagnostic criteria [28]. Local tumour and lymph node staging was performed in accordance with the 7th edition of the TNM classification in the histopathological report [29]. All PET/MR examinations as well as the retrospective analysis of the present data were approved by the local ethics committee as part of general ethical approval for retrospective research in integrated PET/MR. Written informed consent was obtained from all patients before the examinations.
PET/CT
As part of the initial staging, whole-body PET/CT scans were performed on a Biograph mCT scanner (Siemens AG, Healthcare Sector, Erlangen, Germany) with the low-dose or full-dose technique 60 min after injection of a weight-dependent dose of 18F-FDG (mean activity 280 ± 50 MBq). Blood glucose levels had to be below 150 mg/dl. For the full-dose scans, 100 ml of an iodine-based contrast agent (Ultravist©; Bayer Pharma, Berlin, Germany) was injected 70 s before the CT scan. The vendor-specific techniques CareKV and CareDose 4D were used for the low-dose scan (presets 120 kV and 40 mAs) and the full-dose scans (presets 120 kV and 210 mAs). A slice thickness of 5 mm was reconstructed. PET acquisition was performed for 2 min per bed position (seven bed positions for a regular patient) and images were computed using iterative reconstruction (OSEM, three iterations, 21 subsets) and a Gaussian filter of 4 mm. In the full-dose scans, the portal venous phase was used for attenuation correction while low-dose CT scans were used in the low-dose examinations.
PET/MR
PET/MR scans were performed on a Biograph mMR scanner (Siemens AG, Healthcare Sector, Erlangen, Germany). The thoracic examinations were performed either as a dedicated protocol or as part of a whole-body examination (mean waiting time between 18F-FDG injection and PET/MR imaging 130 ± 34 min). During a 20-min PET acquisition of the thorax, the following MR sequences were acquired:
-
A coronal T1 3D Dixon VIBE sequence for four-class segment attenuation correction with TR 3.6 ms, TE1 1.23 ms, TE2 2.46 ms, flip angle 10°, slice thickness 3.12 mm, field of view (FOV) 500 × 500 mm2, matrix size 96 × 96 and acquisition time (TA) 19 s, during inspiration breath-hold.
-
A transverse T2 BLADE turbospin echo (TSE) sequence with TR 4,360 ms, TE 160 ms, slice thickness 5 mm, FOV 400 × 400 mm2, matrix size 384 × 384 and TA 210 s, during free breathing.
-
A transverse T1 fast low-angle shot (FLASH) sequence before contrast agent administration with TR 1,510 ms, TE 2.15 ms, slice thickness 5 mm, FOV 400 × 325 mm2, matrix size 320 × 256 and TA 77 s, during inspiration breath-hold.
-
A coronal T2 steady-state free precession (TrueFISP) sequence with TR 3.75 ms, TE 1.64 ms, slice thickness 6 mm, FOV 330 × 330 mm2, matrix size 320 × 272 with TA 26 s, during inspiration breath-hold.
-
A coronal T2 half-fourier acquired single shot turbo spin echo (HASTE) sequence with TR 649 ms, TE 51 ms, slice thickness 6 mm, FOV 330 × 330 mm2, matrix size 320 × 288 and TA 26 s, during inspiration breath-hold.
-
A contrast-enhanced transverse T1 FLASH sequence with TR 1,700 ms, TE 3.33 ms, slice thickness 7.5 mm, FOV 400 × 366 mm2, matrix size 256 × 205 and TA 46 s and with fat saturation (TI 1,200 ms, during free breathing.
PET data were simultaneously acquired in list mode. Images were reconstructed with the vendor-specific software on the corresponding PET/MR console (Siemens AG, Healthcare Sector, Erlangen, Germany) after the examination using 3D iterative image reconstruction (OSEM, three iterations, 21 subsets, Gaussian filter of 4 mm, matrix size 344 × 344).
Image analysis
T staging and tumour size with different MR sequences
In individual sessions, three different readers analysed each MR sequence and contrast-enhanced CT images in random order together with PET images as fused images on a dedicated Osirix Apple workstation (Apple Inc, Cupertino, CA). To avoid recognition bias, each reader adhered to a minimum time of 3 weeks between the reading sessions for each pulse sequence. In this setting, 3D Dixon VIBE images were assessed in coronal and transverse orientations in separate reading sessions. Before image analysis, artefacts in the PET images (e.g. caused by discrepancies between the μ-map and the non-attenuation-corrected PET images) were excluded. Then, T stage was assessed according to the 7th edition of the TNM classification [29] and maximum tumour diameter was noted by each reader for the different datasets. If a PET-positive lesion was not detectable on the morphological datasets, a diameter of 0 mm was recorded.
Visual detectability and alignment of the PET finding with the primary tumour and lymph nodes with different MR sequences
Image quality for primary tumour visualization and potentially present 18F-FDG-avid lymph nodes was rated using the following two four-point ordinal scales:
-
For visual detectability against the surrounding background: 3 excellent contrast, 2 intermediate contrast, 1 low contrast, 0 not detectable
-
For alignment quality of the PET finding with the morphological correlate 3 excellent alignment, 2 minor misalignment, 1 major misalignment, an 0 complete dislocation/no morphological correlate detectable
For alignment quality, fused PET/MR images were analysed in the orientation of acquisition. In these images, alignment of two datasets was analysed in each spatial direction.
Statistical analysis
McNemar’s test was used to test for differences in T staging accuracy among all MR sequences. To investigate interreader agreement, Fleiss’ kappa was calculated. In a subgroup analysis, T staging accuracies of contrast-enhanced PET/CT and the different MR sequences were compared. As T stage depends highly on tumour size, Bland-Altman analysis was performed to determine the agreement between the actual tumour diameters measured during histopathological examination and the maximum diameters measured with each MR sequence. Here, three patients had to be excluded, two because multiple nodules were present in the resected lung and the lesions measured during histopathological examination could not be identified clearly, one because no precise size was indicated in the histopathological report.
Visual detectability and alignment quality of the PET findings with the morphological correlates were evaluated separately for the primary tumour and lymph node metastases and differences among the MR sequences were evaluated using two-tailed Wilcoxon rank sum tests. As a total of 28 individual tests were necessary to test for differences among the seven MR sequences (T2 BLADE vs. T1 FLASH, T2 BLADE vs. contrast-enhanced T1 FLASH etc.), Bonferroni correction was applied to prevent α-error accumulation and p < 0.0024 was considered statistically significant. In the subgroup analysis in patients with contrast-enhanced PET/CT, p < 0.007 indicated statistical significance after Bonferroni correction. IBM SPSS Statistics 22 (IBM, Armonk, NY, USA) and r (http://www.r-project.org/) were used for statistical analysis.
Results
Patients
According to histopathology, eight patients had stage IA and eight stage IB disease. Stage IIB disease was found in three and stage IIIA disease in seven patients. In two patients, additional pulmonary nodules were found in the same lobe, while one patient had an additional node in a different but ipsilateral lobe. Stage IIIB and stage IV disease was found in one patient each (adenocarcinoma in 17 patients, squamous cell carcinoma in 6, "other" histopathology in 5).
T staging with different MR sequences
According to histopathology, T staging was correct with transverse T2 BLADE in 69 % of patients, with coronal HASTE in 67.9 %, with contrast-enhanced T1 FLASH in 59 %, with TrueFISP in 57.1 %, with non-enhanced T1 FLASH in 50 %, with transverse T1 3D Dixon VIBE in 45.2 % and with coronal T1 3D Dixon VIBE in 47.6 % (Figs. 1 and 2; Online resource 1). While the number of inaccurate evaluations of extrapulmonary spread were comparable among all sequences, inaccurate lesion detection and tumour site estimation were especially problematic with T1 FLASH and with coronal and axial T1 3D Dixon VIBE (Table 1).
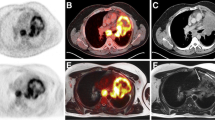
A 64-year-old patient with squamous cell carcinoma in the left lower lobe: a–c PET/CT, d–j PET/MR. While the histopathologically confirmed pT1a lesion is visualized very well on the PET images (c, f), the non-enhanced CT image (a), the T2 BLADE images (d, g, arrow) and the contrast-enhanced T1 FLASH image (h, arrow), it is not detectable on the non-enhanced T1 FLASH image (I) or the T1 3D Dixon VIBE image (j), rendering exact local tumour staging impossible
Interreader agreement was high for all sequences (T2 BLADE 0.85, non-enhanced T1 FLASH 0.82, contrast-enhanced T1 FLASH 0.87, transverse T1 3D Dixon VIBE 0.78, TrueFISP 0.65, T2 HASTE 0.71, coronal T1 3D Dixon VIBE 0.87).
Among the 17 patients who also underwent a contrast-enhanced PET/CT scan, T staging with PET/CT was correct in 73 %. Contrast-enhanced PET/CT was superior to transverse T1 3D Dixon VIBE (p < 0.007), but no significant differences were observed between contrast-enhanced PET/CT and the other MR sequences (p > 0.007).
Tumour size with different MR sequences
Tumour diameter on transverse T2 BLADE correlated best with the actual tumour diameter and showed the smallest limits of agreement in Bland-Altman analysis. Tumour diameter on transverse and coronal T1 3D Dixon VIBE used for attenuation correction had the widest limits of agreement and showed a worse correlation than the diameters determined with the other MR sequences (Figs. 2 and 3, Table 2, Online resource 2).
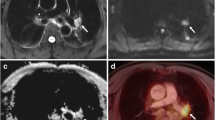
An 84-year-old patient with squamous cell carcinoma in the right upper lobe: a–c PET/CT, d–j PET/MR. On histopathological work-up, the maximum tumour diameter was 33 mm. As no pleural invasion was present (d, e), pathological T stage was 2a. While correct T staging was possible with a maximum tumour diameter of 31 mm on T2 BLADE images (d, h) and 38 mm on T2 HASTE images (j), underestimation of the maximum tumour diameter on T1 3D Dixon VIBE images (g, i; 20 mm in axial orientation) led to downstaging with this MR sequence
Visual detectability and alignment of the PET finding with the primary tumour with different MR sequences
The best detectability of the primary tumour against the surrounding background was found with transverse T2 BLADE (mean 2.55), transverse contrast-enhanced T1 FLASH (mean 2.46) and coronal HASTE (mean 2.67). Detectability was low particularly with non-enhanced T1 FLASH and 3D Dixon VIBE, (Figs. 2 and 4; see Online resources 3 and 4 for further information).
Alignment of the PET findings with the primary tumour worked best with transverse T2 BLADE while problems were observed with non-enhanced T1 FLASH and transverse T1 3D Dixon VIBE (Fig. 5; see Online resources 5 and 6 for further information).
Visual detectability and alignment of the PET findings with lymph nodes with different MR sequences
The visual detectability of lymph node metastases was highest with transverse T2 BLADE and coronal HASTE (Fig. 6; see Online resources 7 and 8 for further information). The best alignment of the PET findings with the morphological correlate in 18F-FDG-avid lymph nodes was found with transverse T2 BLADE (Fig. 7; see Online resources 9 and 10 for further information).
Discussion
To increase patient comfort and reduce costs by avoiding time-consuming acquisition of redundant data, specific MR sequences have to be selected to provide tailored MR imaging protocols in PET/MR hybrid imaging. In this study T2 sequences were able to provide correct T staging in NSCLC patients while minimizing errors in tumour size measurement. On the other hand, correct T staging was achieved in less than 50 % of patients with T1 3D Dixon VIBE MR imaging which is used for MR-based attenuation correction in the context of PET/MR imaging. Relying only on T1 3D Dixon VIBE, as suggested by some authors to shorten the MR protocol, cannot be considered a diagnostic choice for PET/MR in thoracic imaging.
As the suitability of T2 sequences for lung imaging has been thoroughly investigated, the excellent imaging performance of the transverse BLADE and the coronal HASTE sequences is not surprising. Thoracic MR protocols including fast T2 sequences such as HASTE and BLADE have been promoted due to the high image quality with these sequences and their sensitivity in detecting pleural effusion or intraatelectatic tumour growth [9, 30, 31]. While both sequence types feature fast data acquisition during breath-hold, BLADE, furthermore, is acquired in a radial multishot scheme that is inherently insensitive to breathing motion [32].
In this study, both sequences provided high visual detectability of the primary tumour. Alignment between the PET findings and the morphological correlate was excellent particularly with the transverse T2 BLADE sequence. As local staging strongly depends on the maximum tumour diameter in lower tumour stages, the excellent correlation between tumour diameters determined with these two sequences and the actual histopathologically confirmed tumour diameters is of great importance. Furthermore, both sequences allow reliable imaging of lung nodules bigger than 0.5 cm as shown by Schroeder et al. [33] and Lutterbey et al. [34], which is of considerable importance for the detection of pulmonary metastases. The non-enhanced T1 FLASH sequence performed significantly worse in local tumour staging than the transverse T2 BLADE sequence, and provided inferior visual tumour detectability and a considerable number of inaccuracies in tumour diameter evaluation. After contrast agent administration visual detectability of the primary tumour increased. However, due to the unfavourable slice thickness in our protocol, larger differences between the tumour sizes on morphological images and in the histopathological work-up were observed for contrast-enhanced T1, leading to a statistically not significant inferior accuracy in local tumour staging. Diagnostic 3D gradient echo sequences such as VIBE with a high spatial resolution and improved contrast as a result of increased acquisition times have been reported to show a high detectability of pulmonary nodules [8] and have been strongly promoted for thoracic imaging [9, 30, 35].
Chandarana et al. found that even non-enhanced, but still diagnostic VIBE sequences with a high spatial resolution have a high sensitivity for lung lesions >0.5 cm [35]. Therefore, several authors have compared the suitability of PET/CT and PET/MR for thoracic imaging in consecutive acquisitions using the nondiagnostic low-resolution T1 3D Dixon VIBE sequence for attenuation correction. In contrast to a diagnostic VIBE sequence with high spatial resolution, the acquisition time as well as the matrix size were reduced to image a large FOV in one breath-hold per bed position, resulting in low spatial resolution images with decreased image contrast. Hence, these images were originally intended for generation of the patient tissue attenuation correction map, which is part of MR-based attenuation correction in PET/MR hybrid imaging.
Eiber et al. analysed a cohort of 35 patients who underwent PET/CT and MR imaging. The anatomical and morphological allocation of PET-positive findings was slightly better with the T1 3D Dixon VIBE sequence that provided a high spatial resolution (79 × 192 pixels) than with a low-dose CT scan, but the difference was not significant [36]. Furthermore, Stolzmann et al. found no significant differences in the detection rates for pulmonary lung nodules with a low-dose CT scan and the T1 3D Dixon VIBE sequence with a matrix size of 320 × 256 pixels, although significant differences in lesion size were observed [26].
The discrepancy between the findings of the previous studies and those of our study were most likely caused by differences in the MR protocols. In contrast to the VIBE protocol used by Eiber et al. [36] and Stolzmann et al. [26], the VIBE protocol used with the Biograph mMR has faster data acquisition and a considerably lower matrix size of 96 x 96 pixels. This reduction leads to a larger voxel size and reduces the detectability of finer anatomical structures and pulmonary nodules. Furthermore, the sequence parameters of the 3D Dixon VIBE sequence for attenuation correction are optimized for rapid acquisition times rather than diagnostic performance. Therefore, lung nodule detection and evaluation of primary tumour size were significantly worse with the T1 3D Dixon VIBE sequence in the coronal and transverse orientations than with the transverse T2 BLADE, coronal T2 HASTE and transverse contrast-enhanced T1 FLASH sequences, leading to a significantly lower accuracy in local tumour staging than with the transverse T2 BLADE sequence. The present study indicates that the current implementation of the 3D Dixon VIBE sequence for attenuation correction should not be used as the only imaging sequence for T staging in NSCLC patients and its use as a single replacement for other morphological sequences should be reevaluated.
The diagnosis of lymph node metastases depends mainly on locally increased 18F-FDG uptake. Thus differences between PET/CT and PET/MR imaging may not be expected in N staging. This theory is supported by the available literature [13, 37]. However, visual detectability and exact alignment of the two datasets for 18F-FDG-avid lymph nodes are important for further staging examinations (e.g. endobronchial ultrasound-guided transbronchial needle aspiration, follow-up examinations) and treatment planning. In this study, the transverse T2 BLADE sequence performed significantly better than the other MR sequences, including the T1 3D Dixon VIBE sequence. These findings are in line with the results of Jeong et al. in 12 patients, showing that the T1 3D Dixon VIBE sequence performed significantly worse in lymph node staging than contrast-enhanced PET/CT [38]. Significant differences were also observed between the transverse T2 BLADE and the coronal T2 HASTE sequences. These findings are comparable with the results of Both et al. who evaluated lung cancer patients using CT and MR imaging and showed that a transverse T2 TSE sequence was superior to a coronal HASTE sequence for lymph node staging [9].
This study had some limitations. Although all patients had a histopathological work-up, the number of analysed patients was small and the results should therefore be considered as preliminary. Since contrast-enhanced PET/CT is considered the most precise diagnostic method for thoracic imaging, a larger cohort of patients undergoing contrast-enhanced PET/CT and PET/MR imaging is needed to reliably evaluate differences between the two hybrid imaging modalities. Another limitation of this study was the lack of diagnostic VIBE sequences with high spatial resolution as part of the PET/MR protocol. As stated above, these have proven to be of enormous value in lung imaging in recent studies [8, 9, 30] and have already been used by Schwenzer et al. [12] and Fraioli et al. [39] in their studies on lung cancer imaging in PET/MR.
In conclusion, this study showed that the most accurate local tumour staging was achieved with a transverse T2 BLADE sequence, a transverse contrast-enhanced T1 FLASH sequence, a coronal T2 HASTE sequence and a coronal TrueFISP sequence. As the transverse T2 BLADE sequence had the lowest limits of agreement in relation to tumour size, and the highest visual detectability and the best alignment between the PET findings and the morphological correlate for primary tumours and lymph node metastases, it should be considered as a component of every PET/MR protocol for lung cancer evaluation. Although considered as sufficient by some authors, T1 3D Dixon VIBE in the context of this study showed the lowest accuracy in T staging and the widest limits of agreement in relation to tumour size in the transverse and coronal orientations. As visual detectability and alignment between the PET findings and the morphological correlate were low for primary tumours and lymph node metastases with the T1 3D Dixon VIBE sequence, T and N staging in NSCLC patients based on this sequence alone cannot be recommended according to the results of this study.
References
Pieterman RM, van Putten JWG, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61.
MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, et al. High rate of detection of unsuspected distant metastases by PET in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol. 2001;50:287–93.
Van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–92.
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7.
Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–33.
Goeckenjan G, Sitter H, Thomas M, Branscheid D, Flentje M, Griesinger F, et al. Prevention, diagnosis, therapy, and follow-up of lung cancer. Pneumologie. 2010;65:39–59.
Hatabu H, Chen Q, Stock KW, Gefter WB, Itoh H. Fast magnetic resonance imaging of the lung. Eur J Radiol. 1999;29:114–32.
Biederer J, Schoene A, Freitag S, Reuter M, Heller M. Simulated pulmonary nodules implanted in a dedicated porcine chest phantom: sensitivity of MR imaging for detection. Radiology. 2003;227:475–83.
Both M, Schultze J, Reuter M, Bewig B, Hubner R, Bobis I, et al. Fast T1- and T2-weighted pulmonary MR-imaging in patients with bronchial carcinoma. Eur J Radiol. 2005;53:478–88.
Landwehr P, Schulte O, Lackner K. MR imaging of the chest: mediastinum and chest wall. Eur Radiol. 1999;9:1737–44.
Ohno Y, Sugimura K, Hatabu H. MR imaging of lung cancer. Eur J Radiol. 2002;44:172–81.
Schwenzer NF, Schraml C, Müller M, Brendle C, Sauter A, Spengler W, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging – pilot study. Radiology. 2012;264:551–8.
Heusch P, Buchbender C, Köhler J, Nensa F, Gauler T, Gomez B, et al. Thoracic staging in lung cancer: prospective comparison of 18F-FDG PET/MR imaging and 18F-FDG PET/CT. J Nucl Med. 2014;55:373–8.
Heusch P, Nensa F, Schaarschmidt B, Sivanesapillai R, Beiderwellen K, Gomez B, et al. Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging. 2015;42:56–65.
Regier M, Derlin T, Schwarz D, Laqmani A, Henes FO, Groth M, et al. Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)? Eur J Radiol. 2012;81:2913–8.
Heusch P, Buchbender C, Köhler J, Nensa F, Beiderwellen K, Kühl H, et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in hybrid 18F-FDG PET/MRI in non-small cell lung cancer (NSCLC) lesions: initial results. Rofo. 2013;185:1056–62.
Schmidt H, Brendle C, Schraml C, Martirosian P, Bezrukov ID-I, Hetzel J, et al. Correlation of simultaneously acquired diffusion-weighted imaging and 2-deoxy-[18F] fluoro-2-D-glucose positron emission tomography of pulmonary lesions in a dedicated whole-body magnetic resonance/positron emission tomography system. Invest Radiol. 2013;48:247–55.
Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198:75–82.
Werner MK, Parker JA, Kolodny GM, English JR, Palmer MR. Respiratory gating enhances imaging of pulmonary nodules and measurement of tracer uptake in FDG PET/CT. AJR Am J Roentgenol. 2009;193:1640–5.
Goerres GW, Kamel E, Seifert B, Burger C, Buck A, Hany TF, et al. Accuracy of image coregistration of pulmonary lesions in patients with non-small cell lung cancer using an integrated PET/CT system. J Nucl Med. 2002;43:1469–75.
von Schulthess GK, Veit-Haibach P. Workflow considerations in PET/MR imaging. J Nucl Med. 2014;55 Suppl 2:19S–24S.
Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–6.
Beiderwellen K, Gomez B, Buchbender C, Hartung V, Poeppel TD, Nensa F, et al. Depiction and characterization of liver lesions in whole body [18F]-FDG PET/MRI. Eur J Radiol. 2013;82:e669–75.
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–55.
Appenzeller P, Mader C, Huellner MW, Schmidt D, Schmid D, Boss A, et al. PET/CT versus body coil PET/MRI: how low can you go? Insights Imaging. 2013;4:481–90.
Stolzmann P, Veit-Haibach P, Chuck N, Rossi C, Frauenfelder T, Alkadhi H, et al. Detection rate, location, and size of pulmonary nodules in trimodality PET/CT-MR: comparison of low-dose CT and dixon-based MR imaging. Invest Radiol. 2013;48:241–6.
Huellner MW, Appenzeller P, Kuhn FP, Husmann L, Pietsch CM, Burger IA, et al. Whole-body nonenhanced PET/MR versus PET/CT in the staging and restaging of cancers: preliminary observations. Radiology. 2014;273:859–69.
Parkin M, Tyczynski JE, Bofetta P, Samet J, Shields N. Tumours of the lung. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. p. 9–122.
Sobin LH, Gospodarowicz MK, Wittekind C (Eds) Lung. In: TNM classification of malignant tumours. New York: Wiley-Blackwell; 2011. p. 138–143,
Yamashita Y, Yokoyama T, Tomiguchi S, Takahashi M, Ando M. MR imaging of focal lung lesions: elimination of flow and motion artifact by breath-hold ECG-gated and black-blood techniques on T2-weighted turbo SE and STIR sequences. J Magn Reson Imaging. 1999;9:691–8.
Puderbach M, Hintze C, Ley S, Eichinger M, Kauczor H-U, Biederer J. MR imaging of the chest: a practical approach at 1.5T. Eur J Radiol. 2007;64:345–55.
Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42:963–9.
Schroeder T, Ruehm SG, Debatin JF, Ladd ME, Barkhausen J, Goehde SC. Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol. 2005;185:979–84.
Lutterbey G, Leutner C, Gieseke J, Rodenburg J, Elevelt A, Sommer T, et al. Detektion fokaler lungenläsionen mit der magnetresonanz-tomographie mittels T2-gewichteter ultrashort-turbo-spin-echo-sequenz im vergleich zur spiral-computer-tomographie. Rofo. 1998;169:365–9.
Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 2013;268:874–81.
Eiber M, Martinez-Möller A, Souvatzoglou M, Holzapfel K, Pickhard A, Löffelbein D, et al. Value of a dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38:1691–701.
Kohan AA, Kolthammer JA, Vercher-Conejero JL, Rubbert C, Partovi S, Jones R, et al. N staging of lung cancer patients with PET/MRI using a three-segment model attenuation correction algorithm: initial experience. Eur Radiol. 2013;23:3161–9.
Jeong JH, Cho IH, Kong EJ, Chun KA. Evaluation of dixon sequence on hybrid PET/MR compared with contrast-enhanced PET/CT for PET-positive lesions. Nucl Med Mol Imaging. 2014;48:26–32.
Fraioli F, Screaton NJ, Janes SM, Win T, Menezes L, Kayani I, et al. Non-small-cell lung cancer resectability: diagnostic value of PET/MR. Eur J Nucl Med Mol Imaging. 2015;42:49–55.
Compliance with ethical standards
Conflicts of interest
None.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This publication contains parts of the MD thesis of Florian Hild and is therefore in partial fulfilment of the requirements for an MD thesis at the Medical Faculty of the Heinrich-Heine University, Dusseldorf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
(PDF 16 kb)
Online resource 2
(PDF 1926 kb)
Online resource 3
(PDF 1609 kb)
Online resource 4
(PDF 14 kb)
Online resource 5
(PDF 1693 kb)
Online resource 6
(PDF 14 kb)
Online resource 7
(PDF 1652 kb)
Online resource 8
(PDF 14 kb)
Online resource 9
(PDF 1750 kb)
Online resource 10
(PDF 14 kb)
Rights and permissions
About this article
Cite this article
Schaarschmidt, B., Buchbender, C., Gomez, B. et al. Thoracic staging of non-small-cell lung cancer using integrated 18F-FDG PET/MR imaging: diagnostic value of different MR sequences. Eur J Nucl Med Mol Imaging 42, 1257–1267 (2015). https://doi.org/10.1007/s00259-015-3050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3050-5