Abstract
Purpose
To investigate the differences in brain glucose consumption during olfactory stimulation between subjects affected by multiple chemical sensitivity (MCS) and a group of healthy individuals.
Methods
Two 18F-FDG PET/CT scans were performed in 26 subjects (6 men and 20 women; mean age 46.7 ± 11 years) with a clinical diagnosis of MCS and in 11 healthy controls (6 women and 5 men; mean age 45.7 ± 11 years), the first scan after a neutral olfactory stimulation (NS) and the second after a pure olfactory stimulation (OS). Differences in 18F-FDG uptake were analysed by statistical parametric mapping (SPM2).
Results
In controls OS led to an increase in glucose consumption in BA 18 and 19 and a reduction in glucose metabolism in BA 10, 11, 32 and 47. In MCS subjects, OS led to an increase in glucose consumption in BA 20, 23, 18 and 37 and a reduction in glucose metabolism in BA 8, 9 and 10.
Conclusion
The results of our study suggest that cortical activity in subjects with MCS differs from that in healthy individuals during olfactory stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple chemical sensitivity (MCS) is a nonallergic and relatively common chronic disorder in western populations [1]. The prevalence of self-reported chemical sensitivity symptoms in population-based studies ranges from 9 % to 33 % [1], whereas the prevalence of physician-diagnosed MCS and of disabling consequences in the form of social and occupational disruption are much lower, ranging from 0.5 % to 6.3 % [1–3]. Affected subjects complain of nonspecific symptoms attributed to exposure to common volatile chemicals, such as fragranced consumer products, tobacco smoke, freshly printed papers, perfume and pesticides [1]. The triggered symptoms are various and include dysosmia, headache, fatigue, respiratory symptoms, dizziness and/or nausea [3]. There seems to be no clear dose–response relationship between exposure and reaction [4] and no general association between the type of chemical and symptoms. Surprisingly, individuals with MCS have not been shown to differ from non-intolerant individuals in terms of olfactory detection sensitivity or rated intensities of olfactory stimuli [4].
In MCS subjects different patterns of cortical and subcortical brain activation occur during olfactory stimulation. In particular, several perfusional postexposure studies have shown decreased perfusion in the olfactory, cingulus, hippocampus, parahippocampus and temporal cortex regions and in several subcortical areas [5], and abnormal activation of the amygdala, piriform cortex, insular cortex and anterior cingulate cortex during odour processing [6]. However, in the previous study normal olfactory performance was demonstrated using an olfactory screening test in MCS subjects even though most of these patients reported frequent olfactory discomfort during daily life [7].
In normal subjects, the processing of odours (that starts in the olfactory receptor neurons in the olfactory mucosa [8]) mainly involves the piriform cortex, the entorhinal cortex and the periamygdaloid cortex. These structures have been termed the olfactory cortex [9]. Several subcortical areas within the amygdala, including the anterior cortical nucleus and the nucleus of the lateral olfactory tract are also involved [10, 11]. The olfactory cortex projects to a secondary series of structures, including the caudal orbitofrontal cortex (OFC) [9, 10] that receives the majority of corticocortical projections from the caudal portion of the piriform cortex [10, 12]. This structure is considered to constitute the secondary olfactory cortex. Functional imaging studies have shown that the olfactory kinship detection process involves the frontotemporal junction, the insula and the dorsomedial prefrontal cortex, but not the primary or secondary olfactory cortices, the related piriform cortex or the OFC [13] that receive extensive projections subsequently connecting the caudal OFC to other anatomical subsections of the OFC [9]. Two PET/CT studies have shown a significant increase in cerebral blood flow (CBF) in these cortical areas (especially in the piriform cortex and the OFC) in healthy subjects, reflecting active recruitment of these structures during olfactory stimulation [2, 14].
The aim of this study was to compare by means of 18F-FDG PET/CT the metabolic changes in the brain occurring during pure olfactory stimulation (OS) with those occurring during neutral olfactory stimulation (NS) in subjects with MCS and in a cohort of healthy controls (HC subjects) who were identified as normosmic by clinical examination since, to the best of our knowledge, few studies have investigated the usefulness of this imaging modality in olfactory provocative testing.
Materials and methods
HC and MCS subjects
The HC subjects comprised 11 right-handed individuals (6 women and 5 men; mean age 45.7 ± 11 years) without otorhinolaryngological or neurological diseases. Subjects with diabetes, an oncological or HIV history, a neurological or psychiatric or mood disorder, or a history of surgery, radiation or trauma to the brain were excluded from the study. Moreover, we did not consider patients undergoing treatment with drugs that could interfere with 18F-FDG uptake and distribution in the brain [15]. No patient showed liver or renal impairment, and no patient was pregnant or breastfeeding.
The peripheral blood of MCS and HC subjects was tested for the usual parameters. The results of all haematological examinations were normal. A detailed case history was obtained from all subjects. All subjects underwent an ear nose and throat examination with a fibreoptic check of the upper airways. The subjects were assessed using the Mini Mental State Examination and MRI to exclude neurological diseases. Conditions that could potentially lead to the development of olfactory dysfunction were considered as exclusion criteria. Thus, patients with sinonasal disorders or a history of surgery, head trauma, neuropsychiatric disorder (Parkinson’s disease, Alzheimer’s disease, schizophrenia, multiple sclerosis and depression), lower airway and/or lung diseases, active hepatitis, cirrhosis, chronic renal failure, vitamin B12 deficiency, alcohol, tobacco or drug abuse, cerebral vascular accidents, insulin-dependent diabetes mellitus, hypothyroidism and Cushing syndrome were not included in the study.
MCS was diagnosed in 26 subjects (6 men and 20 women; mean age 46.7 ± 11 years) according to the criteria of Cullen [16]. MCS was confirmed in all subjects by the clinic physician at the time of recruitment. Both HC and MCS subjects were evaluated with the multiple forced choice Sniffin’ Sticks screening test [15] and found to be normosmic.
The Ethics Committee of the Tor Vergata University School of Medicine approved the research protocol. The study adhered to the principles of the Declaration of Helsinki and all of the participants provided written informed consent.
Olfactory stimulation and 18F-FDG injection
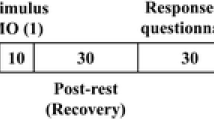
As in our similar study in this field [2], all subjects underwent 18F-FDG PET after receiving the NS which was administered via a common aerosol facial mask in which the ampoule contained only 5 ml saline (0.9 % NaCl; Fig. 1). After 1 month (to allow sufficient time between radiation exposures), the subjects underwent a second 18F-FDG PET scan after receiving a simple OS administered using the same aerosol facial mask in which the ampoule contained a solution of 1.5 ml 100 % vanillin [Dacor Ltd (http://www.herborientis.com/) manufactured and Sarandrea Ltd (http://www.sarandrea.it/) distributed respectively the product that is actually registered under their co-branding] and 5 ml saline (0.9 % NaCl) [2]. Under both conditions the oxygen flow rate was 3.5 l/min. Before the stimulation period each patient lay for 30 min with the eyes closed in a semidarkened and quiet room without any artificial stimulation. Taking into account the kinetics of 18F-FDG in the brain (see below) [17–19] and the fact that during olfactory experiments subjects may automatically control their olfactory exposure by altering their respiration and attention to olfaction during the task condition [20], 18F-FDG was injected after 3 min of a continuous 9-min block stimulation with instructions not to sniff but only inhale the supplied air constantly (see below). The duration of the brain PET acquisition was 15 min and was started 24 min from the end of the olfactory stimulation in all the patients, as shown in Fig. 1.
18F-FDG PET/CT
A Discovery ST16 PET/CT system (GE Medical Systems, TN) was used to assess 18F-FDG brain distribution in all patients using a 3D-mode standard technique and a 128 × 128 matrix [2, 21]. Reconstruction was performed using the 3D ordered-subsets expectation maximization (OSEM) reconstruction method with 20 subsets and four iterations. The system combines a high-speed ultra 16-detector-row (912 detectors per row) CT unit and a PET scanner with 10,080 bismuth germanate crystals in 24 rings (axial full-width at half-maximum 1-cm radius, 5.2 mm in 3D mode, axial field of view 157 mm). All MCS and HC subjects fasted for at least 5 h before intravenous injection of 18F-FDG, and the serum glucose level was less than 95 mg/ml in all of them. All subjects were injected intravenously with 185 – 210 MBq of 18F-FDG and hydrated with 500 ml saline (0.9 % NaCl) [2, 21].
Statistical analysis
Differences in brain 18F-FDG uptake were analysed using statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab 6.5 (Mathworks, Natick, MA). PET data were subjected to affine and nonlinear spatial normalization into MNI space. The spatially normalized set of images were then smoothed with a 12-mm isotropic Gaussian filter to blur individual variations in gyral anatomy and to increase the signal-to-noise ratio. Images were globally normalized using proportional scaling to remove confounding effects on global cerebral glucose consumption changes, with a masking threshold of 0.8. The resulting statistical parametric maps, SPM{t}, were transformed into a normal distribution (SPM{z}) unit. SPM coordinates were corrected to match the Talairach coordinates by the subroutine implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging). Brodmann areas (BA) were then identified with a range of 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocentres, after importing the corrected coordinates, by Talairach client (http://www.talairach.org/index.html).
In accordance with Bennett et al. [22], SPM t-maps were thresholded at p < 0.05, corrected for multiple comparisons with the False Discovery Rate (FDR) option at the voxel level and at p < 0.01 corrected for multiple comparison at the cluster level. Due to the exploratory nature of the study, when statistically significant differences were not found with such conservative thresholds a height threshold of p < 0.001 uncorrected at the voxel level was applied. Only those clusters containing more than 125 (5 × 5 × 5 voxels, i.e. 11 × 11 × 11 mm) contiguous voxels were accepted as significant, based on the calculation of the partial volume effect resulting from the spatial resolution of the PET camera (about twice the FWHM). The voxel-based analyses were performed using a ‘two conditions, one scan/condition, paired t-test’ design model. The following comparisons were assessed: (1) OS vs. NS and vice versa in HC subjects (Tables 2 and 3), and (2) OS vs. NS and vice versa in MCS subjects (Tables 4 and 5).
Two-way analysis of variance was used to assess differences in sex and age. Differences in educational level and occupation were analysed using Fisher’s exact test. A hypothesis was considered valid when the p value was less than or equal to 0.05.
Results
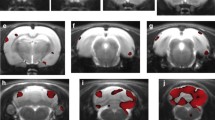
We did not find statistically significant differences in gender, age and the main sociodemographic variables between HC and MCS subjects (Table 1). In HC subjects, the OS led to an increase in glucose consumption in the left cerebellum occipital lobe bilaterally and the left limbic lobe (BA 18 and 19; Table 2 and Fig. 2) and a reduction in glucose metabolism in a large portion of the left frontal and left limbic lobes (BA 10, 11, 32, 38 and 47; Table 3, Fig. 2).
In MCS subjects, OS led to an increase in glucose consumption in the left temporal and occipital lobe bilaterally (BA 20, 23, 18 and 37; Table 4 and Fig. 2) and to a reduction in glucose metabolism in the frontal lobe bilaterally (BA 8, 9 and 10; Table 5 and Fig. 2).
With the exception of the results shown in Table 2 (OS vs. NS comparison in HC subjects), 18F-FDG uptake was not significantly increased or decreased in any subcortical area (Fig. 2).
Discussion
In HC subjects OS led to an increase in glucose consumption in the cuneus, lingual gyrus and parahippocampal region (Table 2), areas that are actively involved in olfaction [8–12]. Our findings are in agreement with those of other functional studies (perfusional PET and SPECT, fMRI) performed during OS in healthy individuals [5, 6, 23–25]. In HC subjects OS led to a decrease in metabolism in several frontal areas including a large portion of the superior and middle frontal gyrus and OFC in agreement with the findings of a previous study (Table 3 and Fig. 2) [2].
Intensive activation/deactivation in the left orbitofrontal gyrus (BA 11 and 47) has been found during emotionally valenced olfactory stimuli suggesting that pleasant and unpleasant emotional judgments are related to recruitment of these areas in the left hemisphere [26]. Several studies have shown that alterations in neural responses are specific to the judgments that will be made about an olfactory stimulus, since the pattern of brain activity induced in one task may not be present during other tasks. In a study in which subjects were asked to perform a task involving pleasantness, fMRI revealed that the medial OFC and pregenual cingulate cortex were activated, while an intensity task resulted in activation of the inferior frontal gyrus [27]. These differences in brain activation began before odour delivery, in an anticipatory fashion following the instructions, suggesting attentional biasing of incoming information in a way that matched the instructions [27]. These findings support previous work indicating that subjective affective judgments activate the secondary olfactory cortex while sensory information is processed in the primary olfactory cortex [28].
Gottfried and Zald showed that lateral and anterior regions of the OFC respond in a preferential manner to binary odour mixtures [29]. By investigating regional CBF in the lateral and anterior OFC, they found that these two regions respond to odour mixtures in different ways. Specifically, activation in the lateral OFC increased with increasing odorant impurity. On the other hand, the anterior region of the OFC was equally activated by binary odour mixtures and deactivated by single odours [29]. Interestingly, these data were further confirmed by Boyle et al. who found that the anterior OFC acts as a sort of on/off switch and this region is similarly activated in response to all odour mixtures and deactivated in response to single odorants [30].
In MCS subjects, a different cortical activation pattern was found during olfactory stimulation (Table 2 and 4, Fig. 2). On the one hand, a similar activation pattern involved both the cuneus and lingual gyrus, and on the other hand, as shown in Table 4, activation of the left inferior temporal gyrus (BA 20) was seen in MCS subjects in contrast to HC subjects. This area is widely connected to the visual cortex, playing a key role in the visual association process [31]. A study performed in subjects with dementia has shown a key role of this brain structure in the cognitive process, with the cortical atrophy of the left inferior temporal gyrus being related to cortical atrophy in deep structures such as the entorhinal cortex and amygdala [32].
Our findings are in partial disagreement with those of the study by Orriols et al. [5, 6]. In their study investigating brain perfusion patterns in a population of five MCS subjects passively breathing a substance able to trigger the syndrome, Orriols et al. did not find any area of significant major brain activity [5]. On the other hand, investigating a larger population of patients (12 patients), Hillert et al. found that although odour-processing brain regions were activated less in MCS subjects than in controls, there was a significant odorant-related increase in activation of the anterior cingulate cortex and cuneus-precuneus, in agreement with the results of our study [6].
In MCS subjects a significant odour-induced deactivation occurred in a large portion of the frontal lobe bilaterally (BA 8, 9 and 10). While other studies have shown a wide cortical deactivation (parietal, temporal and occipital areas) in MCS subjects exposed to odours (acetone) [6], only significant frontal deactivation occurred in MCS subjects in our study (Table 5). Possible explanations for these discrepancies could be related (1) to the type of odorant delivered in the present and the previous study [2] compared to other similar studies [23, 24, 26, 28, 33], and (2) to the particular resting condition in which subjects underwent odorant stimulation. Regarding the first explanation, most compounds used in many studies, such as that of Hillert et al. [6], were acetone (with strong trigeminal properties), vanillin and four other odorants (cedar oil, lavender oil, eugenol and butanol), mainly undiluted [6]. With respect to the second explanation, for the first time in the present study, a large group of MCS subjects underwent olfactory stimulation under resting conditions decoupled from the acquisition phase. Thus were able to create a defined ecological baseline condition to explore olfactory neural underpinnings, avoiding possible cortical activation related to unwanted attentional processes and odour sensitivity enhancement due to the examination environment [34].
Contrary to the description of symptoms in this pathology (that suggests neuronal sensitization as one of the main features [16, 35]), subjects with MCS showed reduced rather than enhanced activation of cerebral regions responsible of secondary processing of odour signals. In particular, as compared to HC subjects, several differences in these deactivation patterns during olfactory stimulation occurred in MCS subjects (Tables 3 and 5). MCS subjects showed reduced glucose consumption in BA 8, 9 and 10 while in HC showed reduced glucose metabolism in BA 11, 32, 38 and 47. The intensive deactivation in the PFC in MCS subjects is not surprising since this structure shows intensive interconnections with the amygdala, hypothalamus, midbrain and pons, and is likely to integrate higher-order brain functions with more developmentally fundamental brain activities such as emotional, visceral or autonomic functions [36] and changes in CBF in this region have already been shown during OS in MCS subjects [33].
During OS, MCS subjects did not show deactivation of the superior temporal gyrus (BA 38), anterior cingulate cortex (BA 32) or middle frontal gyrus (BA 47) in contrast to HC subjects (Tables 3 and 5, Fig. 2), further confirming a different metabolic processing of odour signals as a main characteristic of MCS [6] and ruling out the hypothesis that MCS may be a purely psychosomatic disorder with somatic distress upon exposure to odours [37].
Finally, the detection in our case studies of several secondary olfactory areas in both HC and MCS subjects [8–12, 26] may be due to the relatively slow kinetics of 18F-FDG, that shows activation/deactivation of those areas that are activated late during stimulation and prior to image acquisition, and this is a possible limitation of our study [17–19]. Indeed, the results of the comparison between the metabolic activity in normosmic individuals undergoing an OS and that in normosmic individuals not receiving any OS indicate increased activity in regions associated with OS (i.e. prefrontal, parietal, insular and temporal right hemisphere cortices) which is in accordance with the existing neuroimaging literature on olfactory activation [5, 6, 23–25].
The results of this study suggest that 18F-FDG PET/CT may be a suitable methodology for investigating brain metabolic cortical changes during olfactory tasks. Future studies could evaluate the usefulness of this imaging modality for the investigation of the impact of other stimuli (i.e. visual or auditory) on brain glucose consumption.
Conclusion
The results of this study suggest that brain metabolism differs between HC and MCS subjects during OS, suggesting that MCS subjects process odours differently from HC subjects. In particular, cortical odour processing in MCS subjects is characterized by deactivation that mainly involves the frontal cortex and by active recruitment of the left inferior temporal gyrus. 18F-FDG PET/CT may be a useful imaging modality for investigating variations in brain activity during olfactory tasks.
References
Dantoft TM, Elberling J, Brix S, Szecsi PB, Vesterhauge S, Skovbjerg S. An elevated pro-inflammatory cytokine profile in multiple chemical sensitivity. Psychoneuroendocrinology. 2014;40:140–50.
Alessandrini M, Micarelli A, Chiaravalloti A, Candidi M, Bruno E, Di Pietro B, et al. Cortico-subcortical metabolic correlates of olfactory processing in healthy resting subjects. Sci Rep. 2014;4:5146.
Karnekull SC, Jonsson FU, Larsson M, Olofsson JK. Affected by smells? Environmental chemical responsivity predicts odor perception. Chem Senses. 2011;36:641–8.
Andersson L, Claeson AS, Nyberg L, Stenberg B, Nordin S. Brain responses to olfactory and trigeminal exposure in idiopathic environmental illness (IEI) attributed to smells – an fMRI study. J Psychosom Res. 2014;77:401–8.
Orriols R, Costa R, Cuberas G, Jacas C, Castell J, Sunyer J. Brain dysfunction in multiple chemical sensitivity. J Neurol Sci. 2009;287:72–8.
Hillert L, Musabasic V, Berglund H, Ciumas C, Savic I. Odor processing in multiple chemical sensitivity. Hum Brain Mapp. 2007;28:172–82.
Alessandrini M, Micarelli A, Bruno E, Ottaviani F, Conetta M, Cormano A, et al. Intranasal administration of hyaluronan as a further resource in olfactory performance in multiple chemical sensitivity syndrome. Int J Immunopathol Pharmacol. 2013;26:1019–25.
Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–8.
Price JL. The olfactory system. In: Paxinos G, editor. The human nervous system. San Diego: Academic; 2003. p. 1198–212.
Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–34.
Price JL. Beyond the primary olfactory cortex: olfactory-related areas in the neocortex, thalamus and hypothalamus. Chem Senses. 1985;10:239–58. doi:10.1093/chemse/10.2.239.
Rolls ET, Critchley HD, Treves A. Representation of olfactory information in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1982–96.
Lundstrom JN, Boyle JA, Zatorre RJ, Jones-Gotman M. The neuronal substrates of human olfactory based kin recognition. Hum Brain Mapp. 2009;30:2571–80.
Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–40.
Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34:222–6.
Cullen MR. The worker with multiple chemical sensitivities: an overview. Occup Med. 1987;2:655–61.
Laffon E, Bardies M, Barbet J, Marthan R. Kinetic model analysis for absorbed dose calculation applied to brain in [18F]-fluorodeoxyglucose positron emission tomography imaging. Cancer Biother Radiopharm. 2010;25:665–9.
Schmidt K, Mies G, Sokoloff L. Model of kinetic behavior of deoxyglucose in heterogeneous tissues in brain: a reinterpretation of the significance of parameters fitted to homogeneous tissue models. J Cereb Blood Flow Metab. 1991;11:10–24.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916.
Savic I. Brain imaging studies of the functional organization of human olfaction. Neuroscientist. 2002;8:204–11.
Alessandrini M, Pagani M, Napolitano B, Micarelli A, Candidi M, Bruno E, et al. Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS One. 2013;8:e57596.
Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–22.
Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659:263–6.
Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–4.
Arshamian A, Iannilli E, Gerber JC, Willander J, Persson J, Seo HS, et al. The functional neuroanatomy of odor evoked autobiographical memories cued by odors and words. Neuropsychologia. 2013;51:123–31.
Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–9.
Rolls ET, Grabenhorst F, Margot C, da Silva MA, Velazco MI. Selective attention to affective value alters how the brain processes olfactory stimuli. J Cogn Neurosci. 2008;20:1815–26.
Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703.
Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev. 2005;50:287–304.
Boyle JA, Djordjevic J, Olsson MJ, Lundstrom JN, Jones-Gotman M. The human brain distinguishes between single odorants and binary mixtures. Cereb Cortex. 2009;19:66–71.
Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–107.
Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49:433–42.
Azuma K, Uchiyama I, Takano H, Tanigawa M, Azuma M, Bamba I, et al. Changes in cerebral blood flow during olfactory stimulation in patients with multiple chemical sensitivity: a multi-channel near-infrared spectroscopic study. PLoS One. 2013;8:e80567.
Ahs F, Miller SS, Gordon AR, Lundstrom JN. Aversive learning increases sensory detection sensitivity. Biol Psychol. 2013;92:135–41.
Berg ND, Linneberg A, Dirksen A, Elberling J. Prevalence of self-reported symptoms and consequences related to inhalation of airborne chemicals in a Danish general population. Int Arch Occup Environ Health. 2008;81:881–7.
Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, Goyal N. Neuropsychology of prefrontal cortex. Indian J Psychiatr. 2008;50:202–8.
Bornschein S, Hausteiner C, Zilker T, Forstl H. Psychiatric and somatic disorders and multiple chemical sensitivity (MCS) in 264 ‘environmental patients’. Psychol Med. 2002;32:1387–94.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Marco Alessandrini and Orazio Schillaci contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chiaravalloti, A., Pagani, M., Micarelli, A. et al. Cortical activity during olfactory stimulation in multiple chemical sensitivity: a 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging 42, 733–740 (2015). https://doi.org/10.1007/s00259-014-2969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2969-2